 Found 67 hits with Last Name = 'cook' and Initial = 'pd'
Found 67 hits with Last Name = 'cook' and Initial = 'pd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164644

(2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1CC[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 |r| Show InChI InChI=1S/C10H16N5O11P3/c11-9-8-10(13-4-12-9)15(5-14-8)7-2-1-6(24-7)3-23-28(19,20)26-29(21,22)25-27(16,17)18/h4-7H,1-3H2,(H,19,20)(H,21,22)(H2,11,12,13)(H2,16,17,18)/t6-,7+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164642
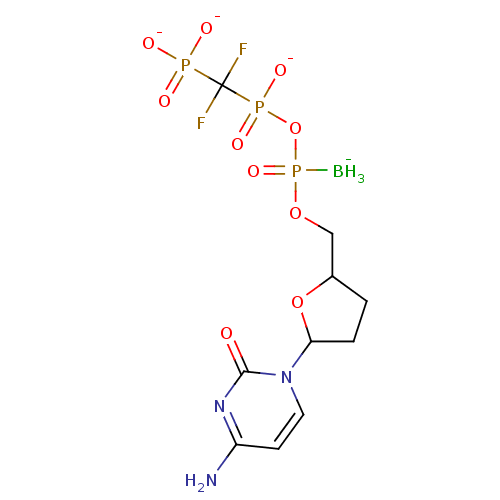
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1ccc(N)nc1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C10H18BF2N3O10P3/c11-29(23,26-28(21,22)10(12,13)27(18,19)20)24-5-6-1-2-8(25-6)16-4-3-7(14)15-9(16)17/h3-4,6,8H,1-2,5H2,11H3,(H,21,22)(H2,14,15,17)(H2,18,19,20)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164639
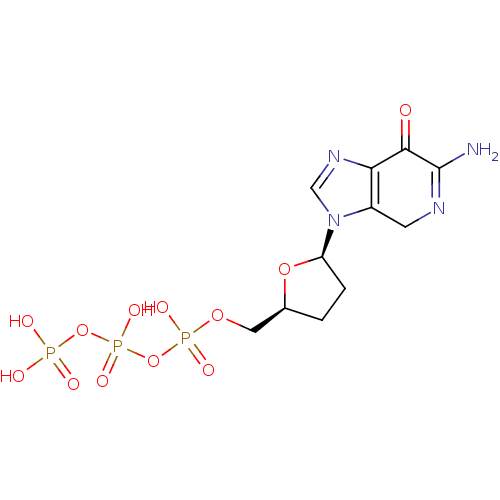
(2'-3'-dideoxy-7-deaza-guaninetriphosphate | CHEMBL...)Show SMILES NC1=NCc2c(ncn2[C@H]2CC[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)C1=O |t:1| Show InChI InChI=1S/C11H17N4O12P3/c12-11-10(16)9-7(3-13-11)15(5-14-9)8-2-1-6(25-8)4-24-29(20,21)27-30(22,23)26-28(17,18)19/h5-6,8H,1-4H2,(H2,12,13)(H,20,21)(H,22,23)(H2,17,18,19)/t6-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164652
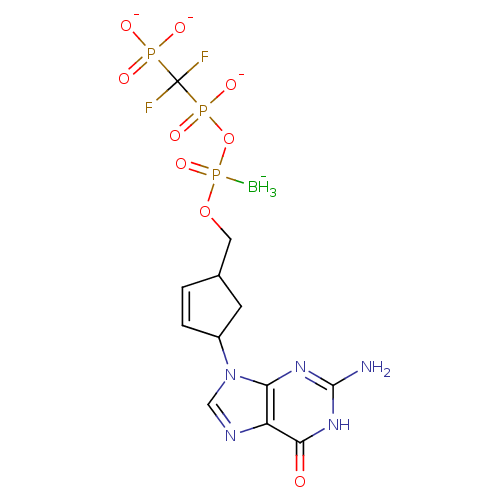
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CC(C=C1)n1cnc2c1nc(N)[nH]c2=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O |c:8| Show InChI InChI=1S/C12H18BF2N5O9P3/c13-32(27,29-31(25,26)12(14,15)30(22,23)24)28-4-6-1-2-7(3-6)20-5-17-8-9(20)18-11(16)19-10(8)21/h1-2,5-7H,3-4H2,13H3,(H,25,26)(H2,22,23,24)(H3,16,18,19,21)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164654
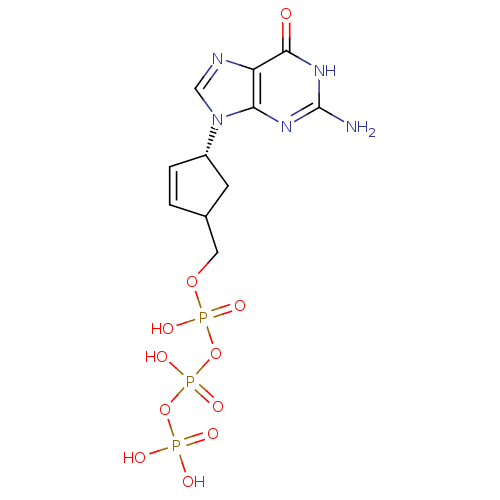
(CHEMBL370031 | [[[4-(2-amino-6-oxo-3,9-dihydropuri...)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C=C1 |c:30| Show InChI InChI=1S/C11H16N5O11P3/c12-11-14-9-8(10(17)15-11)13-5-16(9)7-2-1-6(3-7)4-25-29(21,22)27-30(23,24)26-28(18,19)20/h1-2,5-7H,3-4H2,(H,21,22)(H,23,24)(H2,18,19,20)(H3,12,14,15,17)/t6?,7-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164637
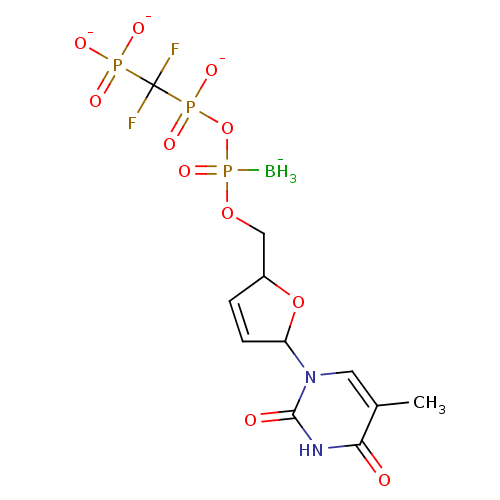
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1OC(C=C1)n1cc(C)c(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O |c:8| Show InChI InChI=1S/C11H17BF2N2O11P3/c1-6-4-16(10(18)15-9(6)17)8-3-2-7(26-8)5-25-30(12,24)27-29(22,23)11(13,14)28(19,20)21/h2-4,7-8H,5H2,1,12H3,(H,22,23)(H,15,17,18)(H2,19,20,21)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164638

