 Found 363 hits with Last Name = 'conde-knape' and Initial = 'k'
Found 363 hits with Last Name = 'conde-knape' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371874
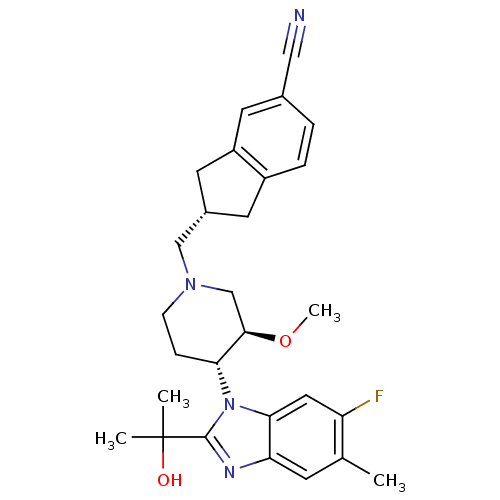
(CHEMBL257733)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(cc3C2)C#N)CC[C@H]1n1c(nc2cc(C)c(F)cc12)C(C)(C)O Show InChI InChI=1S/C28H33FN4O2/c1-17-9-23-25(13-22(17)29)33(27(31-23)28(2,3)34)24-7-8-32(16-26(24)35-4)15-19-11-20-6-5-18(14-30)10-21(20)12-19/h5-6,9-10,13,19,24,26,34H,7-8,11-12,15-16H2,1-4H3/t19-,24+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371872
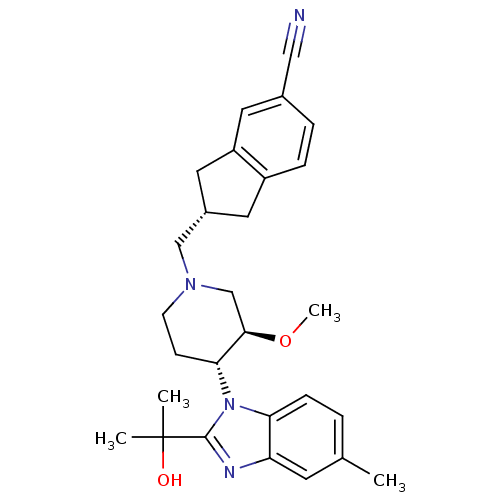
(CHEMBL257280)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(cc3C2)C#N)CC[C@H]1n1c(nc2cc(C)ccc12)C(C)(C)O Show InChI InChI=1S/C28H34N4O2/c1-18-5-8-24-23(11-18)30-27(28(2,3)33)32(24)25-9-10-31(17-26(25)34-4)16-20-13-21-7-6-19(15-29)12-22(21)14-20/h5-8,11-12,20,25-26,33H,9-10,13-14,16-17H2,1-4H3/t20-,25+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371868

