

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388546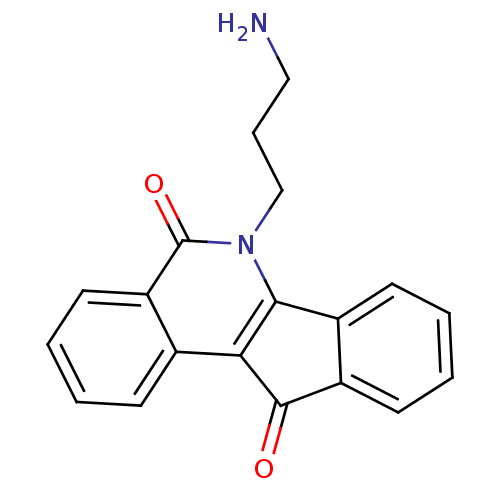 (CHEMBL213072 | CHEMBL333363) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant Tdp1 after 1 hr by FRET assay | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347488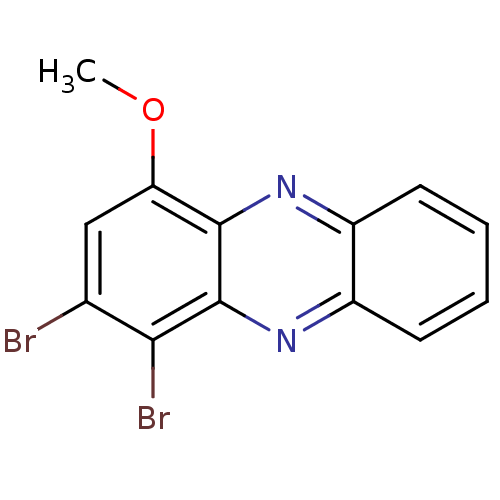 (CHEMBL1802205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347486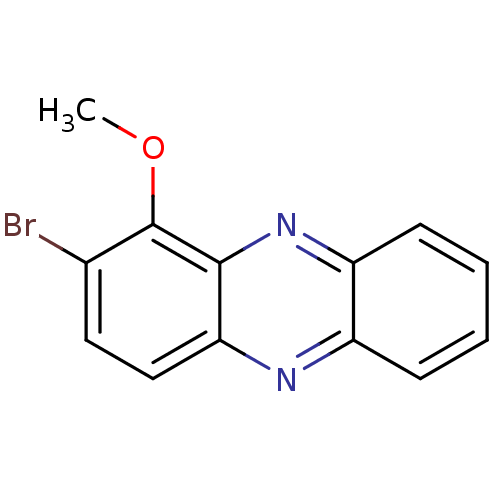 (CHEMBL1802199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347476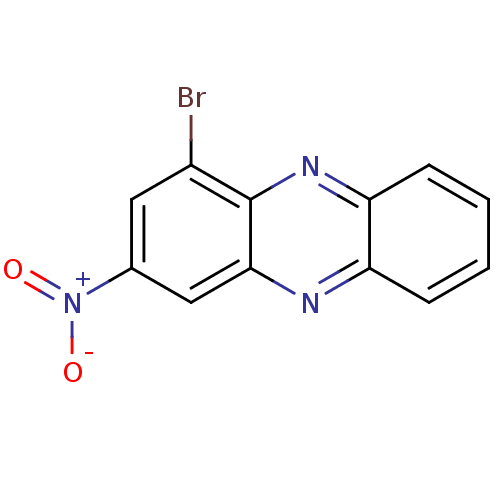 (CHEMBL1802264) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347471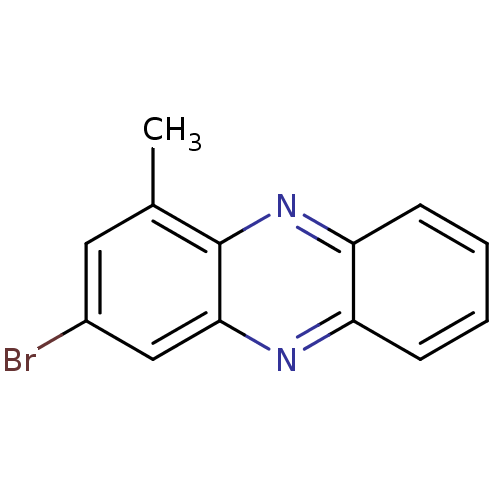 (CHEMBL1802260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347482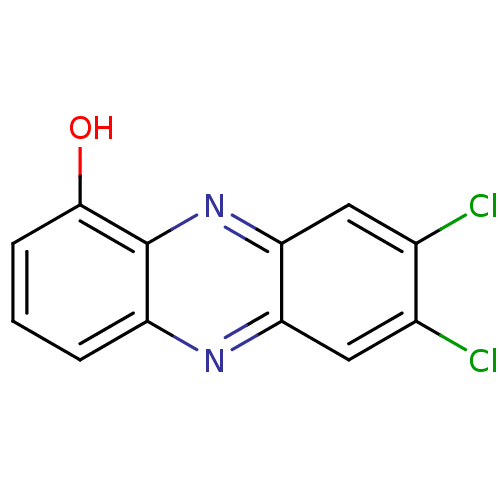 (CHEMBL1802271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347468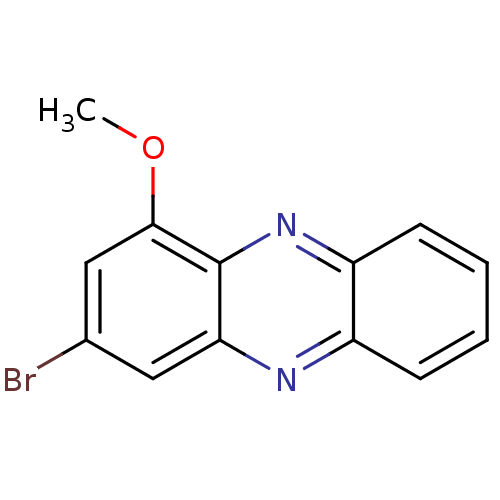 (CHEMBL1802203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206892 (CHEMBL3948489) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206895 (CHEMBL3983017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206890 (CHEMBL3910830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50158383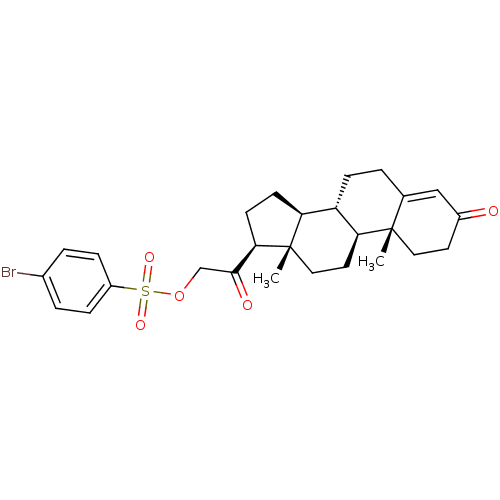 (2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206891 (CHEMBL3921785) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206897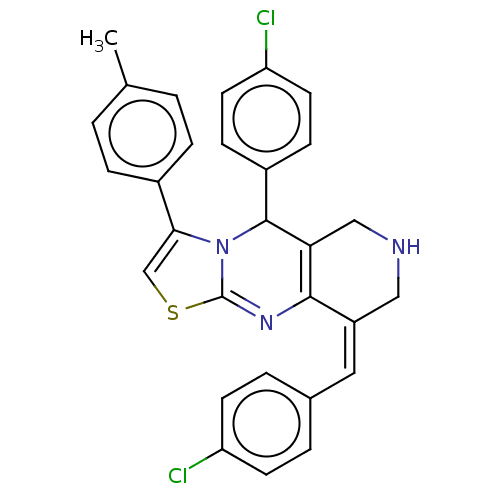 (CHEMBL3945423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206885 (CHEMBL3930722) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347469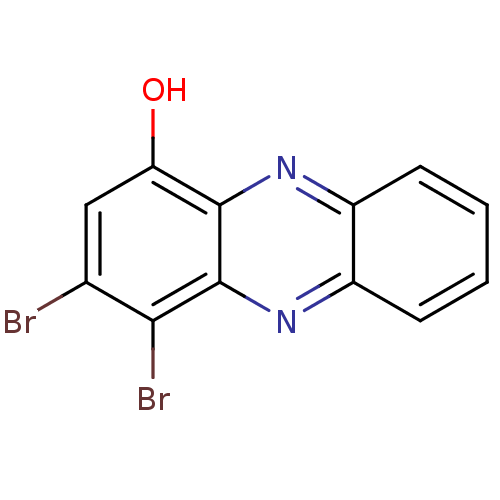 (CHEMBL1802258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206896 (CHEMBL3958208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388544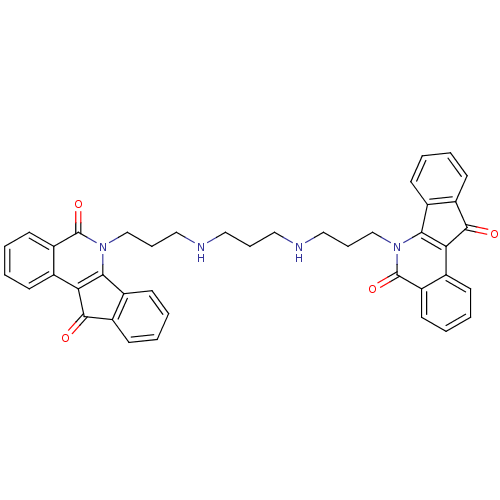 (CHEMBL2057323) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206889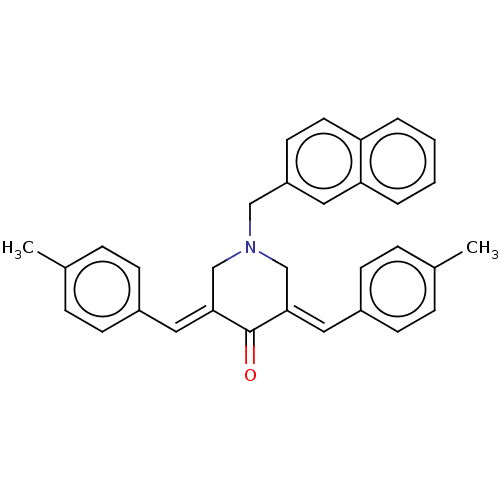 (CHEMBL3928840) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206893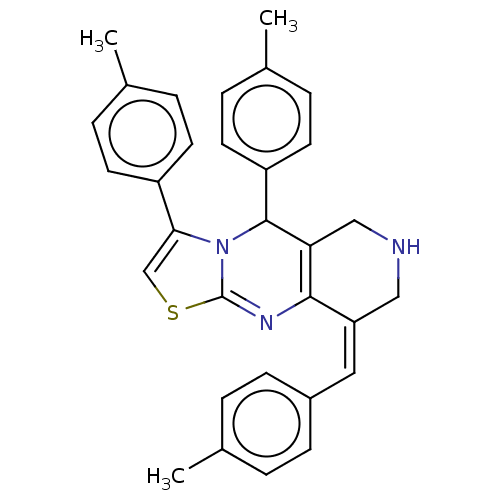 (CHEMBL3901794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388544 (CHEMBL2057323) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human Tdp1 in Tdp1-deficient chicken DT40 whole cell extract using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-p... | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206886 (CHEMBL3955393) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206894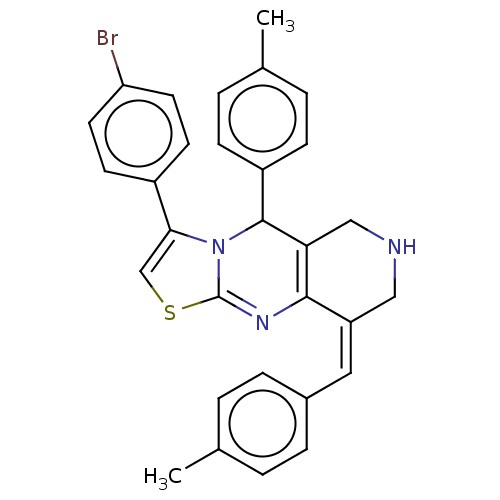 (CHEMBL3972865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347484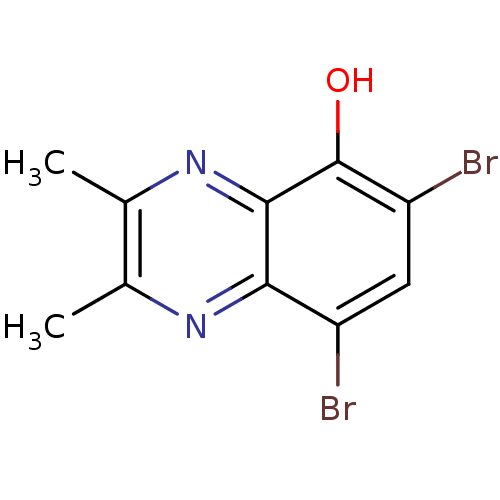 (CHEMBL1802277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206887 (CHEMBL3976003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206888 (CHEMBL3984034) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347472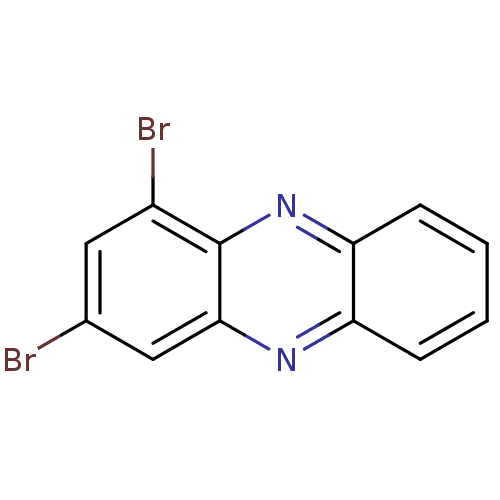 (CHEMBL1802261) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347483 (CHEMBL1802272) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347481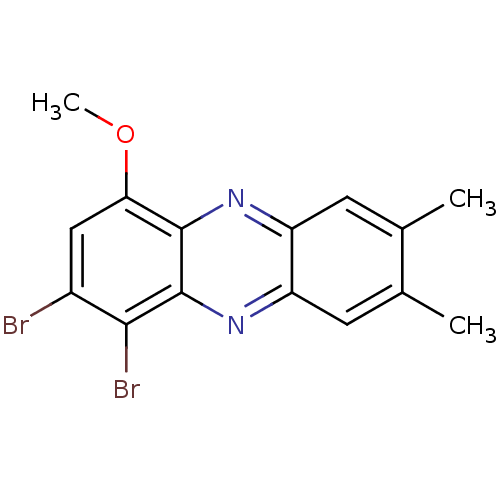 (CHEMBL1802270) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347479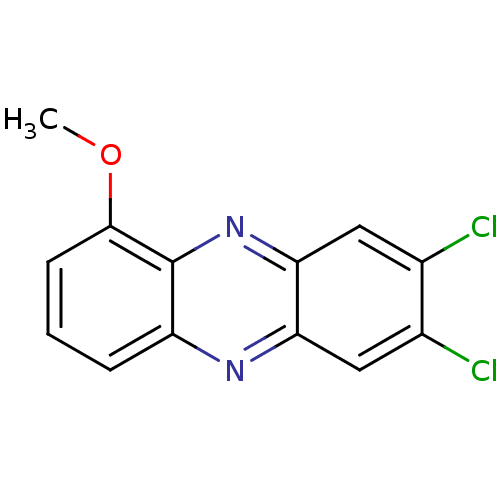 (CHEMBL1802267) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425045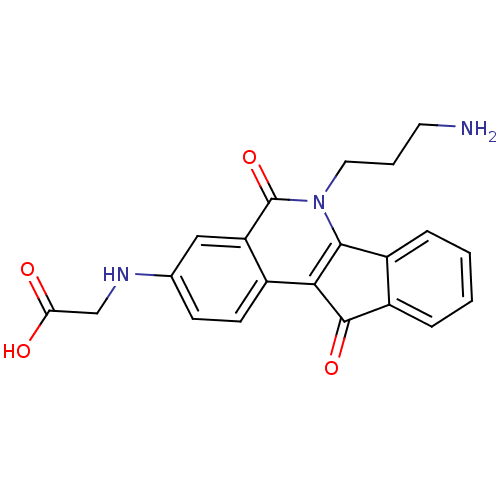 (CHEMBL2312896 | CHEMBL2322964 | US9402842, 43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description A 5′-[32P]-labeled single-stranded DNA oligonucleotide containing a 3′-phosphotyrosine (N14Y) was generated as described by Dexheimer et ... | US Patent US9402842 (2016) BindingDB Entry DOI: 10.7270/Q2H41Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425045 (CHEMBL2312896 | CHEMBL2322964 | US9402842, 43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425045 (CHEMBL2312896 | CHEMBL2322964 | US9402842, 43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description Tdp1 reactions were performed as recently described. Briefly, a 5'-[32P]-labeled single-stranded DNA oligonucleotide containing a 3'-phosphotyrosine ... | US Patent US8912213 (2014) BindingDB Entry DOI: 10.7270/Q2FN14XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425086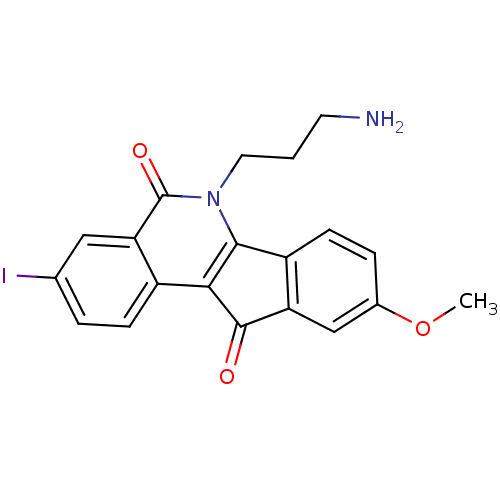 (CHEMBL2312906 | US9402842, 84) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425086 (CHEMBL2312906 | US9402842, 84) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description A 5′-[32P]-labeled single-stranded DNA oligonucleotide containing a 3′-phosphotyrosine (N14Y) was generated as described by Dexheimer et ... | US Patent US9402842 (2016) BindingDB Entry DOI: 10.7270/Q2H41Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM47063 (CHEMBL2059280 | US8912213, 84) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description Tdp1 reactions were performed as recently described. Briefly, a 5'-[32P]-labeled single-stranded DNA oligonucleotide containing a 3'-phosphotyrosine ... | US Patent US8912213 (2014) BindingDB Entry DOI: 10.7270/Q2FN14XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425087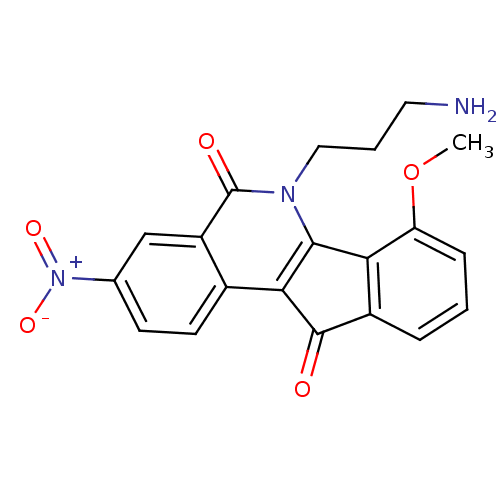 (CHEMBL218884 | US9402842, 55) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM140517 (US8912213, 55) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description Tdp1 reactions were performed as recently described. Briefly, a 5'-[32P]-labeled single-stranded DNA oligonucleotide containing a 3'-phosphotyrosine ... | US Patent US8912213 (2014) BindingDB Entry DOI: 10.7270/Q2FN14XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425087 (CHEMBL218884 | US9402842, 55) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description A 5′-[32P]-labeled single-stranded DNA oligonucleotide containing a 3′-phosphotyrosine (N14Y) was generated as described by Dexheimer et ... | US Patent US9402842 (2016) BindingDB Entry DOI: 10.7270/Q2H41Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425085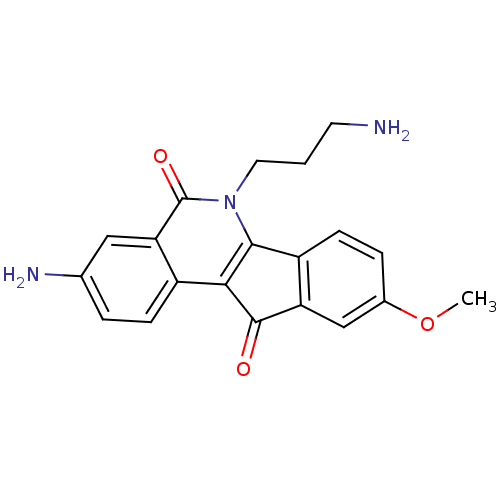 (CHEMBL2312908 | US9402842, 89) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388562 (CHEMBL2059427) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description Tdp1 reactions were performed as recently described. Briefly, a 5'-[32P]-labeled single-stranded DNA oligonucleotide containing a 3'-phosphotyrosine ... | US Patent US8912213 (2014) BindingDB Entry DOI: 10.7270/Q2FN14XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425085 (CHEMBL2312908 | US9402842, 89) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description A 5′-[32P]-labeled single-stranded DNA oligonucleotide containing a 3′-phosphotyrosine (N14Y) was generated as described by Dexheimer et ... | US Patent US9402842 (2016) BindingDB Entry DOI: 10.7270/Q2H41Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50158383 (2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50347478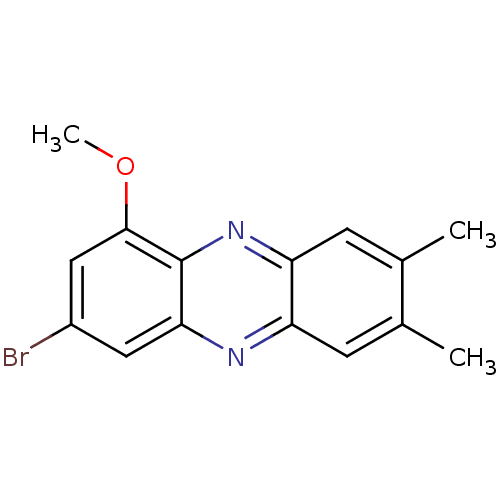 (CHEMBL1802266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human QR2 expressed in Escherichia coli BL21 (DE3) using MTT and NMeH as substrate assessed as formazan formation | J Med Chem 53: 8688-99 (2010) Article DOI: 10.1021/jm1011066 BindingDB Entry DOI: 10.7270/Q2TB178C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425084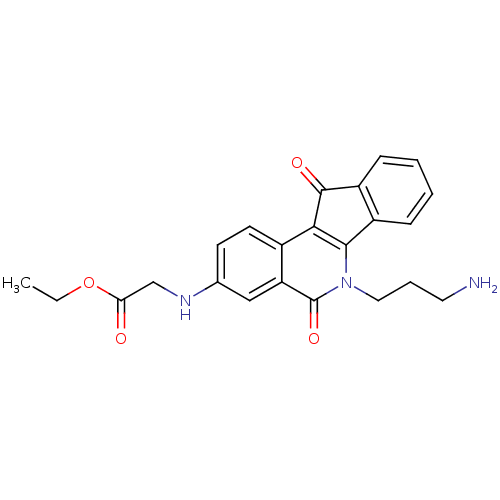 (CHEMBL2312893 | US9402842, 40) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM140516 (US8912213, 54) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description Tdp1 reactions were performed as recently