 Found 2094 hits with Last Name = 'cisar' and Initial = 'js'
Found 2094 hits with Last Name = 'cisar' and Initial = 'js' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598905
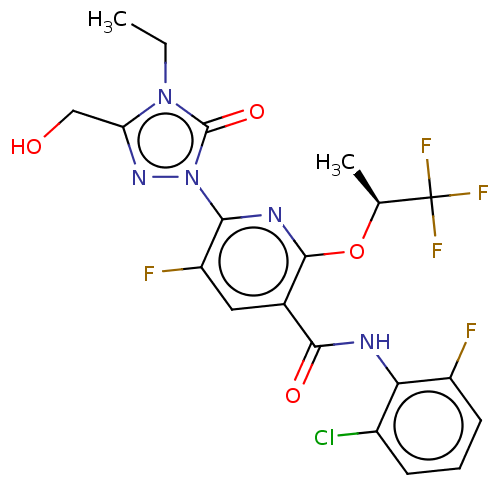
(CHEMBL5180161)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598917
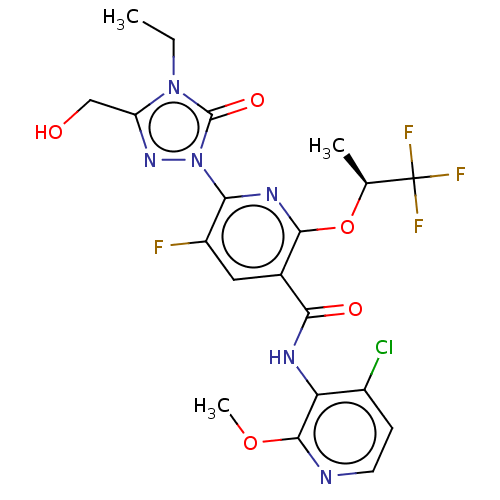
(CHEMBL5171223)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(Cl)ccnc2OC)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598923
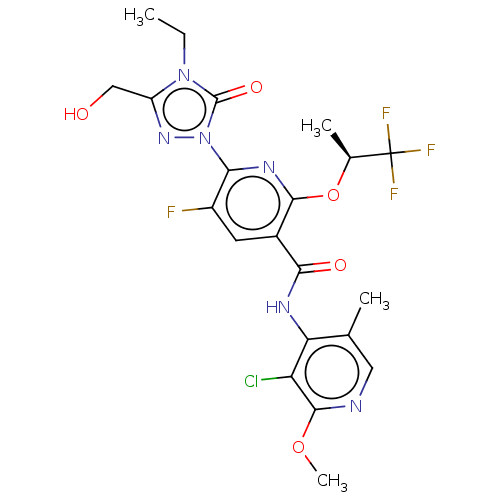
(CHEMBL5193821)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598902

(CHEMBL5187184)Show SMILES CCn1c(CO)nn(-c2nc(OC(C)C)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598922

(CHEMBL5186161)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2C)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598915

(CHEMBL5198030)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(Cl)ccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598926

(CHEMBL5206111)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cc(OC)nc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598913