(CHEMBL269939)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(Br)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C25H30BrN3O/c1-16-4-7-23-22(10-16)27-17(2)29(23)24-8-9-28(15-25(24)30-3)14-18-11-19-5-6-21(26)13-20(19)12-18/h4-7,10,13,18,24-25H,8-9,11-12,14-15H2,1-3H3/t18-,24+,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371871
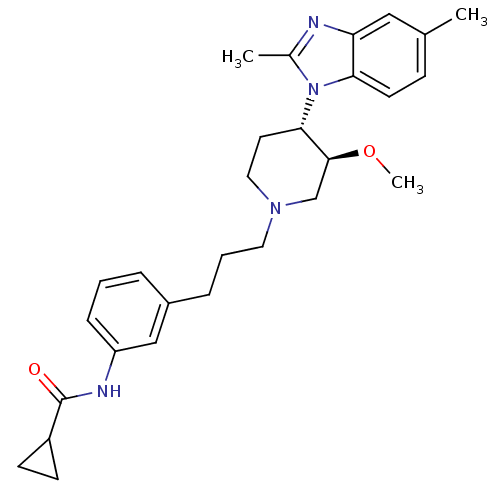
(CHEMBL255112)Show SMILES CO[C@H]1CN(CCCc2cccc(NC(=O)C3CC3)c2)CC[C@@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C28H36N4O2/c1-19-9-12-25-24(16-19)29-20(2)32(25)26-13-15-31(18-27(26)34-3)14-5-7-21-6-4-8-23(17-21)30-28(33)22-10-11-22/h4,6,8-9,12,16-17,22,26-27H,5,7,10-11,13-15,18H2,1-3H3,(H,30,33)/t26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371866
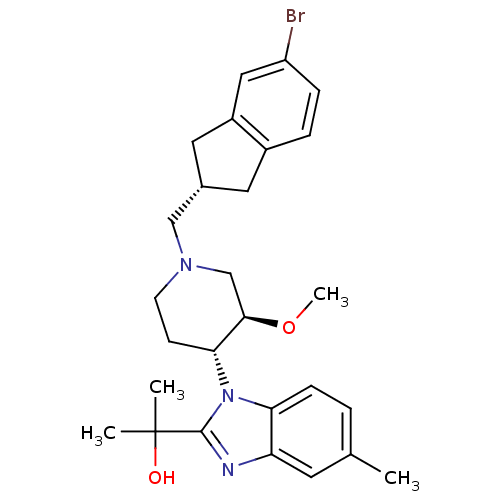
(CHEMBL257906)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(Br)cc3C2)CC[C@H]1n1c(nc2cc(C)ccc12)C(C)(C)O Show InChI InChI=1S/C27H34BrN3O2/c1-17-5-8-23-22(11-17)29-26(27(2,3)32)31(23)24-9-10-30(16-25(24)33-4)15-18-12-19-6-7-21(28)14-20(19)13-18/h5-8,11,14,18,24-25,32H,9-10,12-13,15-16H2,1-4H3/t18-,24+,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371869
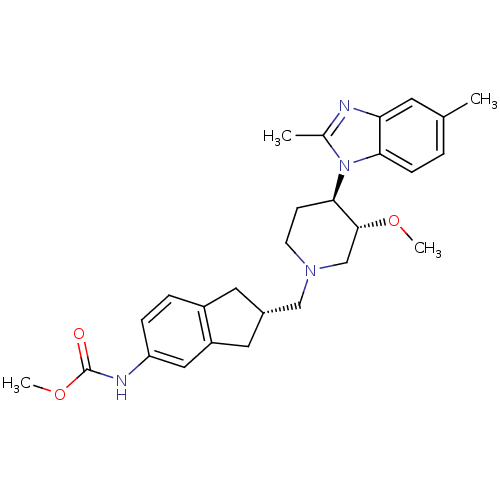
(CHEMBL270151)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(NC(=O)OC)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C27H34N4O3/c1-17-5-8-24-23(11-17)28-18(2)31(24)25-9-10-30(16-26(25)33-3)15-19-12-20-6-7-22(14-21(20)13-19)29-27(32)34-4/h5-8,11,14,19,25-26H,9-10,12-13,15-16H2,1-4H3,(H,29,32)/t19-,25+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371873
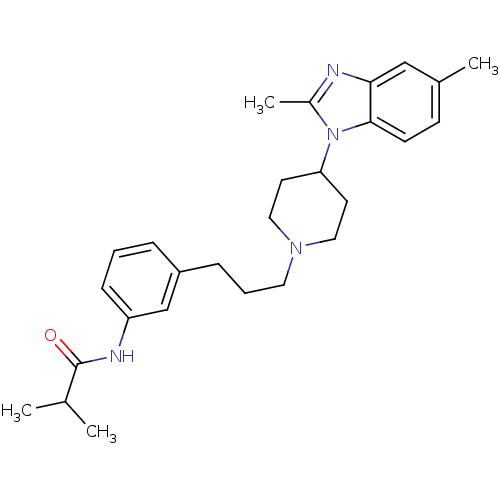
(CHEMBL403730)Show SMILES CC(C)C(=O)Nc1cccc(CCCN2CCC(CC2)n2c(C)nc3cc(C)ccc23)c1 Show InChI InChI=1S/C27H36N4O/c1-19(2)27(32)29-23-9-5-7-22(18-23)8-6-14-30-15-12-24(13-16-30)31-21(4)28-25-17-20(3)10-11-26(25)31/h5,7,9-11,17-19,24H,6,8,12-16H2,1-4H3,(H,29,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371870
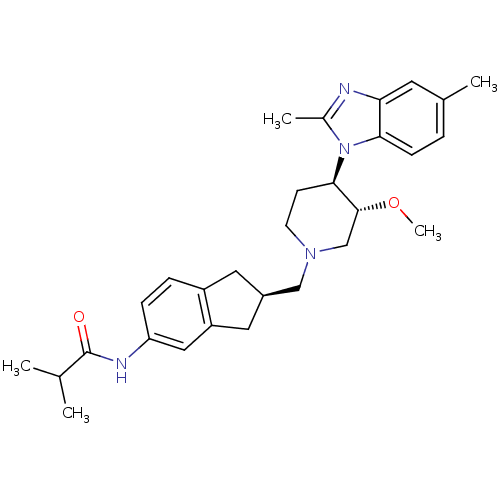
(CHEMBL258260)Show SMILES CO[C@@H]1CN(C[C@@H]2Cc3ccc(NC(=O)C(C)C)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C29H38N4O2/c1-18(2)29(34)31-24-8-7-22-13-21(14-23(22)15-24)16-32-11-10-27(28(17-32)35-5)33-20(4)30-25-12-19(3)6-9-26(25)33/h6-9,12,15,18,21,27-28H,10-11,13-14,16-17H2,1-5H3,(H,31,34)/t21-,27-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371867
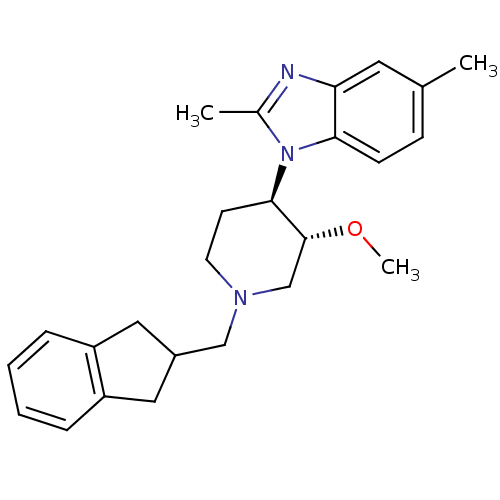
(CHEMBL257905)Show SMILES CO[C@@H]1CN(CC2Cc3ccccc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C25H31N3O/c1-17-8-9-23-22(12-17)26-18(2)28(23)24-10-11-27(16-25(24)29-3)15-19-13-20-6-4-5-7-21(20)14-19/h4-9,12,19,24-25H,10-11,13-16H2,1-3H3/t24-,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Glutamine--fructose-6-phosphate aminotransferase [isomerizing] 1
(Homo sapiens (Human)) | BDBM50356009
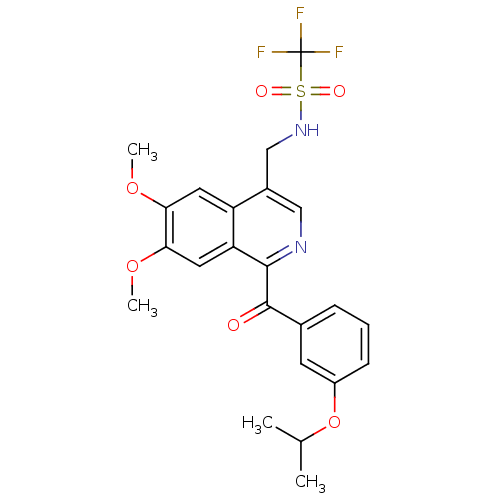
(CHEMBL1911368)Show SMILES COc1cc2c(CNS(=O)(=O)C(F)(F)F)cnc(C(=O)c3cccc(OC(C)C)c3)c2cc1OC Show InChI InChI=1S/C23H23F3N2O6S/c1-13(2)34-16-7-5-6-14(8-16)22(29)21-18-10-20(33-4)19(32-3)9-17(18)15(11-27-21)12-28-35(30,31)23(24,25)26/h5-11,13,28H,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of GFAT in presence of glutamine |
Bioorg Med Chem Lett 21: 6264-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.009
BindingDB Entry DOI: 10.7270/Q21J9B6P |
More data for this
Ligand-Target Pair | |
Glutamine--fructose-6-phosphate aminotransferase [isomerizing] 1
(Homo sapiens (Human)) | BDBM50356009

(CHEMBL1911368)Show SMILES COc1cc2c(CNS(=O)(=O)C(F)(F)F)cnc(C(=O)c3cccc(OC(C)C)c3)c2cc1OC Show InChI InChI=1S/C23H23F3N2O6S/c1-13(2)34-16-7-5-6-14(8-16)22(29)21-18-10-20(33-4)19(32-3)9-17(18)15(11-27-21)12-28-35(30,31)23(24,25)26/h5-11,13,28H,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of GFAT in presence of fructose-6-phosphate |
Bioorg Med Chem Lett 21: 6264-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.009
BindingDB Entry DOI: 10.7270/Q21J9B6P |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50492348