described. Briefly, a 5'-[32P]-labeled single-stranded DNA oligonucleotide containing a 3'-phosphotyrosine ... | US Patent US8912213 (2014) BindingDB Entry DOI: 10.7270/Q2FN14XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425088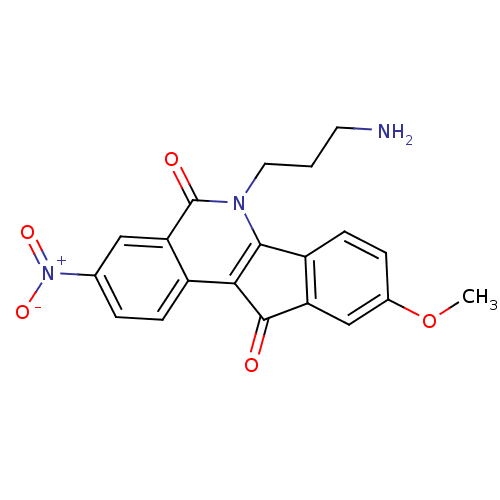 (CHEMBL375623 | US9402842, 54) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425088 (CHEMBL375623 | US9402842, 54) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description A 5′-[32P]-labeled single-stranded DNA oligonucleotide containing a 3′-phosphotyrosine (N14Y) was generated as described by Dexheimer et ... | US Patent US9402842 (2016) BindingDB Entry DOI: 10.7270/Q2H41Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425084 (CHEMBL2312893 | US9402842, 40) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description Tdp1 reactions were performed as recently described. Briefly, a 5'-[32P]-labeled single-stranded DNA oligonucleotide containing a 3'-phosphotyrosine ... | US Patent US8912213 (2014) BindingDB Entry DOI: 10.7270/Q2FN14XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425084 (CHEMBL2312893 | US9402842, 40) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description A 5′-[32P]-labeled single-stranded DNA oligonucleotide containing a 3′-phosphotyrosine (N14Y) was generated as described by Dexheimer et ... | US Patent US9402842 (2016) BindingDB Entry DOI: 10.7270/Q2H41Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 231 total ) | Next | Last >> |