(CHEMBL5193591)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598920

(CHEMBL5209113)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM470454
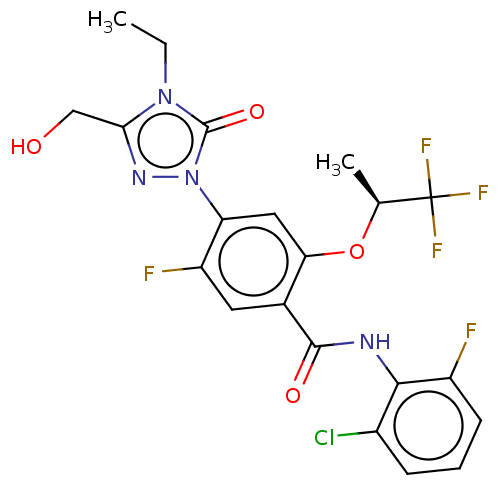
(N-(2-chloro-6-fluorophenyl)-4-[4-ethyl-3-(hydroxym...)Show SMILES CCn1c(CO)nn(-c2cc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O |r| Show InChI InChI=1S/C21H18ClF5N4O4/c1-3-30-17(9-32)29-31(20(30)34)15-8-16(35-10(2)21(25,26)27)11(7-14(15)24)19(33)28-18-12(22)5-4-6-13(18)23/h4-8,10,32H,3,9H2,1-2H3,(H,28,33)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598914
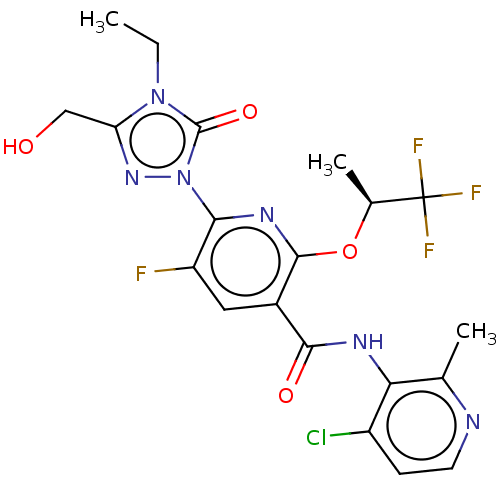
(CHEMBL5197263)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)nccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503331
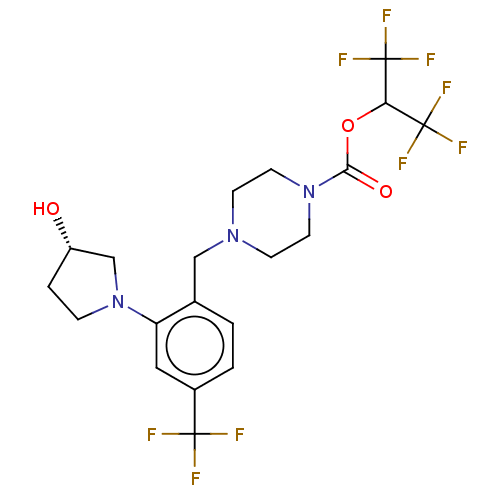
(CHEMBL4522687)Show SMILES O[C@H]1CCN(C1)c1cc(ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C20H22F9N3O3/c21-18(22,23)13-2-1-12(15(9-13)32-4-3-14(33)11-32)10-30-5-7-31(8-6-30)17(34)35-16(19(24,25)26)20(27,28)29/h1-2,9,14,16,33H,3-8,10-11H2/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503336
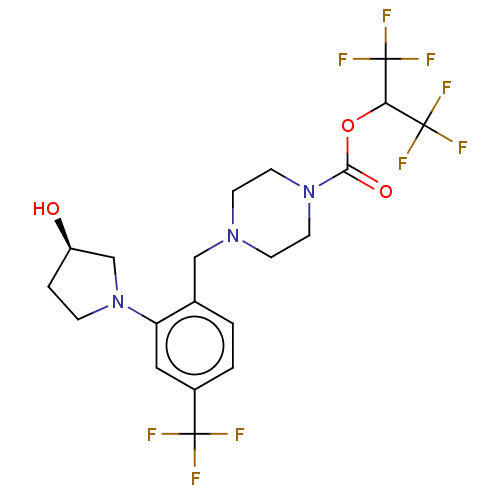
(CHEMBL4437377)Show SMILES O[C@@H]1CCN(C1)c1cc(ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C20H22F9N3O3/c21-18(22,23)13-2-1-12(15(9-13)32-4-3-14(33)11-32)10-30-5-7-31(8-6-30)17(34)35-16(19(24,25)26)20(27,28)29/h1-2,9,14,16,33H,3-8,10-11H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503340
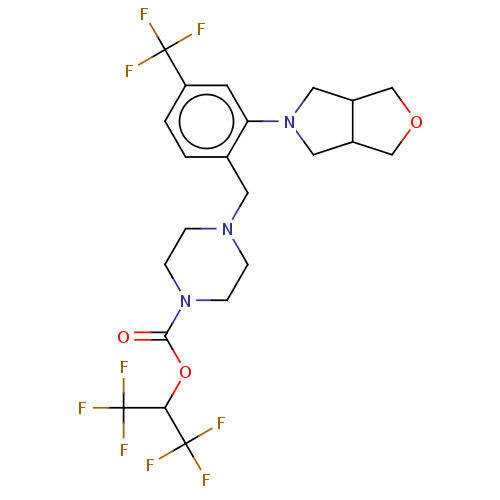
(CHEMBL4468636)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(cc2N2CC3COCC3C2)C(F)(F)F)CC1)C(F)(F)F Show InChI InChI=1S/C22H24F9N3O3/c23-20(24,25)16-2-1-13(17(7-16)34-9-14-11-36-12-15(14)10-34)8-32-3-5-33(6-4-32)19(35)37-18(21(26,27)28)22(29,30)31/h1-2,7,14-15,18H,3-6,8-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM180052
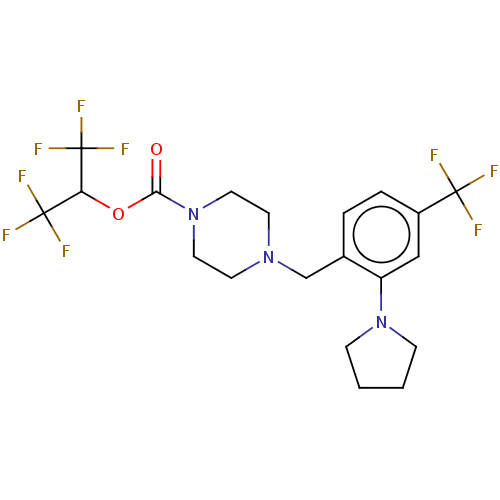
(US9133148, 9aq)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(cc2N2CCCC2)C(F)(F)F)CC1)C(F)(F)F Show InChI InChI=1S/C20H22F9N3O2/c21-18(22,23)14-4-3-13(15(11-14)31-5-1-2-6-31)12-30-7-9-32(10-8-30)17(33)34-16(19(24,25)26)20(27,28)29/h3-4,11,16H,1-2,5-10,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL in human intact PC3 cells preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598919

