 Found 339 hits with Last Name = 'collart' and Initial = 'p'
Found 339 hits with Last Name = 'collart' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176723
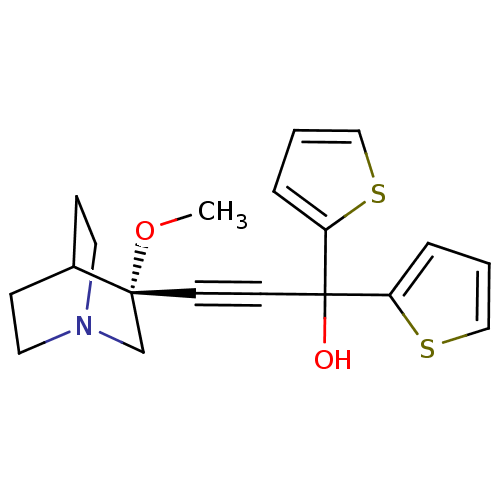
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccs1)c1cccs1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-4.55,6.62,;-3.06,6.24,;-2.65,4.75,;-2.46,3.37,;-.93,4.03,;.43,3.4,;.16,4.79,;-1.19,5.39,;-1.12,7.02,;-.67,5.92,;-4.15,4.35,;-5.64,3.96,;-7.13,3.56,;-7.52,5.04,;-8.62,3.16,;-9.16,1.72,;-10.68,1.79,;-11.09,3.3,;-9.81,4.14,;-6.73,2.08,;-5.29,1.53,;-5.36,.02,;-6.86,-.39,;-7.7,.89,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-10-6-15(18)7-11-20)8-9-19(21,16-4-2-12-23-16)17-5-3-13-24-17/h2-5,12-13,15,21H,6-7,10-11,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176735
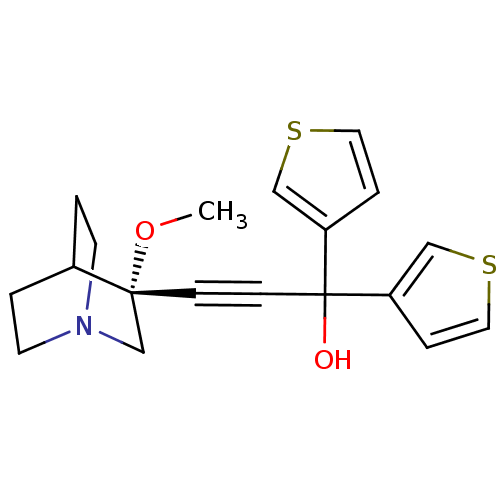
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccsc1)c1ccsc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(9.1,6.91,;10.59,6.52,;11,5.02,;11.19,3.64,;12.73,4.3,;14.09,3.67,;13.82,5.06,;12.47,5.67,;12.54,7.31,;12.98,6.2,;9.5,4.63,;8,4.23,;6.51,3.83,;6.11,5.32,;5.02,3.44,;4.48,2,;2.91,2.09,;2.52,3.58,;3.83,4.41,;6.91,2.35,;8.33,1.8,;8.24,.24,;6.76,-.15,;5.94,1.16,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-8-2-15(18)3-9-20)6-7-19(21,16-4-10-23-12-16)17-5-11-24-13-17/h4-5,10-13,15,21H,2-3,8-9,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176732
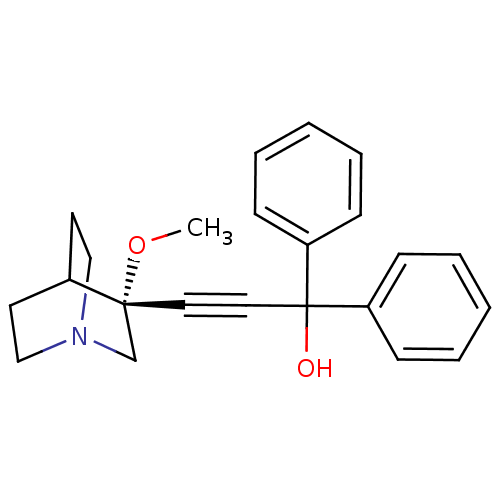
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-diphenylprop...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-2.54,5.9,;-1.05,5.51,;-.64,4.02,;-.44,2.64,;1.09,3.3,;2.46,2.67,;2.18,4.06,;.83,4.66,;.9,6.3,;1.34,5.19,;-2.14,3.62,;-3.63,3.22,;-5.12,2.83,;-5.52,4.31,;-4.73,1.34,;-5.81,.25,;-5.42,-1.23,;-3.93,-1.63,;-2.84,-.54,;-3.24,.94,;-6.61,2.43,;-7.7,3.53,;-9.19,3.13,;-9.59,1.64,;-8.5,.54,;-7.01,.94,)| Show InChI InChI=1S/C23H25NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2-11,19,25H,12-13,16-18H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176723

((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccs1)c1cccs1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-4.55,6.62,;-3.06,6.24,;-2.65,4.75,;-2.46,3.37,;-.93,4.03,;.43,3.4,;.16,4.79,;-1.19,5.39,;-1.12,7.02,;-.67,5.92,;-4.15,4.35,;-5.64,3.96,;-7.13,3.56,;-7.52,5.04,;-8.62,3.16,;-9.16,1.72,;-10.68,1.79,;-11.09,3.3,;-9.81,4.14,;-6.73,2.08,;-5.29,1.53,;-5.36,.02,;-6.86,-.39,;-7.7,.89,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-10-6-15(18)7-11-20)8-9-19(21,16-4-2-12-23-16)17-5-3-13-24-17/h2-5,12-13,15,21H,6-7,10-11,14H2,1H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176718
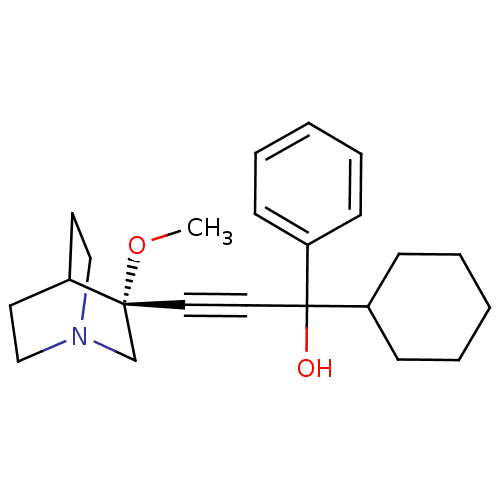
(1-cyclohexyl-3-((R)-3-methoxyquinuclidin-3-yl)-1-p...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(C1CCCCC1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(11.35,5.74,;12.84,5.36,;13.25,3.86,;13.44,2.49,;14.97,3.14,;16.34,2.52,;16.06,3.9,;14.71,4.51,;14.78,6.14,;15.23,5.04,;11.75,3.47,;10.26,3.07,;8.77,2.68,;8.38,4.16,;9.16,1.19,;8.06,.11,;8.45,-1.37,;9.93,-1.78,;11.03,-.69,;10.64,.8,;7.28,2.28,;6.19,3.37,;4.71,2.98,;4.3,1.49,;5.39,.39,;6.88,.8,)| Show InChI InChI=1S/C23H31NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2,4-5,8-9,19,21,25H,3,6-7,10-13,16-18H2,1H3/t22-,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176708
