

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-SCH-23390 from human dopamine D1 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50394089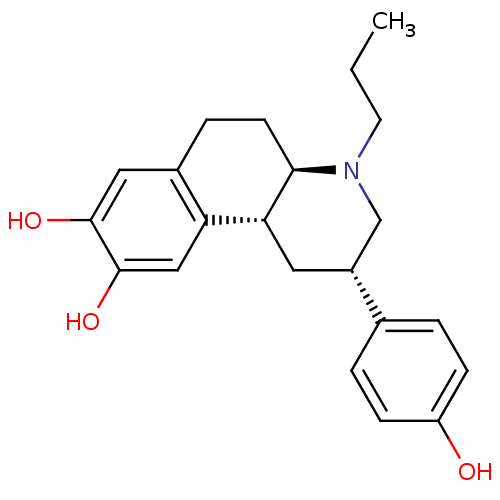 (CHEMBL2158640) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D2long receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50020222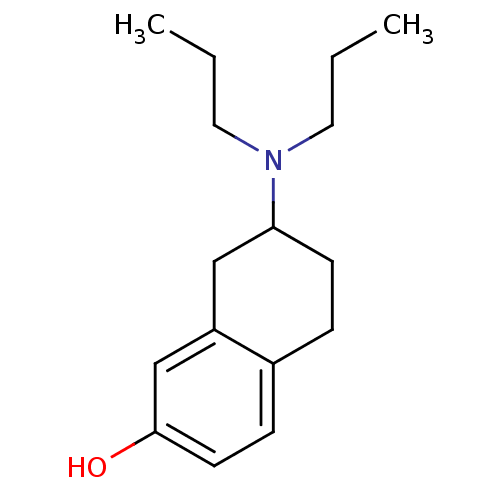 ((+/-)-7-(dipropylamino)-5,6,7,8-tetrahydronaphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50325822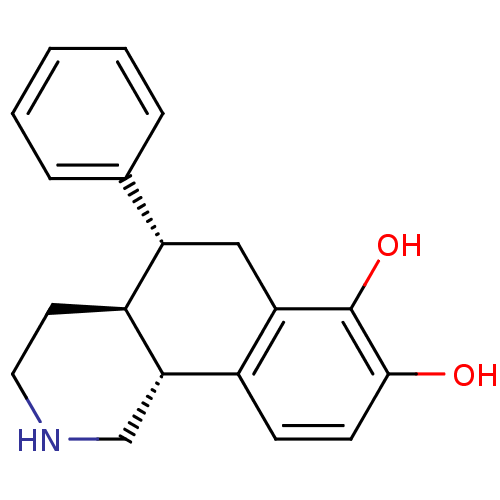 (CHEMBL1224527 | trans-(5R)-5-phenyl-1,2,3,4,4a,5,6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-SCH-23390 from human dopamine D1 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50394091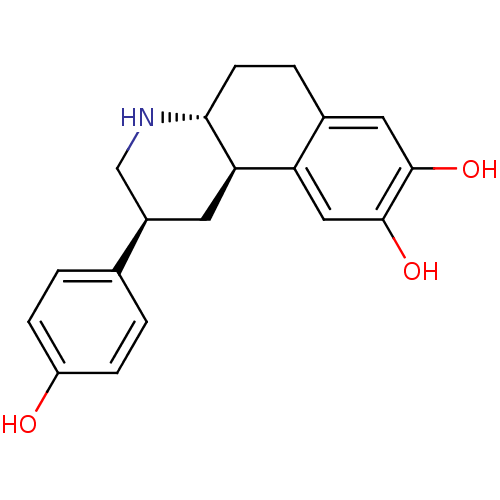 (CHEMBL2158636) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50394089 (CHEMBL2158640) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D2long receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50325822 (CHEMBL1224527 | trans-(5R)-5-phenyl-1,2,3,4,4a,5,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50325822 (CHEMBL1224527 | trans-(5R)-5-phenyl-1,2,3,4,4a,5,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D2long receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50394091 (CHEMBL2158636) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D2long receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50394091 (CHEMBL2158636) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-SCH-23390 from human dopamine D1 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50394089 (CHEMBL2158640) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 921 