(CHEMBL5176109)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cncc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598918
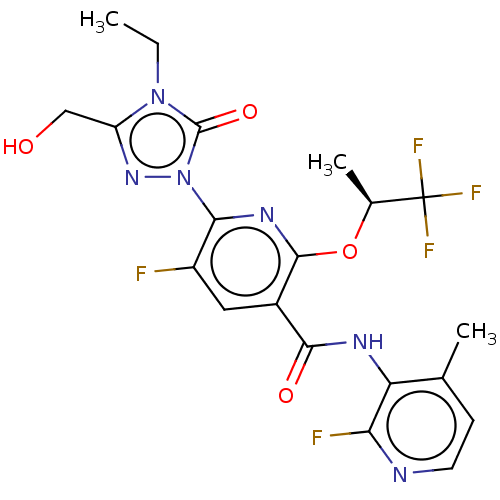
(CHEMBL5207423)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2F)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598916
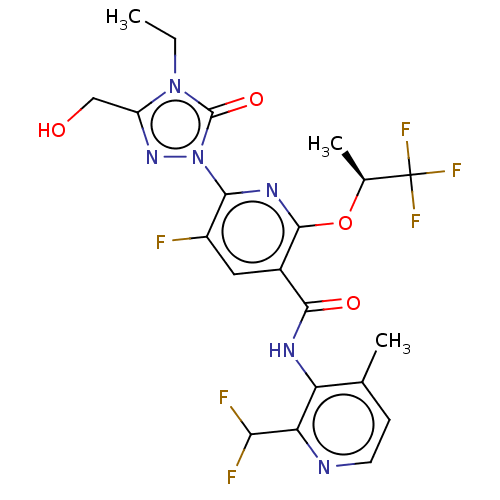
(CHEMBL5205042)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2C(F)F)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598910
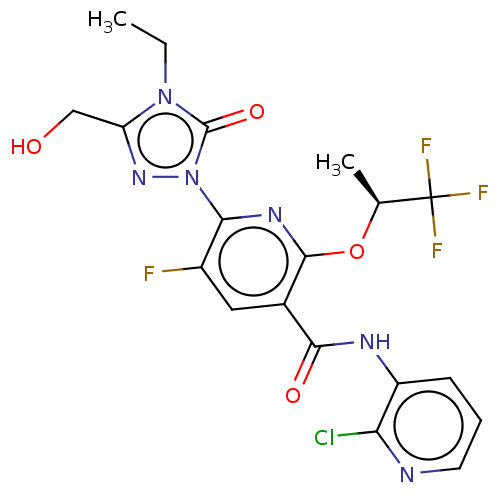
(CHEMBL5197809)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM179948