(CHEMBL2403866)Show SMILES Cc1nc2cc(F)ccc2n1[C@@H]1CC[C@@H](CC1)NC[C@H]1Cc2ccc(cc2C1)C#N |r,wU:11.12,14.19,19.21,(52.97,-7.87,;51.79,-8.86,;50.3,-8.5,;49.48,-9.81,;47.96,-10.09,;47.45,-11.54,;45.93,-11.81,;48.44,-12.72,;49.96,-12.44,;50.49,-10.99,;51.9,-10.41,;53.24,-11.19,;53.18,-12.75,;54.49,-13.57,;55.85,-12.85,;55.91,-11.29,;54.59,-10.47,;57.18,-13.64,;58.52,-12.88,;58.54,-11.34,;57.29,-10.42,;57.78,-8.96,;57.03,-7.63,;57.8,-6.31,;59.35,-6.33,;60.1,-7.66,;59.32,-8.98,;59.78,-10.45,;60.13,-5.01,;60.91,-3.68,)| Show InChI InChI=1S/C25H27FN4/c1-16-29-24-13-21(26)4-9-25(24)30(16)23-7-5-22(6-8-23)28-15-18-11-19-3-2-17(14-27)10-20(19)12-18/h2-4,9-10,13,18,22-23,28H,5-8,11-12,15H2,1H3/t18-,22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH peptide from human MCHR1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 23: 4216-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.017
BindingDB Entry DOI: 10.7270/Q2TX3J95 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50492349
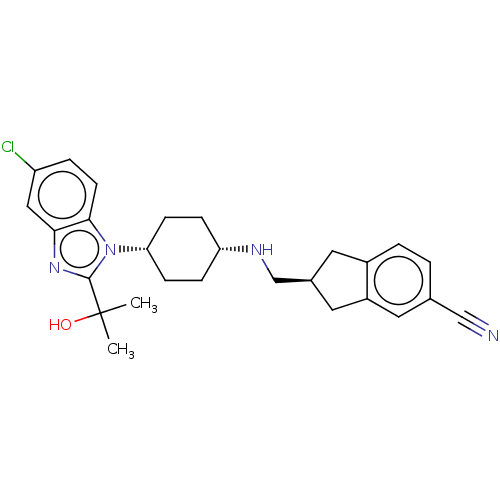
(CHEMBL2403861)Show SMILES CC(C)(O)c1nc2cc(Cl)ccc2n1[C@@H]1CC[C@@H](CC1)NC[C@H]1Cc2ccc(cc2C1)C#N |r,wU:14.15,17.22,22.24,(71.13,-8.4,;71.91,-9.74,;72.68,-8.4,;73.37,-10.27,;70.74,-10.74,;69.24,-10.38,;68.43,-11.69,;66.91,-11.96,;66.39,-13.41,;64.88,-13.69,;67.38,-14.59,;68.9,-14.32,;69.43,-12.87,;70.85,-12.29,;72.19,-13.07,;72.13,-14.63,;73.44,-15.44,;74.8,-14.73,;74.85,-13.16,;73.53,-12.34,;76.13,-15.51,;77.47,-14.76,;77.48,-13.22,;76.24,-12.3,;76.73,-10.84,;75.98,-9.51,;76.75,-8.19,;78.29,-8.2,;79.04,-9.54,;78.26,-10.86,;78.73,-12.32,;79.06,-6.88,;79.85,-5.55,)| Show InChI InChI=1S/C27H31ClN4O/c1-27(2,33)26-31-24-14-21(28)5-10-25(24)32(26)23-8-6-22(7-9-23)30-16-18-12-19-4-3-17(15-29)11-20(19)13-18/h3-5,10-11,14,18,22-23,30,33H,6-9,12-13,16H2,1-2H3/t18-,22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH peptide from human MCHR1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 23: 4216-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.017
BindingDB Entry DOI: 10.7270/Q2TX3J95 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018760
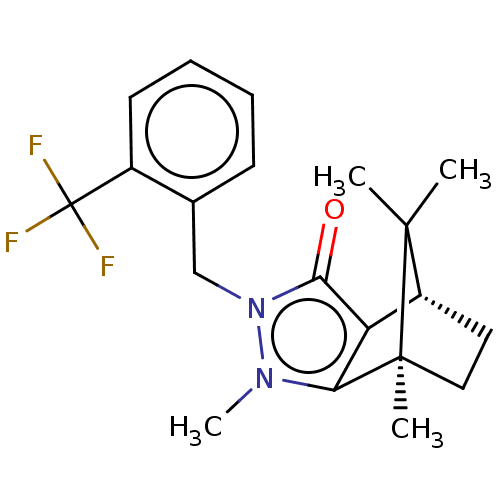
(CHEMBL3291357)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1C(F)(F)F)n3C)C2(C)C |r| Show InChI InChI=1S/C20H23F3N2O/c1-18(2)14-9-10-19(18,3)16-15(14)17(26)25(24(16)4)11-12-7-5-6-8-13(12)20(21,22)23/h5-8,14H,9-11H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018759
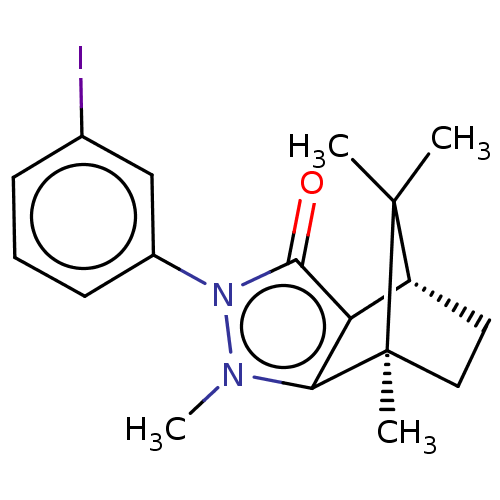
(CHEMBL3291350)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(I)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H21IN2O/c1-17(2)13-8-9-18(17,3)15-14(13)16(22)21(20(15)4)12-7-5-6-11(19)10-12/h5-7,10,13H,8-9H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018761
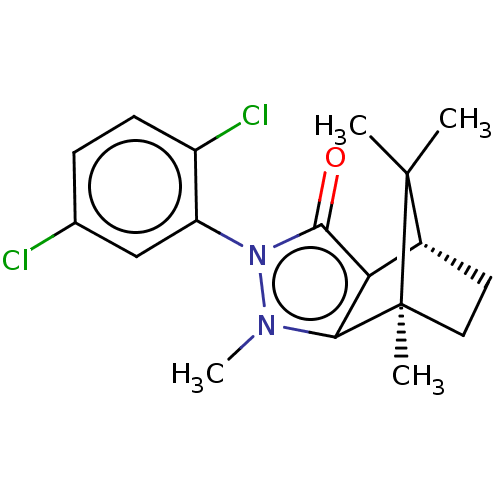
(CHEMBL3291348)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cc(Cl)ccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(16.87,-14.9,;16.87,-13.4,;18.16,-12.66,;18.16,-11.16,;16.87,-10.41,;16.45,-8.98,;15.58,-11.16,;15.58,-12.66,;14.16,-13.12,;13.7,-14.54,;13.28,-11.91,;11.73,-11.9,;10.97,-13.24,;9.43,-13.25,;8.66,-14.58,;8.65,-11.9,;9.43,-10.56,;10.97,-10.57,;11.75,-9.23,;14.16,-10.7,;13.68,-9.23,;17.73,-11.9,;19.22,-12.76,;19.22,-11.04,)| Show InChI InChI=1S/C18H20Cl2N2O/c1-17(2)11-7-8-18(17,3)15-14(11)16(23)22(21(15)4)13-9-10(19)5-6-12(13)20/h5-6,9,11H,7-8H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50492354