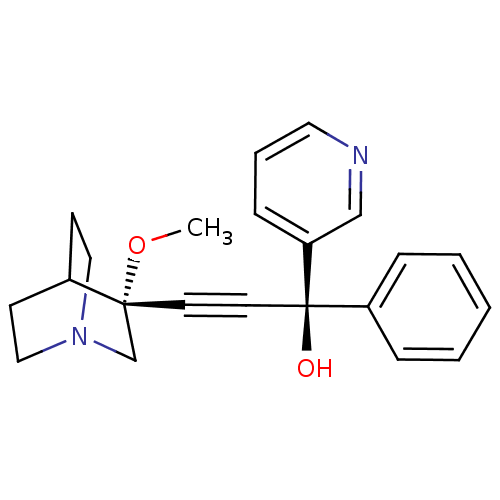
((R)-3-((R)-3-methoxyquinuclidin-3-yl)-1-phenyl-1-(...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#C[C@@](O)(c1ccccc1)c1cccnc1 |wU:12.14,2.1,wD:12.13,2.11,THB:1:2:5.6:9.8,(16.23,-16.03,;17.77,-16,;18.57,-17.32,;19.13,-18.59,;20.42,-17.54,;21.91,-17.77,;21.26,-16.51,;19.8,-16.3,;19.42,-14.71,;20.15,-15.65,;17.23,-18.11,;15.91,-18.89,;14.58,-19.68,;13.8,-18.36,;13.26,-20.47,;11.91,-19.72,;10.59,-20.5,;10.61,-22.05,;11.96,-22.8,;13.28,-22.01,;15.37,-21,;16.9,-20.98,;17.68,-22.31,;16.93,-23.64,;15.4,-23.66,;14.6,-22.35,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3/t21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176712
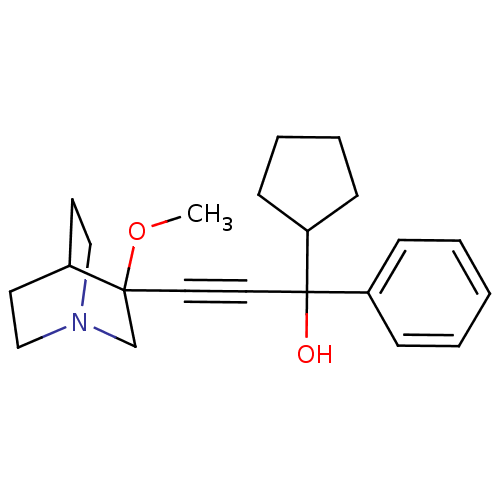
(1-cyclopentyl-3-(3-methoxyquinuclidin-3-yl)-1-phen...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(C1CCCC1)c1ccccc1 |THB:1:2:5.6:9.8,(26.6,5.4,;28.09,5.01,;28.5,3.51,;28.69,2.14,;30.23,2.79,;31.59,2.16,;31.32,3.55,;29.97,4.16,;30.04,5.8,;30.48,4.69,;27,3.12,;25.5,2.72,;24.01,2.33,;23.61,3.81,;24.41,.84,;23.43,-.35,;24.27,-1.64,;25.76,-1.25,;25.84,.29,;22.52,1.93,;21.42,3.02,;19.94,2.63,;19.53,1.13,;20.63,.04,;22.11,.44,)| Show InChI InChI=1S/C22H29NO2/c1-25-21(17-23-15-11-18(21)12-16-23)13-14-22(24,20-9-5-6-10-20)19-7-3-2-4-8-19/h2-4,7-8,18,20,24H,5-6,9-12,15-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176706
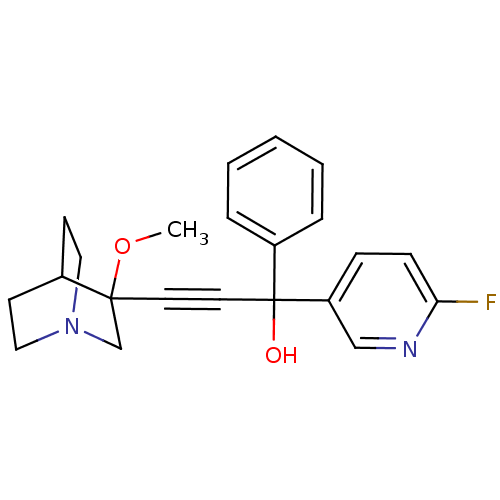
(1-(6-fluoropyridin-3-yl)-3-(3-methoxyquinuclidin-3...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccc(F)nc1 |THB:1:2:5.6:9.8,(7.82,-23.84,;9.35,-23.8,;10.15,-25.13,;10.72,-26.4,;12.01,-25.35,;13.49,-25.58,;12.85,-24.32,;11.39,-24.11,;11.01,-22.52,;11.74,-23.46,;8.82,-25.91,;7.5,-26.7,;6.17,-27.49,;5.39,-26.17,;4.85,-28.28,;3.5,-27.53,;2.18,-28.31,;2.2,-29.85,;3.55,-30.61,;4.87,-29.82,;6.96,-28.81,;8.48,-28.79,;9.27,-30.12,;8.52,-31.45,;9.31,-32.77,;6.98,-31.47,;6.19,-30.16,)| Show InChI InChI=1S/C22H23FN2O2/c1-27-21(16-25-13-9-17(21)10-14-25)11-12-22(26,18-5-3-2-4-6-18)19-7-8-20(23)24-15-19/h2-8,15,17,26H,9-10,13-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.339 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176735

((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccsc1)c1ccsc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(9.1,6.91,;10.59,6.52,;11,5.02,;11.19,3.64,;12.73,4.3,;14.09,3.67,;13.82,5.06,;12.47,5.67,;12.54,7.31,;12.98,6.2,;9.5,4.63,;8,4.23,;6.51,3.83,;6.11,5.32,;5.02,3.44,;4.48,2,;2.91,2.09,;2.52,3.58,;3.83,4.41,;6.91,2.35,;8.33,1.8,;8.24,.24,;6.76,-.15,;5.94,1.16,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-8-2-15(18)3-9-20)6-7-19(21,16-4-10-23-12-16)17-5-11-24-13-17/h4-5,10-13,15,21H,2-3,8-9,14H2,1H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176729
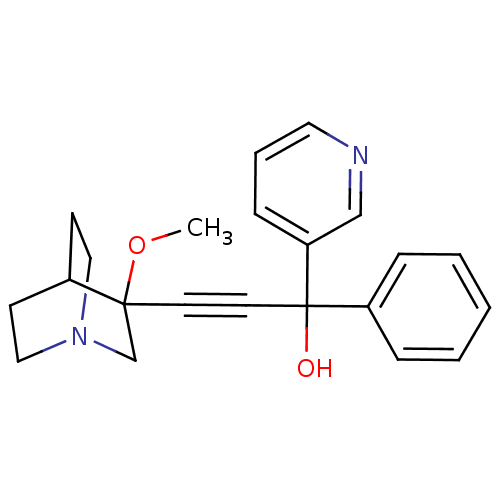