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-SCH-23390 from human dopamine D1 receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50020222 ((+/-)-7-(dipropylamino)-5,6,7,8-tetrahydronaphthal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D2long receptor expressed in HEK293 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 6366-74 (2012) Article DOI: 10.1016/j.bmc.2012.08.058 BindingDB Entry DOI: 10.7270/Q2G161X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytidine deaminase (Homo sapiens (Human)) | BDBM50087289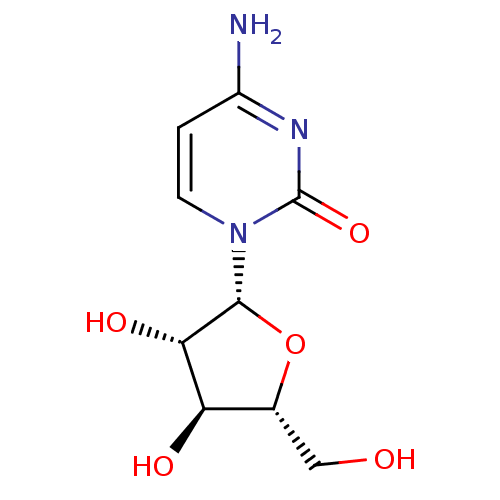 (1-beta-D-Arabinofuranosylcytosine | 4-Amino-1-beta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibitory constant was measured on cytidine/deoxycytidine deaminase | J Med Chem 34: 2607-15 (1991) BindingDB Entry DOI: 10.7270/Q23F4Q8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytidine deaminase (Homo sapiens (Human)) | BDBM50007155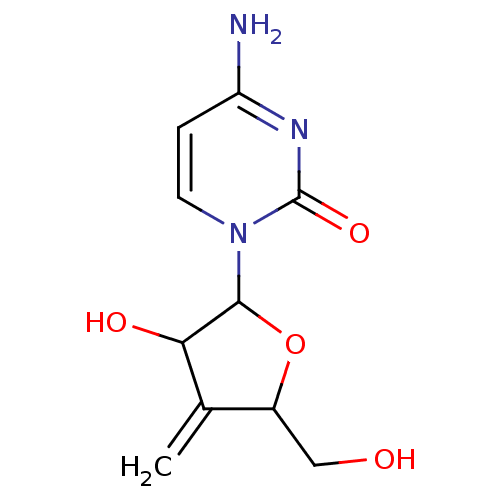 (4-Amino-1-(3-hydroxy-5-hydroxymethyl-4-methylene-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibitory constant was measured on cytidine/deoxycytidine deaminase | J Med Chem 34: 2607-15 (1991) BindingDB Entry DOI: 10.7270/Q23F4Q8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytidine deaminase (Homo sapiens (Human)) | BDBM50421893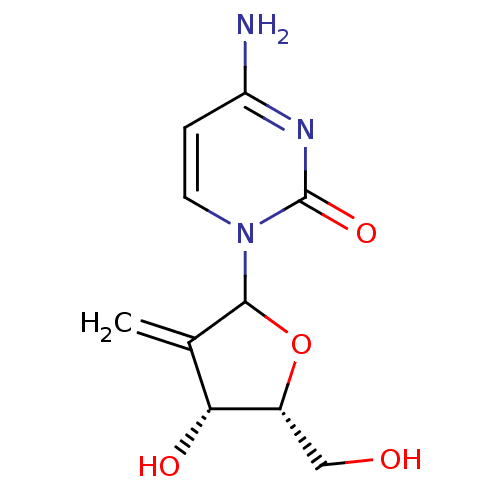 (CHEMBL10128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.69E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibitory constant was measured on cytidine/deoxycytidine deaminase | J Med Chem 34: 2607-15 (1991) BindingDB Entry DOI: 10.7270/Q23F4Q8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytidine deaminase (Homo sapiens (Human)) | BDBM50007156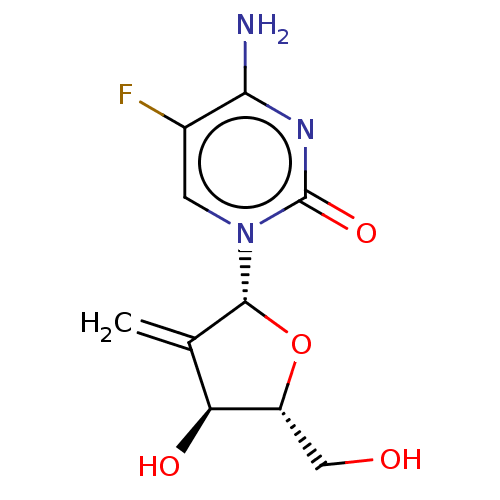 (4-Amino-5-fluoro-1-(4-hydroxy-5-hydroxymethyl-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.51E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibitory constant was measured on cytidine/deoxycytidine deaminase | J Med Chem 34: 2607-15 (1991) BindingDB Entry DOI: 10.7270/Q23F4Q8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50028142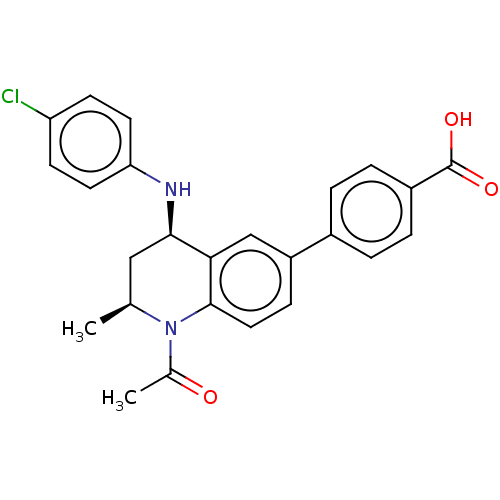 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to full-length BRD4 short isoform (unknown origin) by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRD4 bromodomain 1 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRD4 bromodomain 1/2 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRD3 bromodomain 1 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50042828 (4-Hydroxy-1-(9-mercaptomethyl-10-oxo-azecane-2-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of NEP 24.11 from rat kidney | J Med Chem 36: 3821-8 (1994) BindingDB Entry DOI: 10.7270/Q2ZS2X5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain and PHD finger-containing protein 3 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRPF3 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 7 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | <1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRD7 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016895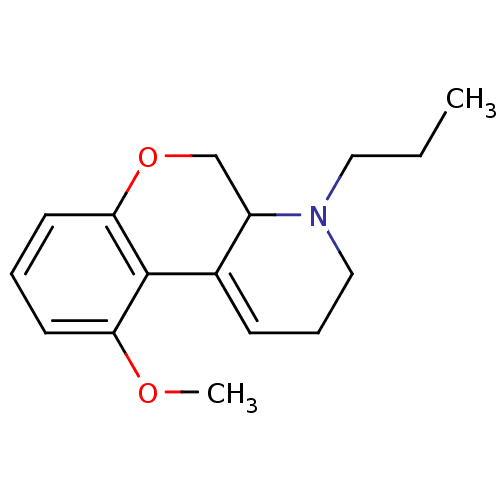 (5-Methoxy-1-propyl-2,3,10,10a-tetrahydro-1H-9-oxa-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H]-8-Hydroxy-2-(di-n-propylamino)tetralin binding to 5-hydroxytryptamine 1A receptor ... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRD3 bromodomain 2 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016900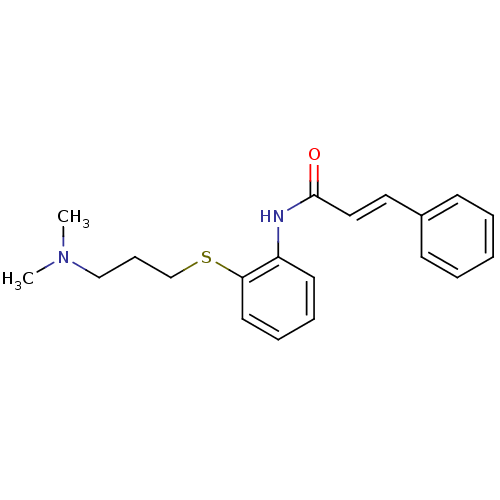 ((E)-N-[2-(3-Dimethylamino-propylsulfanyl)-phenyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H]ketanserin binding to 5-hydroxytryptamine 2 receptor at 1 uM | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50042835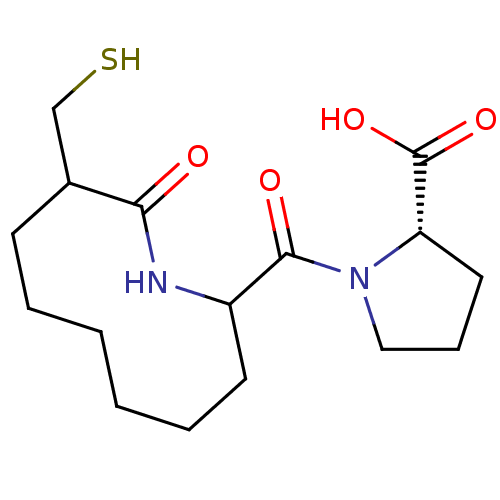 (1-(9-Mercaptomethyl-10-oxo-azecane-2-carbonyl)-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of NEP 24.11 from rat kidney | J Med Chem 36: 3821-8 (1994) BindingDB Entry DOI: 10.7270/Q2ZS2X5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50016897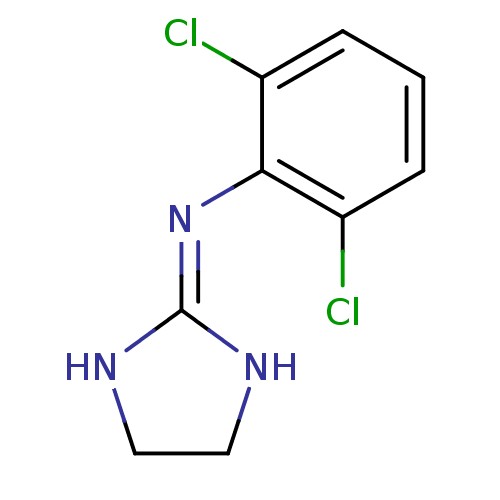 (2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration of the compound necessary to achieve half maximal inhibition of [3H]-clonidine binding to Alpha-2 adrenergic receptor at 1 uM | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50008790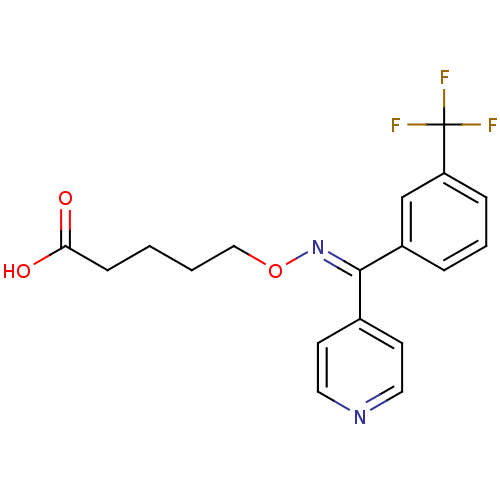 (5-[Pyridin-4-yl-(3-trifluoromethyl-phenyl)-methyle...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of Thromboxane synthase using [14C]-arachidonic acid as radioligand | J Med Chem 34: 1790-7 (1991) BindingDB Entry DOI: 10.7270/Q2BR8R47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50042827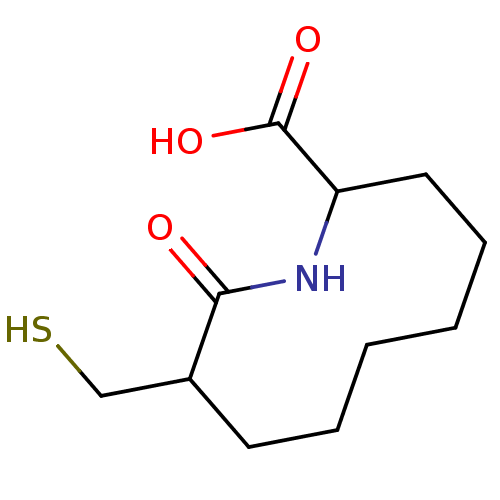 (9-Mercaptomethyl-10-oxo-azecane-2-carboxylic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of NEP 24.11 from rat kidney | J Med Chem 36: 3821-8 (1994) BindingDB Entry DOI: 