(CHEMBL2403862)Show SMILES CC(C)(O)c1nc2cc(Cl)ccc2n1[C@@H]1CC[C@@H](CC1)NC[C@@H]1Cc2ccc(cc2C1)C#N |r,wU:14.15,17.22,wD:22.24,(41.99,-6.39,;42.77,-7.73,;43.54,-6.38,;44.22,-8.25,;41.59,-8.72,;40.1,-8.36,;39.28,-9.67,;37.76,-9.95,;37.25,-11.4,;35.73,-11.68,;38.24,-12.58,;39.76,-12.31,;40.29,-10.85,;41.7,-10.28,;43.04,-11.06,;42.98,-12.61,;44.29,-13.43,;45.65,-12.71,;45.71,-11.15,;44.39,-10.33,;46.98,-13.5,;48.32,-12.74,;48.34,-11.2,;47.09,-10.29,;47.58,-8.83,;46.83,-7.5,;47.6,-6.18,;49.15,-6.19,;49.9,-7.53,;49.12,-8.85,;49.58,-10.31,;49.92,-4.86,;50.7,-3.54,)| Show InChI InChI=1S/C27H31ClN4O/c1-27(2,33)26-31-24-14-21(28)5-10-25(24)32(26)23-8-6-22(7-9-23)30-16-18-12-19-4-3-17(15-29)11-20(19)13-18/h3-5,10-11,14,18,22-23,30,33H,6-9,12-13,16H2,1-2H3/t18-,22-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH peptide from human MCHR1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 23: 4216-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.017
BindingDB Entry DOI: 10.7270/Q2TX3J95 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50341785
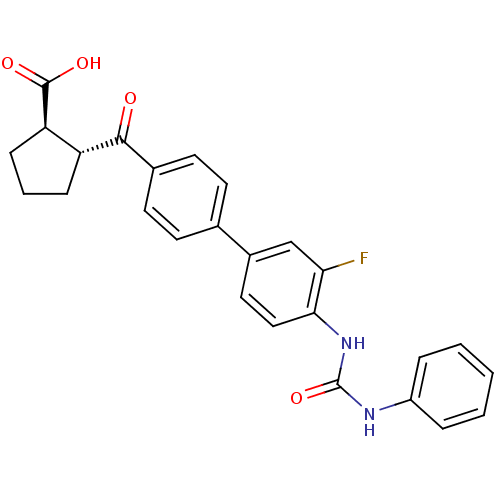
((1R,2R)-2-(3'-fluoro-4'-(3-phenylureido)biphenylca...)Show SMILES OC(=O)[C@@H]1CCC[C@H]1C(=O)c1ccc(cc1)-c1ccc(NC(=O)Nc2ccccc2)c(F)c1 |r| Show InChI InChI=1S/C26H23FN2O4/c27-22-15-18(13-14-23(22)29-26(33)28-19-5-2-1-3-6-19)16-9-11-17(12-10-16)24(30)20-7-4-8-21(20)25(31)32/h1-3,5-6,9-15,20-21H,4,7-8H2,(H,31,32)(H2,28,29,33)/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 |
J Med Chem 54: 2433-46 (2011)
Article DOI: 10.1021/jm101580m
BindingDB Entry DOI: 10.7270/Q2S182TQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018761

(CHEMBL3291348)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cc(Cl)ccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(16.87,-14.9,;16.87,-13.4,;18.16,-12.66,;18.16,-11.16,;16.87,-10.41,;16.45,-8.98,;15.58,-11.16,;15.58,-12.66,;14.16,-13.12,;13.7,-14.54,;13.28,-11.91,;11.73,-11.9,;10.97,-13.24,;9.43,-13.25,;8.66,-14.58,;8.65,-11.9,;9.43,-10.56,;10.97,-10.57,;11.75,-9.23,;14.16,-10.7,;13.68,-9.23,;17.73,-11.9,;19.22,-12.76,;19.22,-11.04,)| Show InChI InChI=1S/C18H20Cl2N2O/c1-17(2)11-7-8-18(17,3)15-14(11)16(23)22(21(15)4)13-9-10(19)5-6-12(13)20/h5-6,9,11H,7-8H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018793
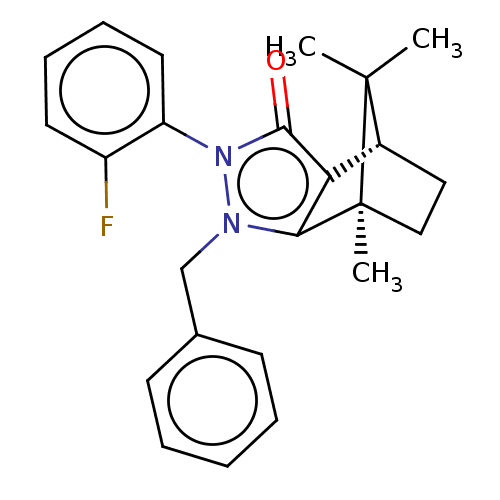
(CHEMBL3291344)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H25FN2O/c1-23(2)17-13-14-24(23,3)21-20(17)22(28)27(19-12-8-7-11-18(19)25)26(21)15-16-9-5-4-6-10-16/h4-12,17H,13-15H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018759