(3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyridin-...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cccnc1 |THB:1:2:5.6:9.8,(-5.03,-13.2,;-3.49,-13.16,;-2.69,-14.49,;-2.13,-15.76,;-.83,-14.71,;.65,-14.94,;0,-13.68,;-1.46,-13.46,;-1.84,-11.87,;-1.11,-12.82,;-4.02,-15.27,;-5.35,-16.06,;-6.67,-16.85,;-7.46,-15.53,;-8,-17.64,;-9.35,-16.88,;-10.67,-17.67,;-10.65,-19.21,;-9.3,-19.97,;-7.98,-19.18,;-5.89,-18.17,;-4.36,-18.15,;-3.58,-19.47,;-4.32,-20.81,;-5.86,-20.83,;-6.65,-19.52,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.407 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176717
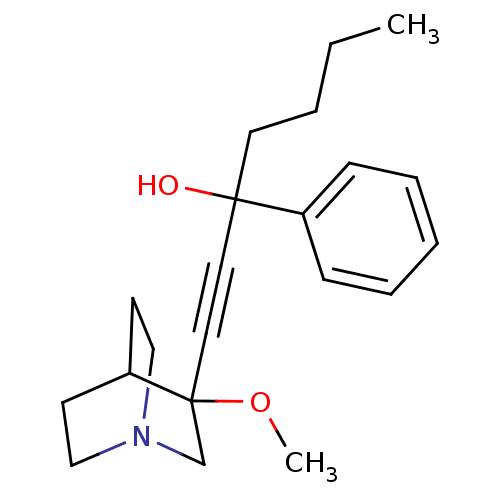
(1-(3-methoxyquinuclidin-3-yl)-3-phenylhept-1-yn-3-...)Show SMILES CCCCC(O)(C#CC1(CN2CCC1CC2)OC)c1ccccc1 |THB:16:8:11.12:15.14,(25.15,-12.89,;23.66,-12.49,;23.27,-11,;24.36,-9.92,;23.96,-8.43,;23.57,-6.94,;25.45,-8.03,;26.94,-7.64,;28.44,-7.24,;28.64,-8.62,;30.17,-7.96,;31.54,-8.59,;31.26,-7.2,;29.91,-6.6,;29.98,-4.96,;30.42,-6.07,;28.03,-5.75,;26.54,-5.36,;22.47,-8.83,;21.38,-7.73,;19.89,-8.13,;19.49,-9.62,;20.58,-10.72,;22.07,-10.31,)| Show InChI InChI=1S/C21H29NO2/c1-3-4-12-20(23,18-8-6-5-7-9-18)13-14-21(24-2)17-22-15-10-19(21)11-16-22/h5-9,19,23H,3-4,10-12,15-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176708

((R)-3-((R)-3-methoxyquinuclidin-3-yl)-1-phenyl-1-(...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#C[C@@](O)(c1ccccc1)c1cccnc1 |wU:12.14,2.1,wD:12.13,2.11,THB:1:2:5.6:9.8,(16.23,-16.03,;17.77,-16,;18.57,-17.32,;19.13,-18.59,;20.42,-17.54,;21.91,-17.77,;21.26,-16.51,;19.8,-16.3,;19.42,-14.71,;20.15,-15.65,;17.23,-18.11,;15.91,-18.89,;14.58,-19.68,;13.8,-18.36,;13.26,-20.47,;11.91,-19.72,;10.59,-20.5,;10.61,-22.05,;11.96,-22.8,;13.28,-22.01,;15.37,-21,;16.9,-20.98,;17.68,-22.31,;16.93,-23.64,;15.4,-23.66,;14.6,-22.35,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176732

((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-diphenylprop...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-2.54,5.9,;-1.05,5.51,;-.64,4.02,;-.44,2.64,;1.09,3.3,;2.46,2.67,;2.18,4.06,;.83,4.66,;.9,6.3,;1.34,5.19,;-2.14,3.62,;-3.63,3.22,;-5.12,2.83,;-5.52,4.31,;-4.73,1.34,;-5.81,.25,;-5.42,-1.23,;-3.93,-1.63,;-2.84,-.54,;-3.24,.94,;-6.61,2.43,;-7.7,3.53,;-9.19,3.13,;-9.59,1.64,;-8.5,.54,;-7.01,.94,)| Show InChI InChI=1S/C23H25NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2-11,19,25H,12-13,16-18H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176711
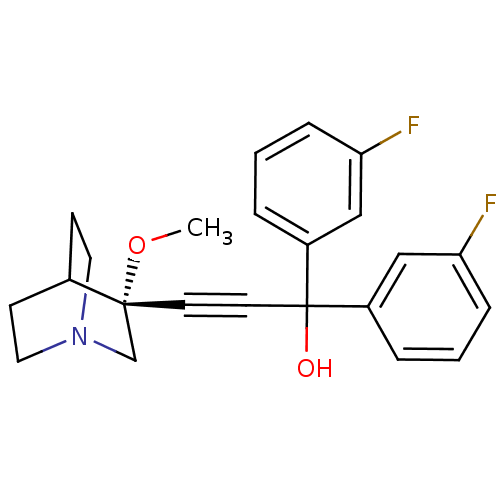
((R)-1,1-bis(3-fluorophenyl)-3-(3-methoxyquinuclidi...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccc(F)c1)c1cccc(F)c1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(26.22,-26.56,;27.7,-26.94,;28.12,-28.44,;28.31,-29.81,;29.84,-29.16,;31.21,-29.79,;30.93,-28.4,;29.58,-27.79,;29.65,-26.16,;30.1,-27.26,;26.62,-28.83,;25.13,-29.23,;23.63,-29.62,;23.24,-28.14,;22.14,-30.02,;21.74,-31.51,;20.25,-31.91,;19.16,-30.81,;19.57,-29.32,;18.48,-28.23,;21.05,-28.93,;24.03,-31.11,;22.94,-32.2,;23.34,-33.68,;24.82,-34.09,;25.91,-32.99,;27.4,-33.39,;25.51,-31.51,)| Show InChI InChI=1S/C23H23F2NO2/c1-28-22(16-26-12-8-17(22)9-13-26)10-11-23(27,18-4-2-6-20(24)14-18)19-5-3-7-21(25)15-19/h2-7,14-15,17,27H,8-9,12-13,16H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176726