10.7270/Q2ZS2X5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50042827 (9-Mercaptomethyl-10-oxo-azecane-2-carboxylic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of NEP 24.11 from rat kidney | J Med Chem 36: 3821-8 (1994) BindingDB Entry DOI: 10.7270/Q2ZS2X5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration of the compound necessary to achieve half maximal inhibition of [3H]-8-Hydroxy-2-(di-n-propylamino)tetralin binding to 5-hydroxytryptam... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRD4 bromodomain 2 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRD2 bromodomain 2 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM29644 ((6aR)-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H](+)-23amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene binding to dopamine recepto... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human BRPF1 by BROMOscan assay | J Med Chem 57: 8111-31 (2014) Article DOI: 10.1021/jm5010539 BindingDB Entry DOI: 10.7270/Q23R0VGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016883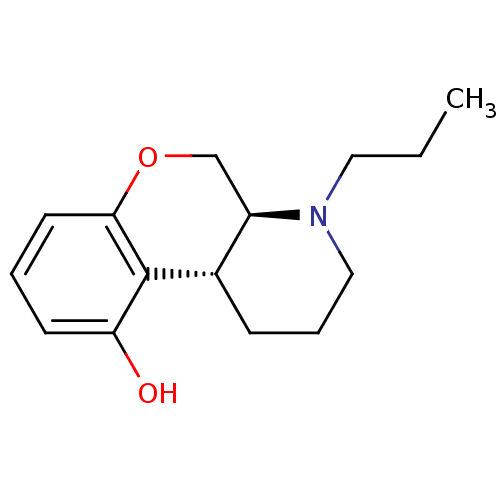 (1-Propyl-2,3,4,4a,10,10a-hexahydro-1H-9-oxa-1-aza-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H]-8-Hydroxy-2-(di-n-propylamino)tetralin binding to 5-hydroxytryptamine 1A receptor ... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM82427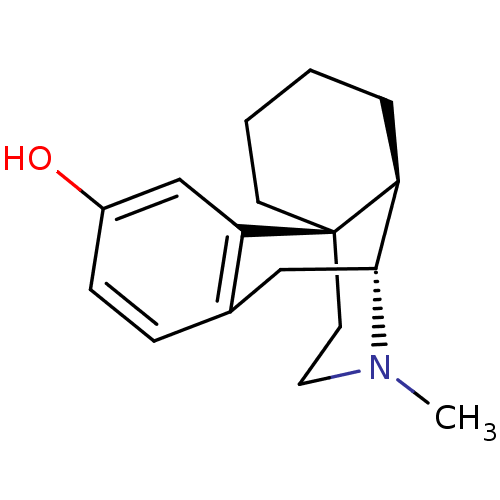 (CAS_5985-38-6 | LEVORPHANOL-tartarate) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from Sprague-Dawley rat cerebellum opioid receptor assessed as relative receptor affinity by scintillation counting | J Med Chem 21: 600-6 (1978) Article DOI: 10.1021/jm00205a003 