(CHEMBL3291350)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(I)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H21IN2O/c1-17(2)13-8-9-18(17,3)15-14(13)16(22)21(20(15)4)12-7-5-6-11(19)10-12/h5-7,10,13H,8-9H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50492361
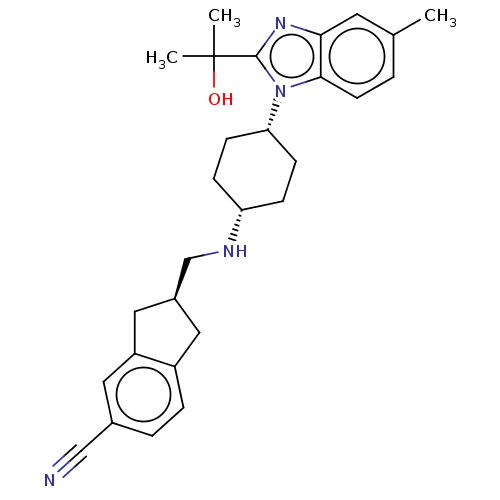
(CHEMBL2403864)Show SMILES Cc1ccc2n([C@@H]3CC[C@@H](CC3)NC[C@H]3Cc4ccc(cc4C3)C#N)c(nc2c1)C(C)(C)O |r,wU:6.5,9.12,14.14,(36.65,-12.27,;38.17,-12,;39.16,-13.18,;40.68,-12.9,;41.21,-11.45,;42.62,-10.88,;43.96,-11.65,;43.9,-13.21,;45.22,-14.03,;46.57,-13.31,;46.63,-11.75,;45.31,-10.93,;47.9,-14.1,;49.24,-13.34,;49.26,-11.8,;48.01,-10.88,;48.5,-9.42,;47.75,-8.1,;48.53,-6.78,;50.07,-6.79,;50.82,-8.13,;50.04,-9.44,;50.5,-10.91,;50.84,-5.46,;51.62,-4.14,;42.52,-9.32,;41.02,-8.96,;40.21,-10.27,;38.68,-10.55,;43.69,-8.32,;42.91,-6.99,;44.46,-6.98,;45.14,-8.85,)| Show InChI InChI=1S/C28H34N4O/c1-18-4-11-26-25(12-18)31-27(28(2,3)33)32(26)24-9-7-23(8-10-24)30-17-20-14-21-6-5-19(16-29)13-22(21)15-20/h4-6,11-13,20,23-24,30,33H,7-10,14-15,17H2,1-3H3/t20-,23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH peptide from human MCHR1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 23: 4216-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.017
BindingDB Entry DOI: 10.7270/Q2TX3J95 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018769
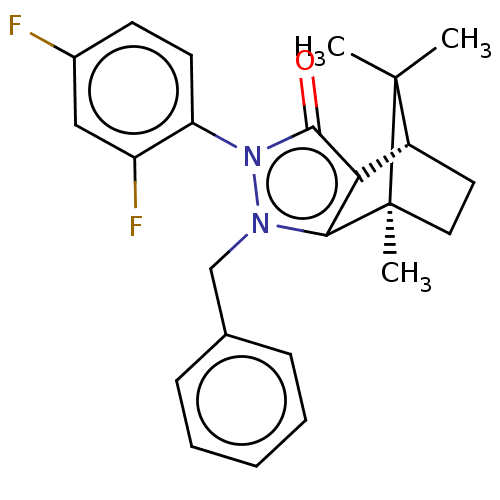
(CHEMBL3291346)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;9.91,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H24F2N2O/c1-23(2)17-11-12-24(23,3)21-20(17)22(29)28(19-10-9-16(25)13-18(19)26)27(21)14-15-7-5-4-6-8-15/h4-10,13,17H,11-12,14H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018795
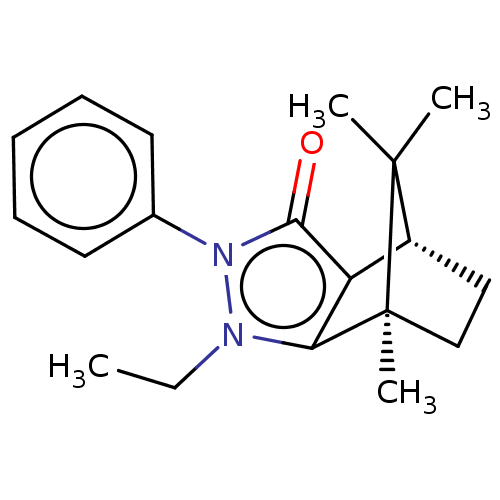
(CHEMBL3291339)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3CC)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-5-20-16-15(14-11-12-19(16,4)18(14,2)3)17(22)21(20)13-9-7-6-8-10-13/h6-10,14H,5,11-12H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018794
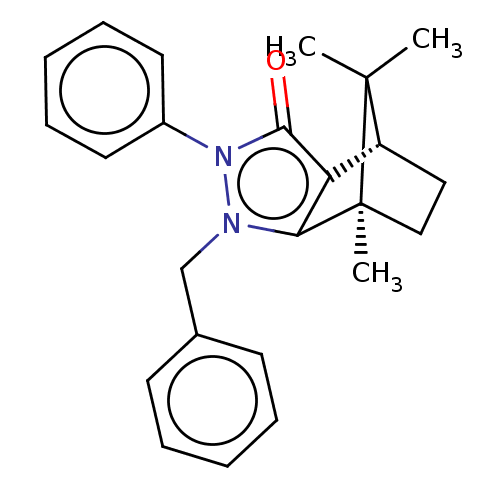
(CHEMBL3291340)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3Cc1ccccc1)C2(C)C |r| Show InChI InChI=1S/C24H26N2O/c1-23(2)19-14-15-24(23,3)21-20(19)22(27)26(18-12-8-5-9-13-18)25(21)16-17-10-6-4-7-11-17/h4-13,19H,14-16H2,1-3H3/t19-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018760

(CHEMBL3291357)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1C(F)(F)F)n3C)C2(C)C |r| Show InChI InChI=1S/C20H23F3N2O/c1-18(2)14-9-10-19(18,3)16-15(14)17(26)25(24(16)4)11-12-7-5-6-8-13(12)20(21,22)23/h5-8,14H,9-11H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018797
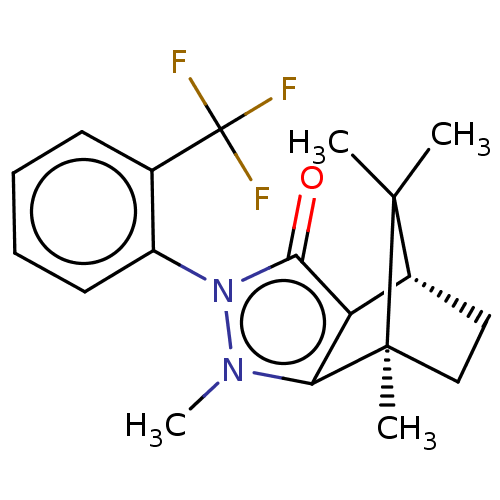
(CHEMBL3291345)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1C(F)(F)F)n3C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;13.37,-6.66,;14.46,-5.57,;11.88,-6.27,;12.97,-5.16,;16.96,-8.3,;16.48,-6.83,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C19H21F3N2O/c1-17(2)12-9-10-18(17,3)15-14(12)16(25)24(23(15)4)13-8-6-5-7-11(13)19(20,21)22/h5-8,12H,9-10H2,1-4H3/t12-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018793

(CHEMBL3291344)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H25FN2O/c1-23(2)17-13-14-24(23,3)21-20(17)22(28)27(19-12-8-7-11-18(19)25)26(21)15-16-9-5-4-6-10-16/h4-12,17H,13-15H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018769

(CHEMBL3291346)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;9.91,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H24F2N2O/c1-23(2)17-11-12-24(23,3)21-20(17)22(29)28(19-10-9-16(25)13-18(19)26)27(21)14-15-7-5-4-6-8-15/h4-10,13,17H,11-12,14H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018797

(CHEMBL3291345)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1C(F)(F)F)n3C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;13.37,-6.66,;14.46,-5.57,;11.88,-6.27,;12.97,-5.16,;16.96,-8.3,;16.48,-6.83,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C19H21F3N2O/c1-17(2)12-9-10-18(17,3)15-14(12)16(25)24(23(15)4)13-8-6-5-7-11(13)19(20,21)22/h5-8,12H,9-10H2,1-4H3/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50492360