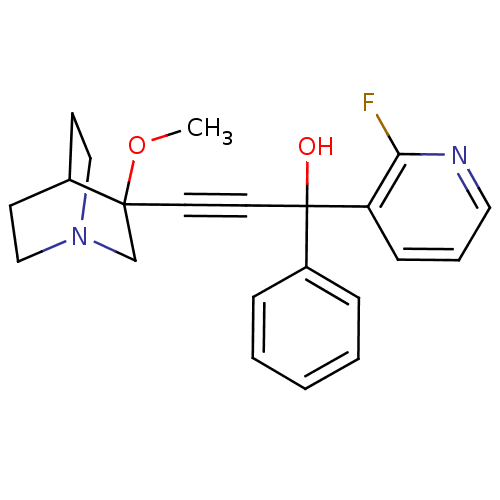
(1-(2-fluoropyridin-3-yl)-3-(3-methoxyquinuclidin-3...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cccnc1F |THB:1:2:5.6:9.8,(22.68,-23.08,;24.21,-23.04,;25.02,-24.36,;25.58,-25.63,;26.87,-24.58,;28.36,-24.82,;27.71,-23.56,;26.25,-23.34,;25.87,-21.75,;26.6,-22.69,;23.68,-25.15,;22.36,-25.94,;21.03,-26.73,;20.25,-25.41,;19.71,-27.52,;18.36,-26.76,;17.04,-27.55,;17.06,-29.09,;18.41,-29.85,;19.73,-29.06,;21.82,-28.05,;21.07,-29.39,;21.85,-30.71,;23.39,-30.69,;24.14,-29.35,;23.35,-28.03,;24.1,-26.68,)| Show InChI InChI=1S/C22H23FN2O2/c1-27-21(16-25-14-9-17(21)10-15-25)11-12-22(26,18-6-3-2-4-7-18)19-8-5-13-24-20(19)23/h2-8,13,17,26H,9-10,14-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50073179
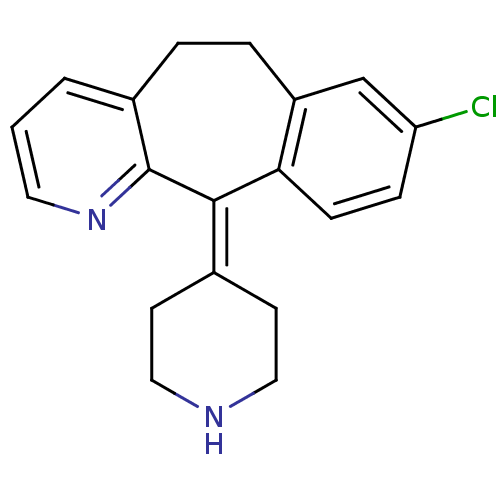
(8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7]-[#6]-[#6]-2)c1 Show InChI InChI=1S/C19H19ClN2/c20-16-5-6-17-15(12-16)4-3-14-2-1-9-22-19(14)18(17)13-7-10-21-11-8-13/h1-2,5-6,9,12,21H,3-4,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176714
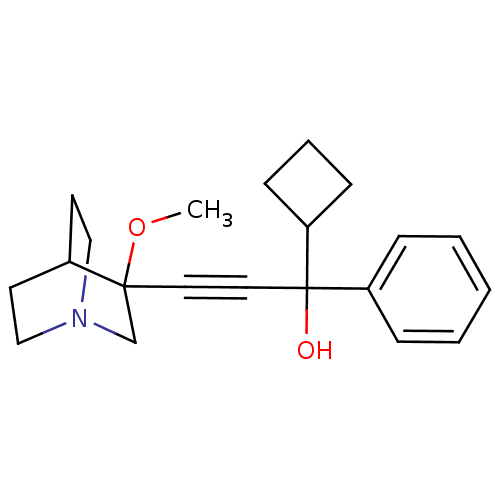
(1-cyclobutyl-3-(3-methoxyquinuclidin-3-yl)-1-pheny...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(C1CCC1)c1ccccc1 |THB:1:2:5.6:9.8,(-3.4,-5.54,;-1.91,-5.93,;-1.5,-7.42,;-1.31,-8.8,;.23,-8.14,;1.59,-8.77,;1.32,-7.38,;-.03,-6.78,;.04,-5.14,;.48,-6.25,;-3,-7.82,;-4.49,-8.21,;-5.98,-8.61,;-6.38,-7.12,;-5.59,-10.1,;-6.36,-11.43,;-5.03,-12.2,;-4.26,-10.87,;-7.48,-9.01,;-8.57,-7.91,;-10.05,-8.31,;-10.46,-9.8,;-9.36,-10.9,;-7.88,-10.5,)| Show InChI InChI=1S/C21H27NO2/c1-24-20(16-22-14-10-17(20)11-15-22)12-13-21(23,19-8-5-9-19)18-6-3-2-4-7-18/h2-4,6-7,17,19,23H,5,8-11,14-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50421552
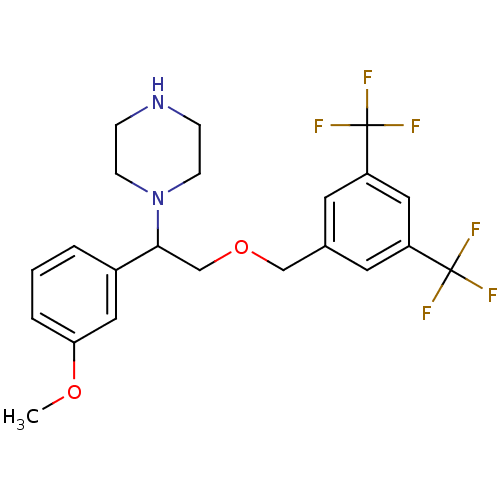
(CHEMBL108200)Show SMILES COc1cccc(c1)C(COCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)N1CCNCC1 Show InChI InChI=1S/C22H24F6N2O2/c1-31-19-4-2-3-16(11-19)20(30-7-5-29-6-8-30)14-32-13-15-9-17(21(23,24)25)12-18(10-15)22(26,27)28/h2-4,9-12,20,29H,5-8,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22548
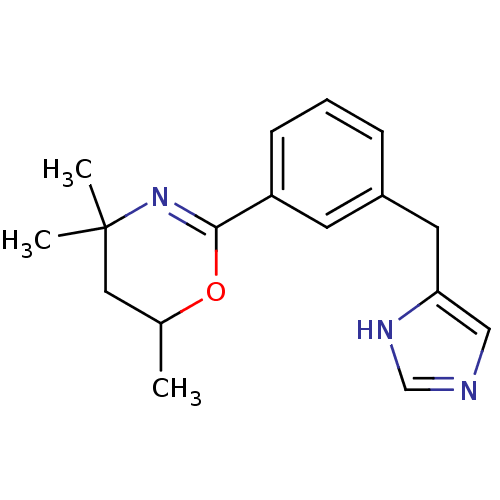
(2-[3-(1H-imidazol-4-ylmethyl)phenyl]-4,4,6-trimeth...)Show SMILES CC1CC(C)(C)N=C(O1)c1cccc(Cc2cnc[nH]2)c1 |c:6| Show InChI InChI=1S/C17H21N3O/c1-12-9-17(2,3)20-16(21-12)14-6-4-5-13(7-14)8-15-10-18-11-19-15/h4-7,10-12H,8-9H2,1-3H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -51.4 | n/a | n/a | 2 | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176704
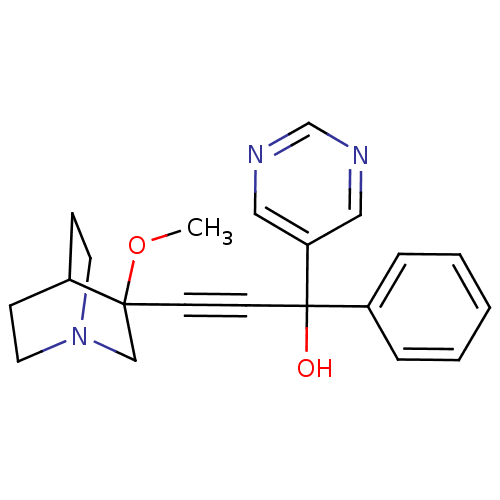
(3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyrimidi...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cncnc1 |THB:1:2:5.6:9.8,(8.21,-34.77,;9.74,-34.74,;10.54,-36.06,;11.11,-37.33,;12.4,-36.28,;13.88,-36.51,;13.24,-35.25,;11.78,-35.04,;11.4,-33.45,;12.13,-34.39,;9.21,-36.85,;7.89,-37.63,;6.56,-38.42,;5.78,-37.1,;5.24,-39.21,;3.89,-38.46,;2.57,-39.24,;2.59,-40.78,;3.94,-41.54,;5.26,-40.75,;7.35,-39.74,;8.88,-39.72,;9.66,-41.04,;8.91,-42.38,;7.37,-42.4,;6.58,-41.09,)| Show InChI InChI=1S/C21H23N3O2/c1-26-20(15-24-11-7-17(20)8-12-24)9-10-21(25,18-5-3-2-4-6-18)19-13-22-16-23-14-19/h2-6,13-14,16-17,25H,7-8,11-12,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176722