BindingDB Entry DOI: 10.7270/Q2WM1HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM82427 (CAS_5985-38-6 | LEVORPHANOL-tartarate) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from Sprague-Dawley rat cerebellum opioid receptor assessed as relative receptor affinity by scintillation counting | J Med Chem 21: 600-6 (1978) Article DOI: 10.1021/jm00205a003 BindingDB Entry DOI: 10.7270/Q2WM1HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50008798 (7-(3-Pyridin-3-yl-bicyclo[2.2.1]hept-2-yl)-heptano...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description In vitro inhibition of thromboxane synthase using [14C]-arachidonic acid | J Med Chem 34: 1790-7 (1991) BindingDB Entry DOI: 10.7270/Q2BR8R47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50016882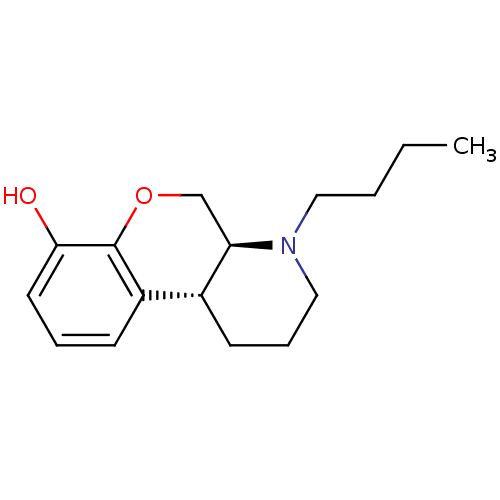 (1-Butyl-2,3,4,4a,10,10a-hexahydro-1H-9-oxa-1-aza-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H](+)-23amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene binding to dopamine recepto... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50016888 (1-Propyl-2,3,4,4a,10,10a-hexahydro-1H-9-oxa-1-aza-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H](+)-23amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene binding to dopamine recepto... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016890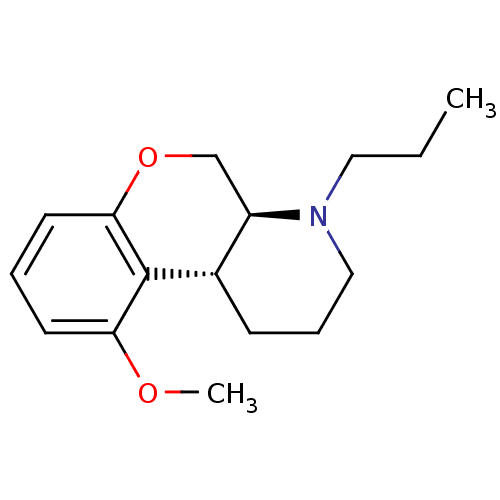 (5-Methoxy-1-propyl-2,3,4,4a,10,10a-hexahydro-1H-9-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H]-8-Hydroxy-2-(di-n-propylamino)tetralin binding to 5-hydroxytryptamine 1A receptor ... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50042832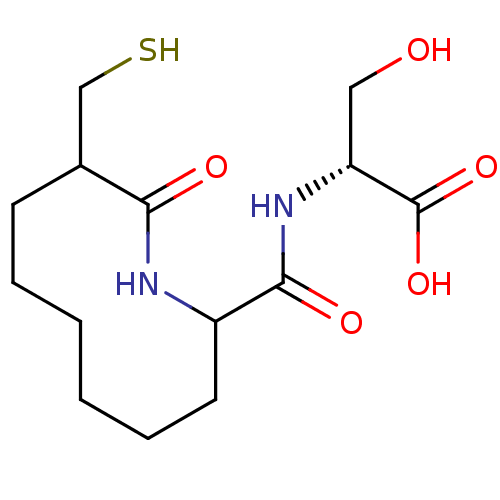 (3-Hydroxy-2-[(9-mercaptomethyl-10-oxo-azecane-2-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of NEP 24.11 from rat kidney | J Med Chem 36: 3821-8 (1994) BindingDB Entry DOI: 10.7270/Q2ZS2X5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50228062 (CHEMBL165290) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H](+)-23amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene binding to dopamine recepto... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50042831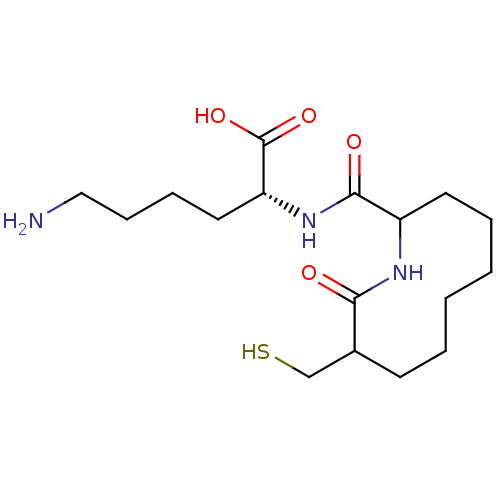 (6-Amino-2-[(9-mercaptomethyl-10-oxo-azecane-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of NEP 24.11 from rat kidney | J Med Chem 36: 3821-8 (1994) BindingDB Entry DOI: 10.7270/Q2ZS2X5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50016885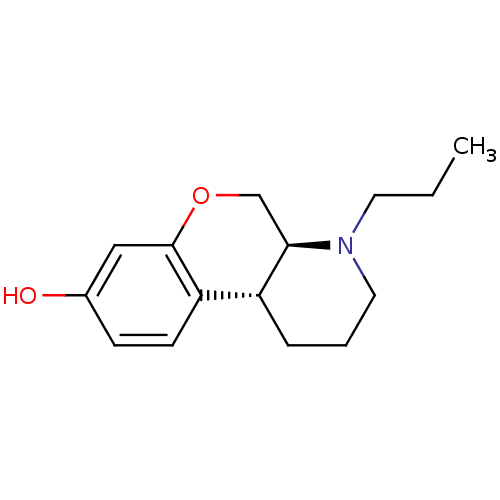 (1-Propyl-2,3,4,4a,10,10a-hexahydro-1H-9-oxa-1-aza-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration necessary to achieve half maximal inhibition of [3H](+)-23amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene binding to dopamine recepto... | J Med Chem 32: 720-7 (1989) BindingDB Entry DOI: 10.7270/Q2639NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50497043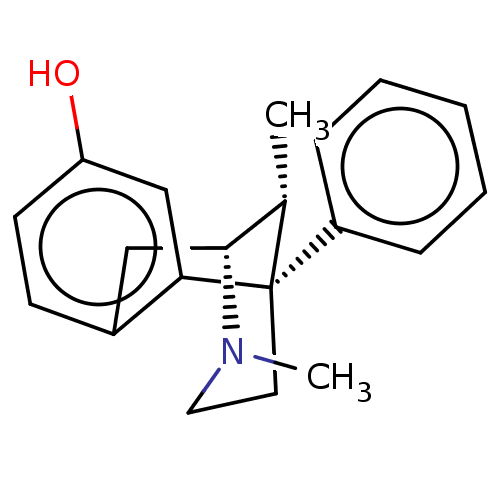 (CHEMBL3249802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from Sprague-Dawley rat cerebellum opioid receptor assessed as relative receptor affinity by scintillation counting | J Med Chem 21: 600-6 (1978) Article DOI: 10.1021/jm00205a003 BindingDB Entry DOI: 10.7270/Q2WM1HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 396 total ) | Next | Last >> |