(US9133148, 2l)Show SMILES FC(F)(F)C(OC(=O)N1CCN(CC1)C(c1cccnc1)c1cccnc1)C(F)(F)F Show InChI InChI=1S/C19H18F6N4O2/c20-18(21,22)16(19(23,24)25)31-17(30)29-9-7-28(8-10-29)15(13-3-1-5-26-11-13)14-4-2-6-27-12-14/h1-6,11-12,15-16H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM180039

(US9133148, 9ad)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2cccc(Cl)c2N2CCOCC2)CC1)C(F)(F)F Show InChI InChI=1S/C19H22ClF6N3O3/c20-14-3-1-2-13(15(14)28-8-10-31-11-9-28)12-27-4-6-29(7-5-27)17(30)32-16(18(21,22)23)19(24,25)26/h1-3,16H,4-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503335
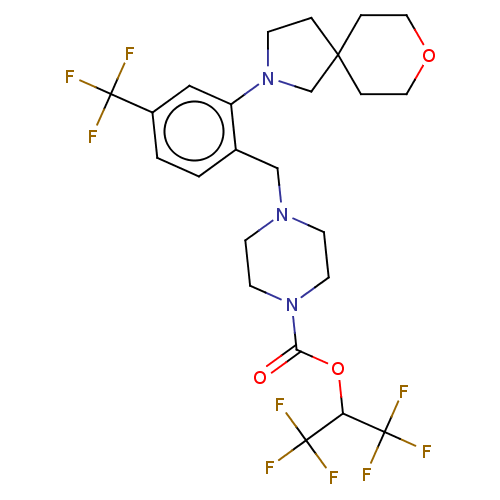
(CHEMBL4450649)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(cc2N2CCC3(C2)CCOCC3)C(F)(F)F)CC1)C(F)(F)F Show InChI InChI=1S/C24H28F9N3O3/c25-22(26,27)17-2-1-16(18(13-17)36-6-3-21(15-36)4-11-38-12-5-21)14-34-7-9-35(10-8-34)20(37)39-19(23(28,29)30)24(31,32)33/h1-2,13,19H,3-12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503334
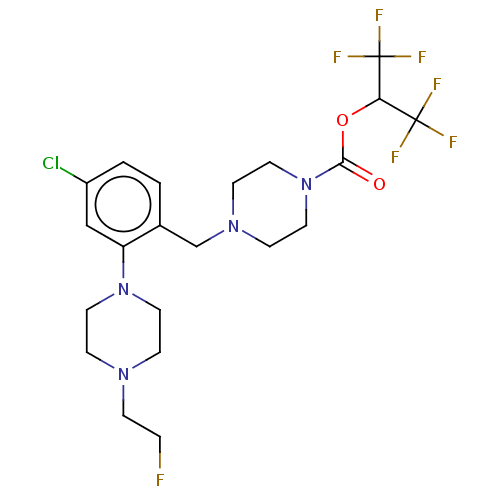
(CHEMBL4468639)Show SMILES FCCN1CCN(CC1)c1cc(Cl)ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H26ClF7N4O2/c22-16-2-1-15(17(13-16)32-9-5-30(4-3-23)6-10-32)14-31-7-11-33(12-8-31)19(34)35-18(20(24,25)26)21(27,28)29/h1-2,13,18H,3-12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598908

(CHEMBL5193091)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cnccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM180078