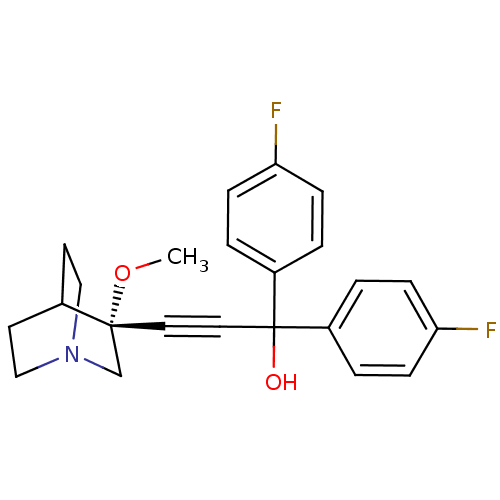
((R)-1,1-bis(4-fluorophenyl)-3-(3-methoxyquinuclidi...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccc(F)cc1)c1ccc(F)cc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(25.27,-13.99,;26.76,-14.38,;27.17,-15.87,;27.37,-17.25,;28.9,-16.59,;30.27,-17.22,;29.99,-15.83,;28.64,-15.23,;28.71,-13.59,;29.15,-14.7,;25.67,-16.27,;24.18,-16.66,;22.69,-17.06,;22.3,-15.57,;21.2,-17.46,;20.11,-16.36,;18.62,-16.76,;18.22,-18.25,;16.73,-18.64,;19.31,-19.34,;20.8,-18.94,;23.09,-18.55,;22,-19.63,;22.39,-21.12,;23.88,-21.52,;24.28,-23.01,;24.97,-20.43,;24.57,-18.95,)| Show InChI InChI=1S/C23H23F2NO2/c1-28-22(16-26-14-10-17(22)11-15-26)12-13-23(27,18-2-6-20(24)7-3-18)19-4-8-21(25)9-5-19/h2-9,17,27H,10-11,14-16H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000240
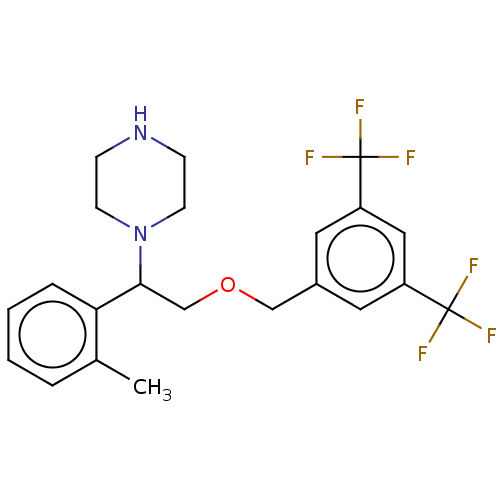
(CHEMBL435796)Show SMILES Cc1ccccc1C(COCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)N1CCNCC1 Show InChI InChI=1S/C22H24F6N2O/c1-15-4-2-3-5-19(15)20(30-8-6-29-7-9-30)14-31-13-16-10-17(21(23,24)25)12-18(11-16)22(26,27)28/h2-5,10-12,20,29H,6-9,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176729

(3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyridin-...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cccnc1 |THB:1:2:5.6:9.8,(-5.03,-13.2,;-3.49,-13.16,;-2.69,-14.49,;-2.13,-15.76,;-.83,-14.71,;.65,-14.94,;0,-13.68,;-1.46,-13.46,;-1.84,-11.87,;-1.11,-12.82,;-4.02,-15.27,;-5.35,-16.06,;-6.67,-16.85,;-7.46,-15.53,;-8,-17.64,;-9.35,-16.88,;-10.67,-17.67,;-10.65,-19.21,;-9.3,-19.97,;-7.98,-19.18,;-5.89,-18.17,;-4.36,-18.15,;-3.58,-19.47,;-4.32,-20.81,;-5.86,-20.83,;-6.65,-19.52,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176716
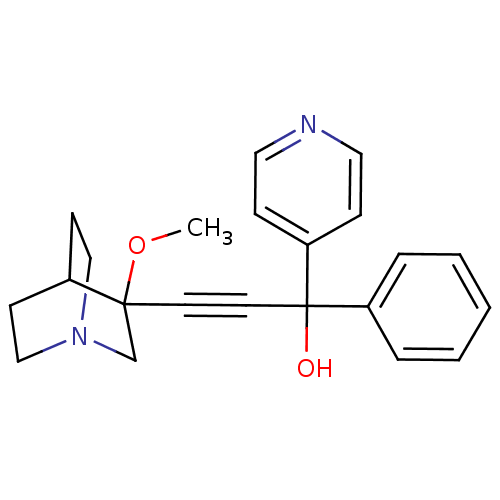
(3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyridin-...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccncc1 |THB:1:2:5.6:9.8,(8.98,-12.42,;10.51,-12.38,;11.32,-13.7,;11.88,-14.97,;13.17,-13.92,;14.65,-14.16,;14.01,-12.9,;12.55,-12.68,;12.17,-11.1,;12.9,-12.04,;9.98,-14.49,;8.66,-15.28,;7.34,-16.07,;6.55,-14.75,;6.01,-16.85,;4.66,-16.1,;3.35,-16.88,;3.37,-18.43,;4.71,-19.18,;6.03,-18.39,;8.12,-17.38,;9.66,-17.37,;10.44,-18.69,;9.69,-20.03,;8.16,-20.06,;7.37,-18.73,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-15-9-18(21)10-16-24)11-12-22(25,19-5-3-2-4-6-19)20-7-13-23-14-8-20/h2-8,13-14,18,25H,9-10,15-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50109647
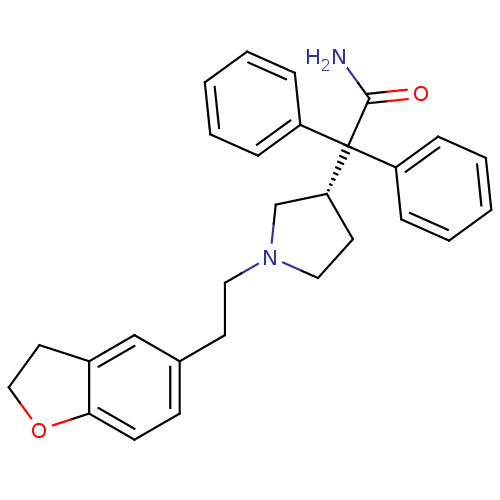
(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50165019
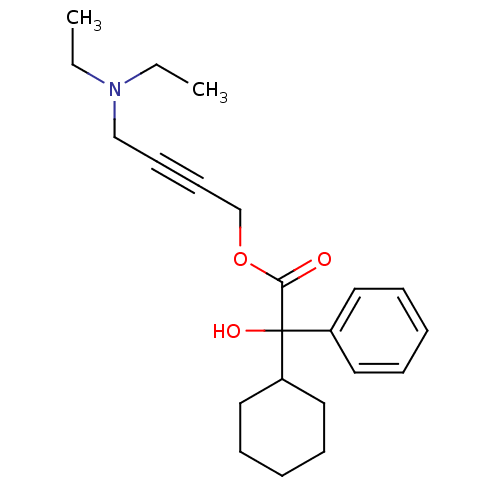
(4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...)Show InChI InChI=1S/C22H31NO3/c1-3-23(4-2)17-11-12-18-26-21(24)22(25,19-13-7-5-8-14-19)20-15-9-6-10-16-20/h5,7-8,13-14,20,25H,3-4,6,9-10,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000241
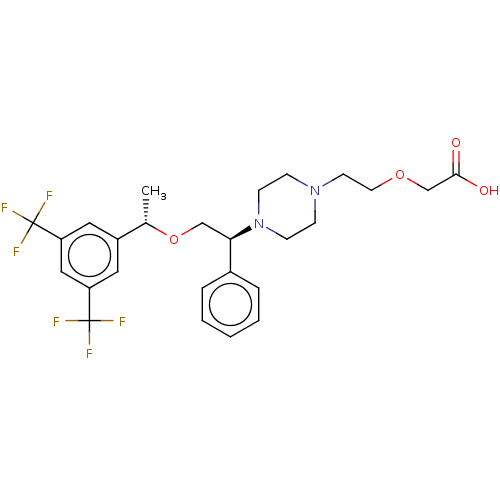