(CHEMBL2403856)Show SMILES Cc1nc2cc(C)ccc2n1[C@@H]1CC[C@@H](CC1)NC[C@H]1Cc2ccc(cc2C1)C#N |r,wU:11.12,14.19,19.21,(37.22,-25.78,;36.05,-26.77,;34.55,-26.4,;33.74,-27.72,;32.21,-27.99,;31.7,-29.44,;30.18,-29.72,;32.69,-30.62,;34.21,-30.35,;34.74,-28.9,;36.16,-28.32,;37.49,-29.1,;37.43,-30.66,;38.75,-31.47,;40.1,-30.76,;40.16,-29.19,;38.84,-28.37,;41.43,-31.54,;42.77,-30.79,;42.79,-29.25,;41.54,-28.33,;42.03,-26.87,;41.28,-25.54,;42.06,-24.22,;43.6,-24.23,;44.35,-25.57,;43.57,-26.89,;44.04,-28.35,;44.37,-22.91,;45.15,-21.58,)| Show InChI InChI=1S/C26H30N4/c1-17-3-10-26-25(11-17)29-18(2)30(26)24-8-6-23(7-9-24)28-16-20-13-21-5-4-19(15-27)12-22(21)14-20/h3-5,10-12,20,23-24,28H,6-9,13-14,16H2,1-2H3/t20-,23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH peptide from human MCHR1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 23: 4216-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.017
BindingDB Entry DOI: 10.7270/Q2TX3J95 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018798
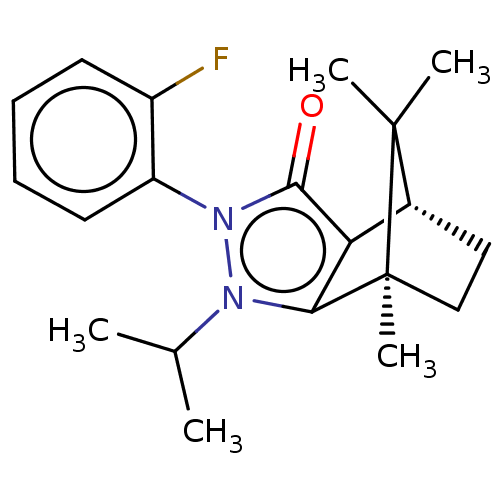
(CHEMBL3291342)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3C(C)C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;15.7,-5.48,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H25FN2O/c1-12(2)22-17-16(13-10-11-20(17,5)19(13,3)4)18(24)23(22)15-9-7-6-8-14(15)21/h6-9,12-13H,10-11H2,1-5H3/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018802
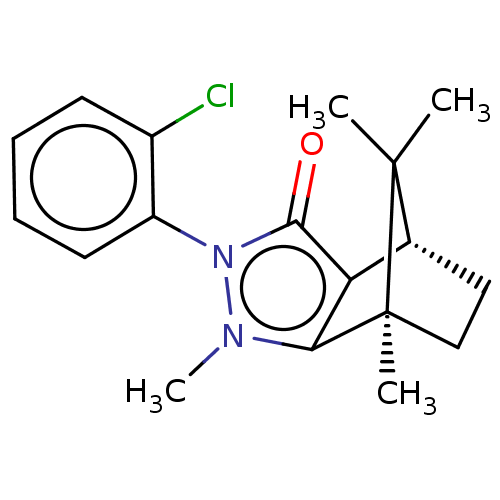
(CHEMBL3291354)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(22.13,-16.72,;22.13,-15.22,;23.42,-14.48,;23.42,-12.98,;22.13,-12.23,;21.71,-10.79,;20.84,-12.98,;20.84,-14.48,;19.42,-14.94,;18.96,-16.36,;18.54,-13.73,;16.99,-13.72,;16.23,-12.39,;14.69,-12.38,;13.91,-13.72,;14.69,-15.07,;16.23,-15.06,;17.01,-16.4,;19.42,-12.52,;18.94,-11.05,;22.99,-13.72,;24.49,-14.58,;24.49,-12.86,)| Show InChI InChI=1S/C18H21ClN2O/c1-17(2)11-9-10-18(17,3)15-14(11)16(22)21(20(15)4)13-8-6-5-7-12(13)19/h5-8,11H,9-10H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018799
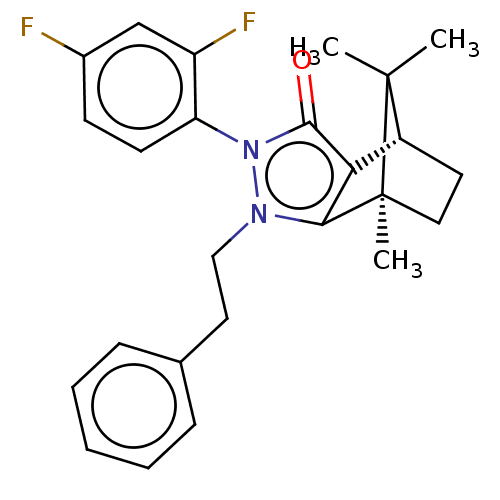
(CHEMBL3291347)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3CCc1ccccc1)C2(C)C |r,wU:4.4,1.0,(16.88,-14.91,;16.88,-13.41,;18.18,-12.67,;18.18,-11.17,;16.89,-10.42,;16.46,-8.98,;15.59,-11.17,;15.59,-12.67,;14.17,-13.14,;13.71,-14.55,;13.29,-11.92,;11.75,-11.92,;10.98,-13.26,;9.44,-13.26,;8.66,-11.91,;7.11,-11.91,;9.44,-10.58,;10.98,-10.58,;11.76,-9.24,;14.17,-10.71,;13.69,-9.24,;14.72,-8.09,;14.24,-6.63,;15.26,-5.48,;14.77,-4.02,;13.26,-3.71,;12.23,-4.88,;12.73,-6.33,;17.75,-11.92,;19.24,-12.77,;19.24,-11.05,)| Show InChI InChI=1S/C25H26F2N2O/c1-24(2)18-11-13-25(24,3)22-21(18)23(30)29(20-10-9-17(26)15-19(20)27)28(22)14-12-16-7-5-4-6-8-16/h4-10,15,18H,11-14H2,1-3H3/t18-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018803
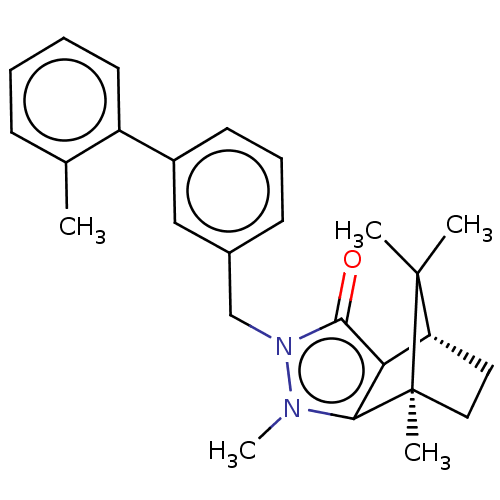
(CHEMBL3291358)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1cccc(c1)-c1ccccc1C)n3C)C2(C)C |r| Show InChI InChI=1S/C26H30N2O/c1-17-9-6-7-12-20(17)19-11-8-10-18(15-19)16-28-24(29)22-21-13-14-26(4,25(21,2)3)23(22)27(28)5/h6-12,15,21H,13-14,16H2,1-5H3/t21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50341766
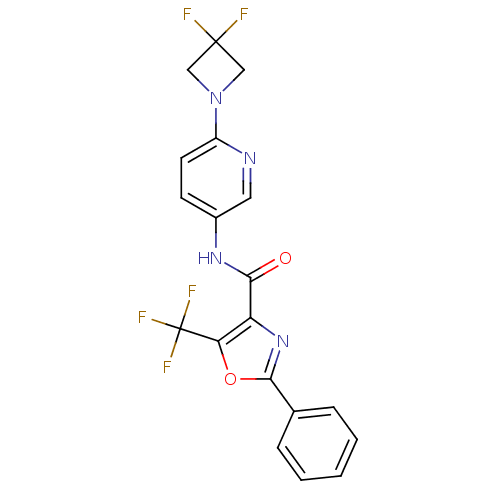
(2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...)Show SMILES FC(F)(F)c1oc(nc1C(=O)Nc1ccc(nc1)N1CC(F)(F)C1)-c1ccccc1 Show InChI InChI=1S/C19H13F5N4O2/c20-18(21)9-28(10-18)13-7-6-12(8-25-13)26-16(29)14-15(19(22,23)24)30-17(27-14)11-4-2-1-3-5-11/h1-8H,9-10H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay |
J Med Chem 54: 2433-46 (2011)
Article DOI: 10.1021/jm101580m
BindingDB Entry DOI: 10.7270/Q2S182TQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018794