(US9133148, 10g)Show SMILES Cn1cc(CN2CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)c(n1)-c1ccccc1Cl Show InChI InChI=1S/C19H19ClF6N4O2/c1-28-10-12(15(27-28)13-4-2-3-5-14(13)20)11-29-6-8-30(9-7-29)17(31)32-16(18(21,22)23)19(24,25)26/h2-5,10,16H,6-9,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598907
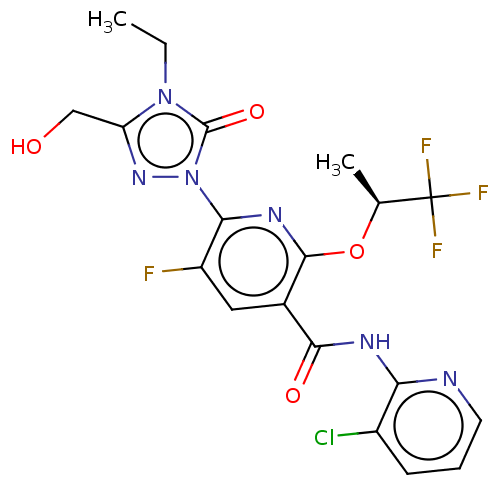
(CHEMBL5178023)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2ncccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598912

(CHEMBL5170313)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cc(C)cnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503339
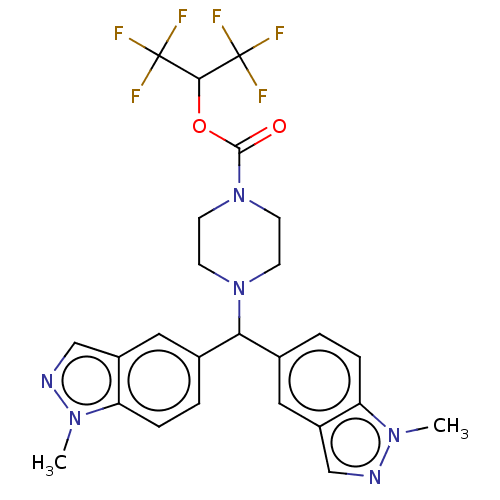
(CHEMBL4469632)Show SMILES Cn1ncc2cc(ccc12)C(N1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc2n(C)ncc2c1 Show InChI InChI=1S/C25H24F6N6O2/c1-34-19-5-3-15(11-17(19)13-32-34)21(16-4-6-20-18(12-16)14-33-35(20)2)36-7-9-37(10-8-36)23(38)39-22(24(26,27)28)25(29,30)31/h3-6,11-14,21-22H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598906

(CHEMBL5181951)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2ccccc2F)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Mus musculus (mouse)) | BDBM50503336

(CHEMBL4437377)Show SMILES O[C@@H]1CCN(C1)c1cc(ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C20H22F9N3O3/c21-18(22,23)13-2-1-12(15(9-13)32-4-3-14(33)11-32)10-30-5-7-31(8-6-30)17(34)35-16(19(24,25)26)20(27,28)29/h1-2,9,14,16,33H,3-8,10-11H2/t14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503333

(CHEMBL4539731)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1cc(ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)C(F)(F)F)O2 |r| Show InChI InChI=1S/C22H24F9N3O3/c23-20(24,25)14-2-1-13(17(9-14)34-11-15-3-4-16(12-34)36-15)10-32-5-7-33(8-6-32)19(35)37-18(21(26,27)28)22(29,30)31/h1-2,9,15-16,18H,3-8,10-12H2/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50598923

(CHEMBL5193821)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598924
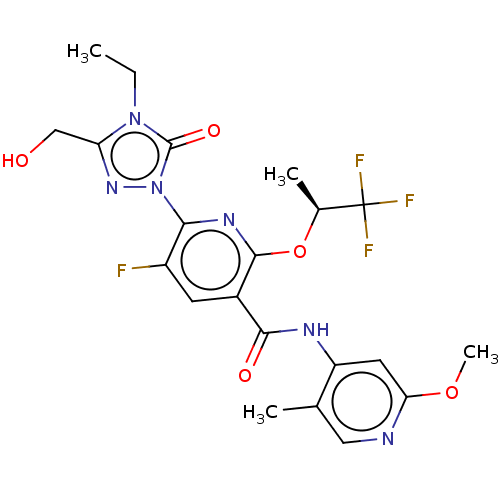
(CHEMBL5201357)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cc(OC)ncc2C)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598925