(CHEMBL138824)Show SMILES C[C@H](OC[C@@H](N1CCN(CCOCC(O)=O)CC1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H30F6N2O4/c1-18(20-13-21(25(27,28)29)15-22(14-20)26(30,31)32)38-16-23(19-5-3-2-4-6-19)34-9-7-33(8-10-34)11-12-37-17-24(35)36/h2-6,13-15,18,23H,7-12,16-17H2,1H3,(H,35,36)/t18-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000252
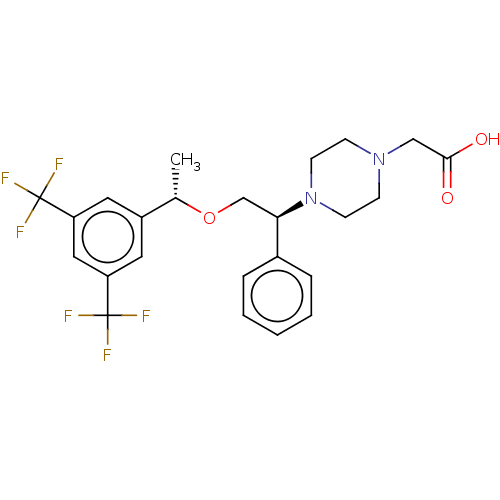
(CHEMBL136347)Show SMILES C[C@H](OC[C@@H](N1CCN(CC(O)=O)CC1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H26F6N2O3/c1-16(18-11-19(23(25,26)27)13-20(12-18)24(28,29)30)35-15-21(17-5-3-2-4-6-17)32-9-7-31(8-10-32)14-22(33)34/h2-6,11-13,16,21H,7-10,14-15H2,1H3,(H,33,34)/t16-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000238
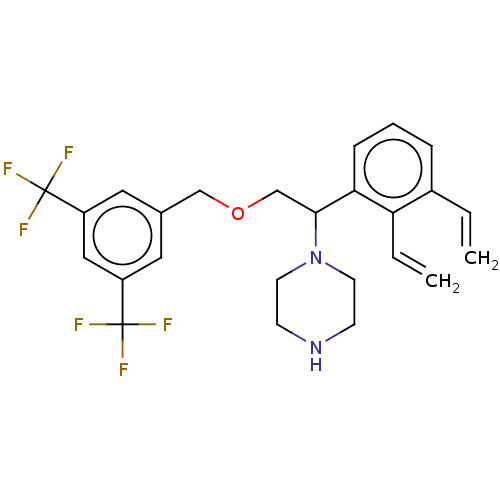
(CHEMBL336305)Show SMILES FC(F)(F)c1cc(COCC(N2CCNCC2)c2cccc(C=C)c2C=C)cc(c1)C(F)(F)F Show InChI InChI=1S/C25H26F6N2O/c1-3-18-6-5-7-22(21(18)4-2)23(33-10-8-32-9-11-33)16-34-15-17-12-19(24(26,27)28)14-20(13-17)25(29,30)31/h3-7,12-14,23,32H,1-2,8-11,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50421561
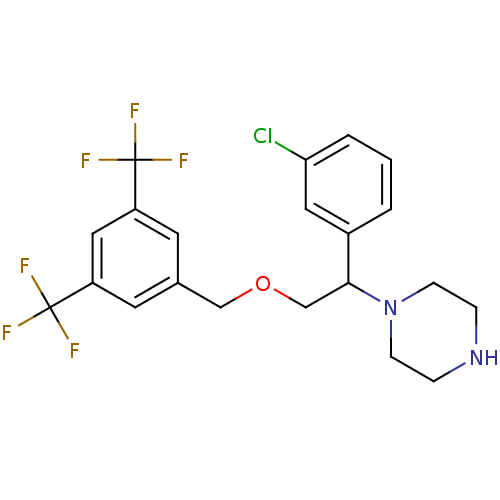
(CHEMBL419742)Show SMILES FC(F)(F)c1cc(COCC(N2CCNCC2)c2cccc(Cl)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C21H21ClF6N2O/c22-18-3-1-2-15(10-18)19(30-6-4-29-5-7-30)13-31-12-14-8-16(20(23,24)25)11-17(9-14)21(26,27)28/h1-3,8-11,19,29H,4-7,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50421567
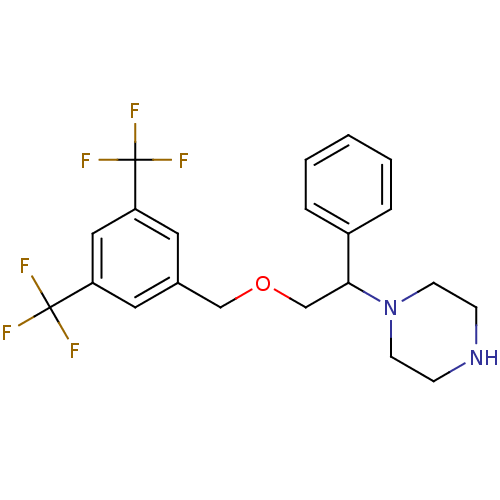
(CHEMBL109937)Show SMILES FC(F)(F)c1cc(COCC(N2CCNCC2)c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C21H22F6N2O/c22-20(23,24)17-10-15(11-18(12-17)21(25,26)27)13-30-14-19(16-4-2-1-3-5-16)29-8-6-28-7-9-29/h1-5,10-12,19,28H,6-9,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50421562
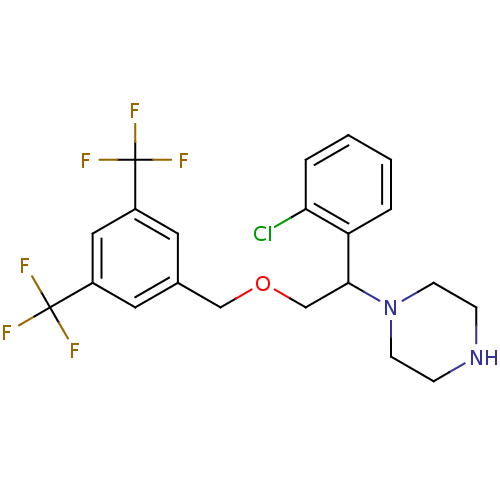
(CHEMBL108889)Show SMILES FC(F)(F)c1cc(COCC(N2CCNCC2)c2ccccc2Cl)cc(c1)C(F)(F)F Show InChI InChI=1S/C21H21ClF6N2O/c22-18-4-2-1-3-17(18)19(30-7-5-29-6-8-30)13-31-12-14-9-15(20(23,24)25)11-16(10-14)21(26,27)28/h1-4,9-11,19,29H,5-8,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000236
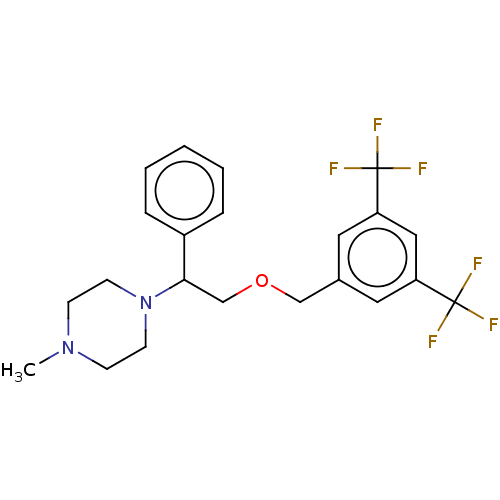
(CHEMBL140080)Show SMILES CN1CCN(CC1)C(COCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C22H24F6N2O/c1-29-7-9-30(10-8-29)20(17-5-3-2-4-6-17)15-31-14-16-11-18(21(23,24)25)13-19(12-16)22(26,27)28/h2-6,11-13,20H,7-10,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176718

(1-cyclohexyl-3-((R)-3-methoxyquinuclidin-3-yl)-1-p...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(C1CCCCC1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(11.35,5.74,;12.84,5.36,;13.25,3.86,;13.44,2.49,;14.97,3.14,;16.34,2.52,;16.06,3.9,;14.71,4.51,;14.78,6.14,;15.23,5.04,;11.75,3.47,;10.26,3.07,;8.77,2.68,;8.38,4.16,;9.16,1.19,;8.06,.11,;8.45,-1.37,;9.93,-1.78,;11.03,-.69,;10.64,.8,;7.28,2.28,;6.19,3.37,;4.71,2.98,;4.3,1.49,;5.39,.39,;6.88,.8,)| Show InChI InChI=1S/C23H31NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2,4-5,8-9,19,21,25H,3,6-7,10-13,16-18H2,1H3/t22-,23?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
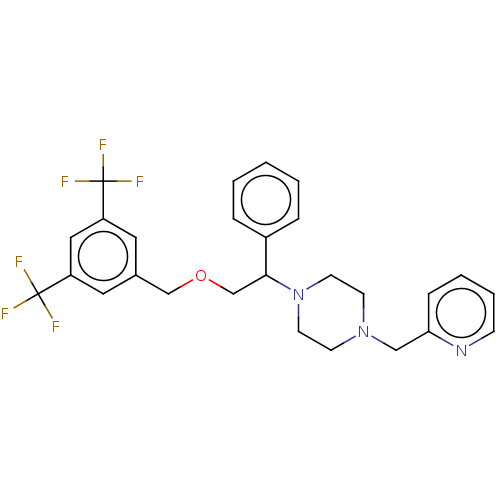