(CHEMBL3291340)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3Cc1ccccc1)C2(C)C |r| Show InChI InChI=1S/C24H26N2O/c1-23(2)19-14-15-24(23,3)21-20(19)22(27)26(18-12-8-5-9-13-18)25(21)16-17-10-6-4-7-11-17/h4-13,19H,14-16H2,1-3H3/t19-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018799

(CHEMBL3291347)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3CCc1ccccc1)C2(C)C |r,wU:4.4,1.0,(16.88,-14.91,;16.88,-13.41,;18.18,-12.67,;18.18,-11.17,;16.89,-10.42,;16.46,-8.98,;15.59,-11.17,;15.59,-12.67,;14.17,-13.14,;13.71,-14.55,;13.29,-11.92,;11.75,-11.92,;10.98,-13.26,;9.44,-13.26,;8.66,-11.91,;7.11,-11.91,;9.44,-10.58,;10.98,-10.58,;11.76,-9.24,;14.17,-10.71,;13.69,-9.24,;14.72,-8.09,;14.24,-6.63,;15.26,-5.48,;14.77,-4.02,;13.26,-3.71,;12.23,-4.88,;12.73,-6.33,;17.75,-11.92,;19.24,-12.77,;19.24,-11.05,)| Show InChI InChI=1S/C25H26F2N2O/c1-24(2)18-11-13-25(24,3)22-21(18)23(30)29(20-10-9-17(26)15-19(20)27)28(22)14-12-16-7-5-4-6-8-16/h4-10,15,18H,11-14H2,1-3H3/t18-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018795

(CHEMBL3291339)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3CC)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-5-20-16-15(14-11-12-19(16,4)18(14,2)3)17(22)21(20)13-9-7-6-8-10-13/h6-10,14H,5,11-12H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018804
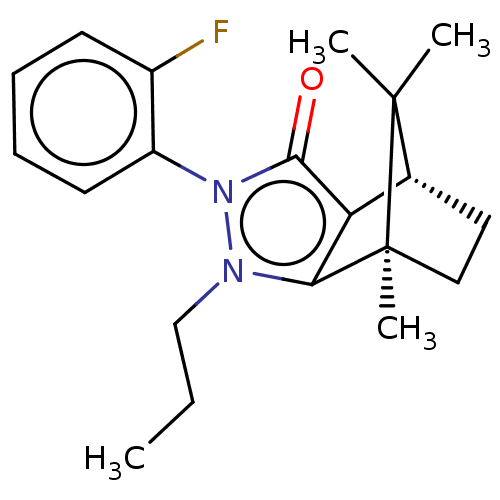
(CHEMBL3291341)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3CCC)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;17.04,-4.21,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H25FN2O/c1-5-12-22-17-16(13-10-11-20(17,4)19(13,2)3)18(24)23(22)15-9-7-6-8-14(15)21/h6-9,13H,5,10-12H2,1-4H3/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371868

(CHEMBL269939)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(Br)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C25H30BrN3O/c1-16-4-7-23-22(10-16)27-17(2)29(23)24-8-9-28(15-25(24)30-3)14-18-11-19-5-6-21(26)13-20(19)12-18/h4-7,10,13,18,24-25H,8-9,11-12,14-15H2,1-3H3/t18-,24+,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH peptide from human MCHR1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 23: 4216-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.017
BindingDB Entry DOI: 10.7270/Q2TX3J95 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018803

(CHEMBL3291358)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1cccc(c1)-c1ccccc1C)n3C)C2(C)C |r| Show InChI InChI=1S/C26H30N2O/c1-17-9-6-7-12-20(17)19-11-8-10-18(15-19)16-28-24(29)22-21-13-14-26(4,25(21,2)3)23(22)27(28)5/h6-12,15,21H,13-14,16H2,1-5H3/t21-,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50492355
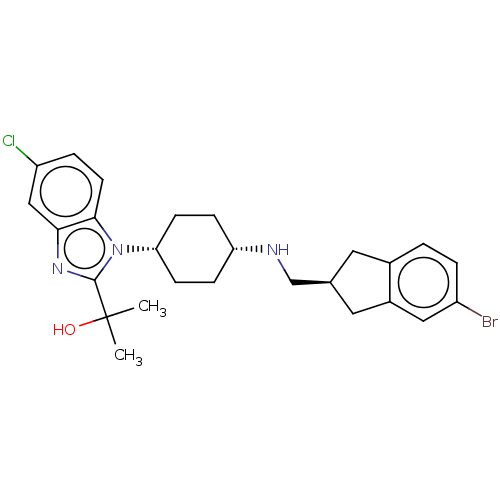
(CHEMBL2403859)Show SMILES CC(C)(O)c1nc2cc(Cl)ccc2n1[C@@H]1CC[C@@H](CC1)NC[C@H]1Cc2ccc(Br)cc2C1 |r,wU:14.15,17.22,22.24,(11.65,-5.98,;12.43,-7.32,;13.2,-5.98,;13.88,-7.85,;11.26,-8.32,;9.76,-7.96,;8.95,-9.27,;7.42,-9.54,;6.91,-11,;5.39,-11.27,;7.9,-12.17,;9.42,-11.9,;9.95,-10.45,;11.36,-9.87,;12.7,-10.65,;12.64,-12.21,;13.96,-13.02,;15.31,-12.31,;15.37,-10.74,;14.05,-9.92,;16.64,-13.09,;17.98,-12.34,;18,-10.8,;16.75,-9.88,;17.24,-8.42,;16.49,-7.09,;17.26,-5.77,;18.81,-5.78,;19.59,-4.46,;19.56,-7.12,;18.78,-8.44,;19.24,-9.91,)| Show InChI InChI=1S/C26H31BrClN3O/c1-26(2,32)25-30-23-14-20(28)5-10-24(23)31(25)22-8-6-21(7-9-22)29-15-16-11-17-3-4-19(27)13-18(17)12-16/h3-5,10,13-14,16,21-22,29,32H,6-9,11-12,15H2,1-2H3/t16-,21-,22+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH peptide from human MCHR1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 23: 4216-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.017
BindingDB Entry DOI: 10.7270/Q2TX3J95 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018798