(CHEMBL5197655)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2ccnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503342

(CHEMBL4436074)Show SMILES CS(=O)(=O)c1ccc(CN2CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)c(c1)N1CCCC1 Show InChI InChI=1S/C20H25F6N3O4S/c1-34(31,32)15-5-4-14(16(12-15)28-6-2-3-7-28)13-27-8-10-29(11-9-27)18(30)33-17(19(21,22)23)20(24,25)26/h4-5,12,17H,2-3,6-11,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503327
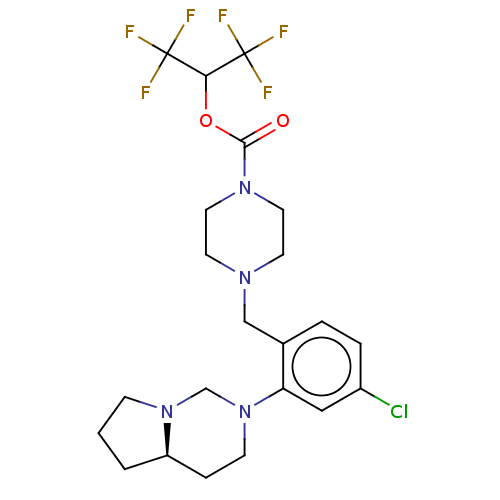
(CHEMBL4435014)Show SMILES [H][C@]12CCCN1CN(CC2)c1cc(Cl)ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C22H27ClF6N4O2/c23-16-4-3-15(18(12-16)33-7-5-17-2-1-6-32(17)14-33)13-30-8-10-31(11-9-30)20(34)35-19(21(24,25)26)22(27,28)29/h3-4,12,17,19H,1-2,5-11,13-14H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM179938

(US9133148, 2b)Show SMILES FC(F)(F)C(OC(=O)N1CCN(CC1)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C21H18Cl2F6N2O2/c22-15-5-1-13(2-6-15)17(14-3-7-16(23)8-4-14)30-9-11-31(12-10-30)19(32)33-18(20(24,25)26)21(27,28)29/h1-8,17-18H,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503344

(CHEMBL4439400)Show SMILES [H][C@]12CC[C@]([H])(COC1)N2c1cc(ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C22H24F9N3O3/c23-20(24,25)14-2-1-13(17(9-14)34-15-3-4-16(34)12-36-11-15)10-32-5-7-33(8-6-32)19(35)37-18(21(26,27)28)22(29,30)31/h1-2,9,15-16,18H,3-8,10-12H2/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM180052

(US9133148, 9aq)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(cc2N2CCCC2)C(F)(F)F)CC1)C(F)(F)F Show InChI InChI=1S/C20H22F9N3O2/c21-18(22,23)14-4-3-13(15(11-14)31-5-1-2-6-31)12-30-7-9-32(10-8-30)17(33)34-16(19(24,25)26)20(27,28)29/h3-4,11,16H,1-2,5-10,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Mus musculus (mouse)) | BDBM50503333

(CHEMBL4539731)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1cc(ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)C(F)(F)F)O2 |r| Show InChI InChI=1S/C22H24F9N3O3/c23-20(24,25)14-2-1-13(17(9-14)34-11-15-3-4-16(12-34)36-15)10-32-5-7-33(8-6-32)19(35)37-18(21(26,27)28)22(29,30)31/h1-2,9,15-16,18H,3-8,10-12H2/t15-,16+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598911
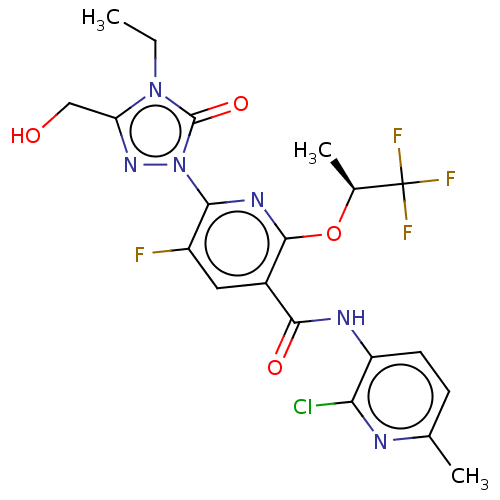
(CHEMBL5184263)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2ccc(C)nc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Mus musculus (mouse)) | BDBM50503331