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000239
(CHEMBL342266)Show SMILES FC(F)(F)c1cc(COCC(N2CCN(Cc3ccccn3)CC2)c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C27H27F6N3O/c28-26(29,30)22-14-20(15-23(16-22)27(31,32)33)18-37-19-25(21-6-2-1-3-7-21)36-12-10-35(11-13-36)17-24-8-4-5-9-34-24/h1-9,14-16,25H,10-13,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22538
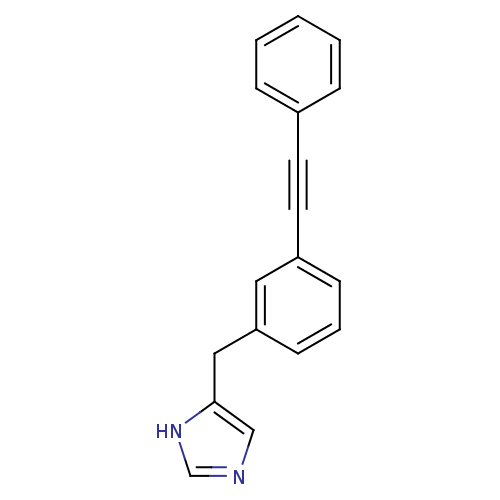
(4-Benzyl-1H-imidazole derivative, 19 | 4-{[3-(2-ph...)Show InChI InChI=1S/C18H14N2/c1-2-5-15(6-3-1)9-10-16-7-4-8-17(11-16)12-18-13-19-14-20-18/h1-8,11,13-14H,12H2,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176704

(3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyrimidi...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cncnc1 |THB:1:2:5.6:9.8,(8.21,-34.77,;9.74,-34.74,;10.54,-36.06,;11.11,-37.33,;12.4,-36.28,;13.88,-36.51,;13.24,-35.25,;11.78,-35.04,;11.4,-33.45,;12.13,-34.39,;9.21,-36.85,;7.89,-37.63,;6.56,-38.42,;5.78,-37.1,;5.24,-39.21,;3.89,-38.46,;2.57,-39.24,;2.59,-40.78,;3.94,-41.54,;5.26,-40.75,;7.35,-39.74,;8.88,-39.72,;9.66,-41.04,;8.91,-42.38,;7.37,-42.4,;6.58,-41.09,)| Show InChI InChI=1S/C21H23N3O2/c1-26-20(15-24-11-7-17(20)8-12-24)9-10-21(25,18-5-3-2-4-6-18)19-13-22-16-23-14-19/h2-6,13-14,16-17,25H,7-8,11-12,15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000255
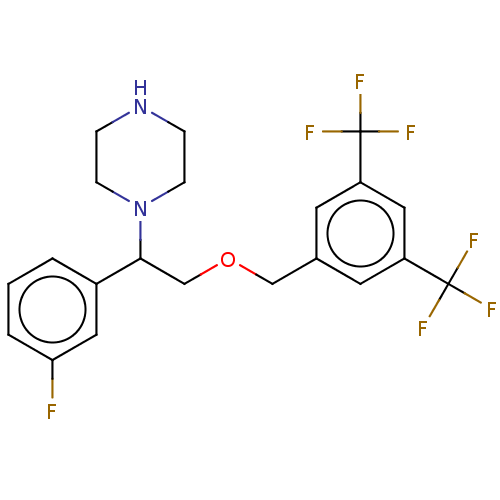
(CHEMBL139088)Show SMILES Fc1cccc(c1)C(COCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)N1CCNCC1 Show InChI InChI=1S/C21H21F7N2O/c22-18-3-1-2-15(10-18)19(30-6-4-29-5-7-30)13-31-12-14-8-16(20(23,24)25)11-17(9-14)21(26,27)28/h1-3,8-11,19,29H,4-7,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000248
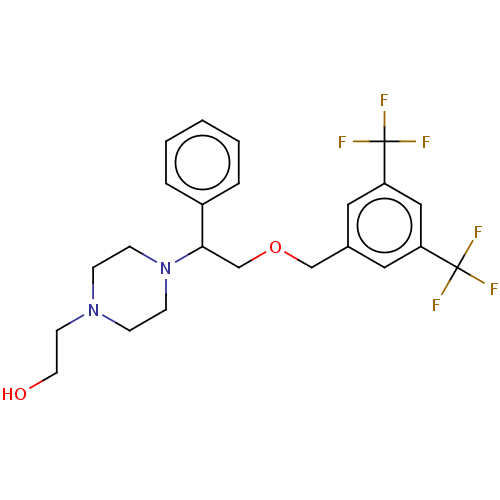
(CHEMBL139750)Show SMILES OCCN1CCN(CC1)C(COCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C23H26F6N2O2/c24-22(25,26)19-12-17(13-20(14-19)23(27,28)29)15-33-16-21(18-4-2-1-3-5-18)31-8-6-30(7-9-31)10-11-32/h1-5,12-14,21,32H,6-11,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176733
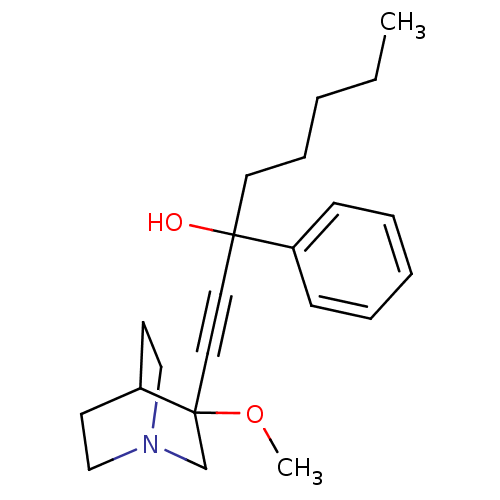
(1-(3-methoxyquinuclidin-3-yl)-3-phenyloct-1-yn-3-o...)Show SMILES CCCCCC(O)(C#CC1(CN2CCC1CC2)OC)c1ccccc1 |THB:17:9:12.13:16.15,(11.05,-11.38,;9.96,-12.47,;8.47,-12.07,;8.08,-10.58,;9.17,-9.49,;8.77,-8,;8.38,-6.51,;10.27,-7.61,;11.76,-7.21,;13.26,-6.81,;13.45,-8.19,;14.99,-7.54,;16.36,-8.17,;16.08,-6.77,;14.73,-6.17,;14.8,-4.53,;15.24,-5.64,;12.85,-5.32,;11.36,-4.93,;7.28,-8.4,;6.19,-7.31,;4.7,-7.7,;4.3,-9.19,;5.39,-10.29,;6.88,-9.89,)| Show InChI InChI=1S/C22H31NO2/c1-3-4-8-13-21(24,19-9-6-5-7-10-19)14-15-22(25-2)18-23-16-11-20(22)12-17-23/h5-7,9-10,20,24H,3-4,8,11-13,16-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000221

(CHEMBL336251)Show SMILES CCCCN1CCN(CC1)C(COCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C25H30F6N2O/c1-2-3-9-32-10-12-33(13-11-32)23(20-7-5-4-6-8-20)18-34-17-19-14-21(24(26,27)28)16-22(15-19)25(29,30)31/h4-8,14-16,23H,2-3,9-13,17-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000243
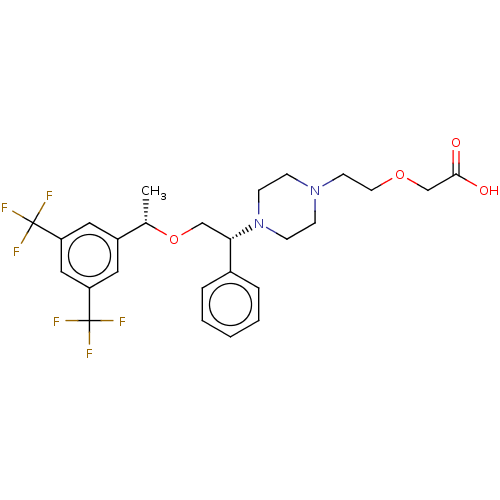