(CHEMBL3291342)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3C(C)C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;15.7,-5.48,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H25FN2O/c1-12(2)22-17-16(13-10-11-20(17,5)19(13,3)4)18(24)23(22)15-9-7-6-8-14(15)21/h6-9,12-13H,10-11H2,1-5H3/t13-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018806

(CHEMBL3291343)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3CC=C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;17.04,-4.21,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H23FN2O/c1-5-12-22-17-16(13-10-11-20(17,4)19(13,2)3)18(24)23(22)15-9-7-6-8-14(15)21/h5-9,13H,1,10-12H2,2-4H3/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018807
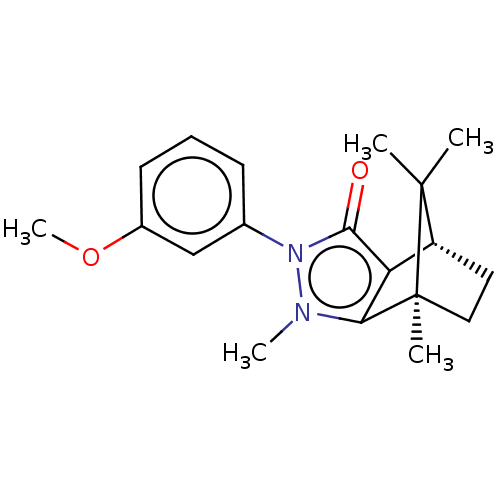
(CHEMBL3291355)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(OC)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C19H24N2O2/c1-18(2)14-9-10-19(18,3)16-15(14)17(22)21(20(16)4)12-7-6-8-13(11-12)23-5/h6-8,11,14H,9-10H2,1-5H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50341768

(4-{5-[(2-Phenyl-5-trifluoromethyloxazole-4-carbony...)Show SMILES COC(=O)N1CCN(CC1)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cn1 Show InChI InChI=1S/C22H20F3N5O4/c1-33-21(32)30-11-9-29(10-12-30)16-8-7-15(13-26-16)27-19(31)17-18(22(23,24)25)34-20(28-17)14-5-3-2-4-6-14/h2-8,13H,9-12H2,1H3,(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay |
J Med Chem 54: 2433-46 (2011)
Article DOI: 10.1021/jm101580m
BindingDB Entry DOI: 10.7270/Q2S182TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50492348

(CHEMBL2403866)Show SMILES Cc1nc2cc(F)ccc2n1[C@@H]1CC[C@@H](CC1)NC[C@H]1Cc2ccc(cc2C1)C#N |r,wU:11.12,14.19,19.21,(52.97,-7.87,;51.79,-8.86,;50.3,-8.5,;49.48,-9.81,;47.96,-10.09,;47.45,-11.54,;45.93,-11.81,;48.44,-12.72,;49.96,-12.44,;50.49,-10.99,;51.9,-10.41,;53.24,-11.19,;53.18,-12.75,;54.49,-13.57,;55.85,-12.85,;55.91,-11.29,;54.59,-10.47,;57.18,-13.64,;58.52,-12.88,;58.54,-11.34,;57.29,-10.42,;57.78,-8.96,;57.03,-7.63,;57.8,-6.31,;59.35,-6.33,;60.1,-7.66,;59.32,-8.98,;59.78,-10.45,;60.13,-5.01,;60.91,-3.68,)| Show InChI InChI=1S/C25H27FN4/c1-16-29-24-13-21(26)4-9-25(24)30(16)23-7-5-22(6-8-23)28-15-18-11-19-3-2-17(14-27)10-20(19)12-18/h2-4,9-10,13,18,22-23,28H,5-8,11-12,15H2,1H3/t18-,22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel by patch clamp technique |
Bioorg Med Chem Lett 23: 4216-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.017
BindingDB Entry DOI: 10.7270/Q2TX3J95 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018809
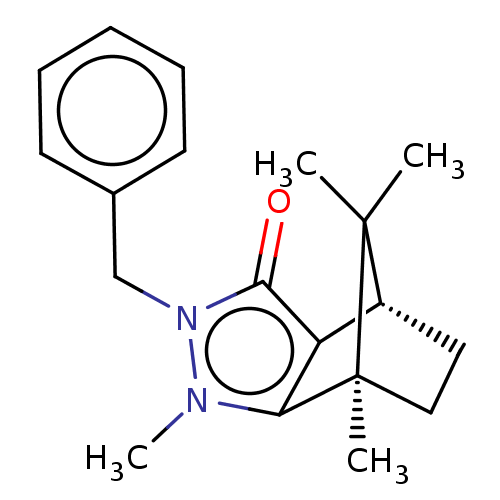
(CHEMBL3291356)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1)n3C)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-18(2)14-10-11-19(18,3)16-15(14)17(22)21(20(16)4)12-13-8-6-5-7-9-13/h5-9,14H,10-12H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018808
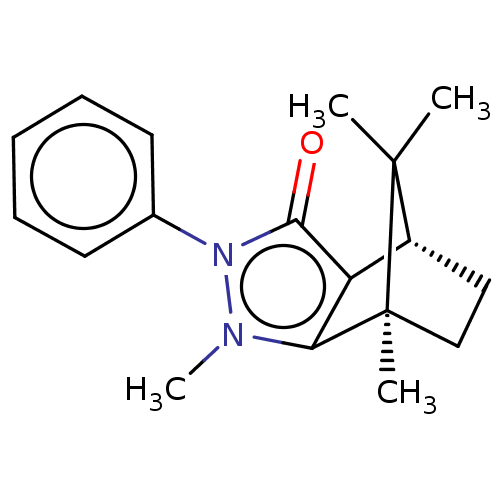
(CHEMBL3291337)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H22N2O/c1-17(2)13-10-11-18(17,3)15-14(13)16(21)20(19(15)4)12-8-6-5-7-9-12/h5-9,13H,10-11H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data