(CHEMBL4522687)Show SMILES O[C@H]1CCN(C1)c1cc(ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C20H22F9N3O3/c21-18(22,23)13-2-1-12(15(9-13)32-4-3-14(33)11-32)10-30-5-7-31(8-6-30)17(34)35-16(19(24,25)26)20(27,28)29/h1-2,9,14,16,33H,3-8,10-11H2/t14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503337

(CHEMBL4517565)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(Cl)cc2N2CC3CCC(C2)O3)CC1)C(F)(F)F Show InChI InChI=1S/C21H24ClF6N3O3/c22-14-2-1-13(17(9-14)31-11-15-3-4-16(12-31)33-15)10-29-5-7-30(8-6-29)19(32)34-18(20(23,24)25)21(26,27)28/h1-2,9,15-16,18H,3-8,10-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598921

(CHEMBL5209156)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cnc(OC)c2C#N)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Mus musculus (mouse)) | BDBM50503334

(CHEMBL4468639)Show SMILES FCCN1CCN(CC1)c1cc(Cl)ccc1CN1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H26ClF7N4O2/c22-16-2-1-15(17(13-16)32-9-5-30(4-3-23)6-10-32)14-31-7-11-33(12-8-31)19(34)35-18(20(24,25)26)21(27,28)29/h1-2,13,18H,3-12,14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM180033

(US9133148, 9x)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(Br)cc2N2CCCC2)CC1)C(F)(F)F Show InChI InChI=1S/C19H22BrF6N3O2/c20-14-4-3-13(15(11-14)28-5-1-2-6-28)12-27-7-9-29(10-8-27)17(30)31-16(18(21,22)23)19(24,25)26/h3-4,11,16H,1-2,5-10,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Mus musculus (mouse)) | BDBM50503340

(CHEMBL4468636)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(cc2N2CC3COCC3C2)C(F)(F)F)CC1)C(F)(F)F Show InChI InChI=1S/C22H24F9N3O3/c23-20(24,25)16-2-1-13(17(7-16)34-9-14-11-36-12-15(14)10-34)8-32-3-5-33(6-4-32)19(35)37-18(21(26,27)28)22(29,30)31/h1-2,7,14-15,18H,3-6,8-12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503345

(CHEMBL4550205)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(cc2N2CCCCC2)C(F)(F)F)CC1)C(F)(F)F Show InChI InChI=1S/C21H24F9N3O2/c22-19(23,24)15-5-4-14(16(12-15)32-6-2-1-3-7-32)13-31-8-10-33(11-9-31)18(34)35-17(20(25,26)27)21(28,29)30/h4-5,12,17H,1-3,6-11,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM180029

(US9133148, 9t)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(Cl)cc2N2CCOCC2)CC1)C(F)(F)F Show InChI InChI=1S/C19H22ClF6N3O3/c20-14-2-1-13(15(11-14)28-7-9-31-10-8-28)12-27-3-5-29(6-4-27)17(30)32-16(18(21,22)23)19(24,25)26/h1-2,11,16H,3-10,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM180076

(US9133148, 10e)Show SMILES Cn1cc(CN2CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)c(n1)-c1ccccc1 Show InChI InChI=1S/C19H20F6N4O2/c1-27-11-14(15(26-27)13-5-3-2-4-6-13)12-28-7-9-29(10-8-28)17(30)31-16(18(20,21)22)19(23,24)25/h2-6,11,16H,7-10,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data