(CHEMBL423913)Show SMILES C[C@H](OC[C@H](N1CCN(CCOCC(O)=O)CC1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H30F6N2O4/c1-18(20-13-21(25(27,28)29)15-22(14-20)26(30,31)32)38-16-23(19-5-3-2-4-6-19)34-9-7-33(8-10-34)11-12-37-17-24(35)36/h2-6,13-15,18,23H,7-12,16-17H2,1H3,(H,35,36)/t18-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22540
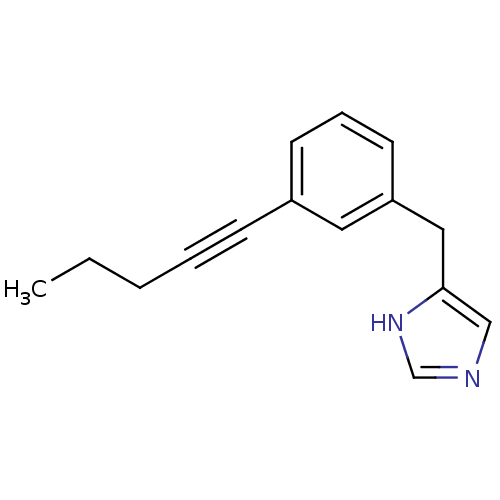
(4-Benzyl-1H-imidazole derivative, 21 | 4-{[3-(pent...)Show InChI InChI=1S/C15H16N2/c1-2-3-4-6-13-7-5-8-14(9-13)10-15-11-16-12-17-15/h5,7-9,11-12H,2-3,10H2,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176712

(1-cyclopentyl-3-(3-methoxyquinuclidin-3-yl)-1-phen...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(C1CCCC1)c1ccccc1 |THB:1:2:5.6:9.8,(26.6,5.4,;28.09,5.01,;28.5,3.51,;28.69,2.14,;30.23,2.79,;31.59,2.16,;31.32,3.55,;29.97,4.16,;30.04,5.8,;30.48,4.69,;27,3.12,;25.5,2.72,;24.01,2.33,;23.61,3.81,;24.41,.84,;23.43,-.35,;24.27,-1.64,;25.76,-1.25,;25.84,.29,;22.52,1.93,;21.42,3.02,;19.94,2.63,;19.53,1.13,;20.63,.04,;22.11,.44,)| Show InChI InChI=1S/C22H29NO2/c1-25-21(17-23-15-11-18(21)12-16-23)13-14-22(24,20-9-5-6-10-20)19-7-3-2-4-8-19/h2-4,7-8,18,20,24H,5-6,9-12,15-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000250
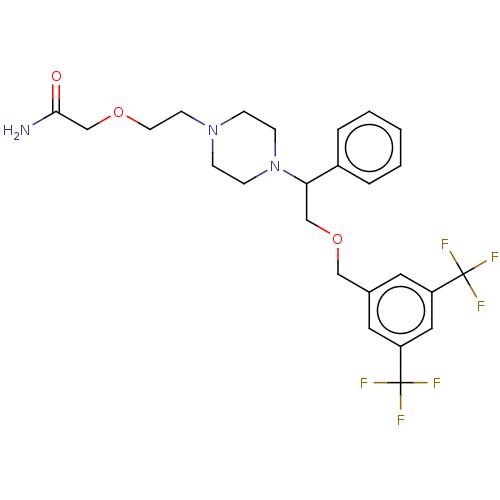
(CHEMBL337789)Show SMILES NC(=O)COCCN1CCN(CC1)C(COCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C25H29F6N3O3/c26-24(27,28)20-12-18(13-21(14-20)25(29,30)31)15-37-16-22(19-4-2-1-3-5-19)34-8-6-33(7-9-34)10-11-36-17-23(32)35/h1-5,12-14,22H,6-11,15-17H2,(H2,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 13: 437-42 (2003)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2MC91J0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176726

(1-(2-fluoropyridin-3-yl)-3-(3-methoxyquinuclidin-3...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cccnc1F |THB:1:2:5.6:9.8,(22.68,-23.08,;24.21,-23.04,;25.02,-24.36,;25.58,-25.63,;26.87,-24.58,;28.36,-24.82,;27.71,-23.56,;26.25,-23.34,;25.87,-21.75,;26.6,-22.69,;23.68,-25.15,;22.36,-25.94,;21.03,-26.73,;20.25,-25.41,;19.71,-27.52,;18.36,-26.76,;17.04,-27.55,;17.06,-29.09,;18.41,-29.85,;19.73,-29.06,;21.82,-28.05,;21.07,-29.39,;21.85,-30.71,;23.39,-30.69,;24.14,-29.35,;23.35,-28.03,;24.1,-26.68,)| Show InChI InChI=1S/C22H23FN2O2/c1-27-21(16-25-14-9-17(21)10-15-25)11-12-22(26,18-6-3-2-4-7-18)19-8-5-13-24-20(19)23/h2-8,13,17,26H,9-10,14-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176710
((S)-1-cyclohexyl-3-((R)-3-methoxyquinuclidin-3-yl)...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#C[C@](O)(C1CCCCC1)c1ccccc1 |wU:12.14,2.1,wD:12.13,2.11,THB:1:2:5.6:9.8,(13.9,-6.16,;15.38,-6.55,;15.79,-8.04,;15.99,-9.42,;17.52,-8.76,;18.88,-9.39,;18.61,-8,;17.26,-7.4,;17.33,-5.76,;17.77,-6.87,;14.3,-8.44,;12.81,-8.83,;11.32,-9.23,;10.92,-7.74,;11.71,-10.71,;10.61,-11.8,;11,-13.27,;12.48,-13.68,;13.57,-12.6,;13.18,-11.11,;9.83,-9.62,;8.73,-8.53,;7.25,-8.93,;6.85,-10.42,;7.94,-11.51,;9.42,-11.11,)| Show InChI InChI=1S/C23H31NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2,4-5,8-9,19,21,25H,3,6-7,10-13,16-18H2,1H3/t22-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data