(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1OC(CC1F)n1cc(C)c(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C11H18BF3N2O11P3/c1-5-3-17(10(19)16-9(5)18)8-2-6(13)7(27-8)4-26-31(12,25)28-30(23,24)11(14,15)29(20,21)22/h3,6-8H,2,4H2,1,12H3,(H,23,24)(H,16,18,19)(H2,20,21,22)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164647

(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1cnc2c(N)ncnc12)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C11H18BF2N5O9P3/c12-31(25,28-30(23,24)11(13,14)29(20,21)22)26-3-6-1-2-7(27-6)19-5-18-8-9(15)16-4-17-10(8)19/h4-7H,1-3H2,12H3,(H,23,24)(H2,15,16,17)(H2,20,21,22)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50145605

(4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...)Show InChI InChI=1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50370655
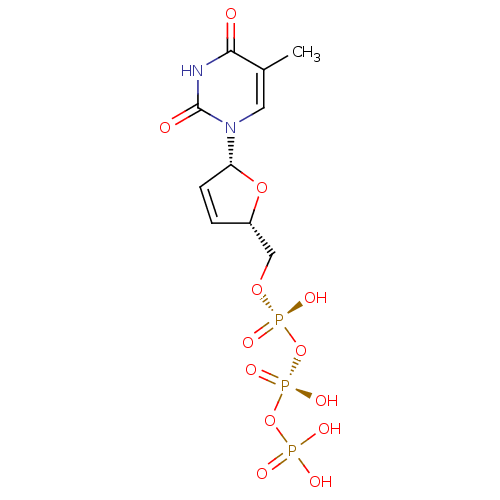
(CHEMBL485652)Show SMILES Cc1cn([C@@H]2O[C@H](CO[P@@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)C=C2)c(=O)[nH]c1=O |r,c:21| Show InChI InChI=1S/C10H15N2O13P3/c1-6-4-12(10(14)11-9(6)13)8-3-2-7(23-8)5-22-27(18,19)25-28(20,21)24-26(15,16)17/h2-4,7-8H,5H2,1H3,(H,18,19)(H,20,21)(H,11,13,14)(H2,15,16,17)/t7-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164648
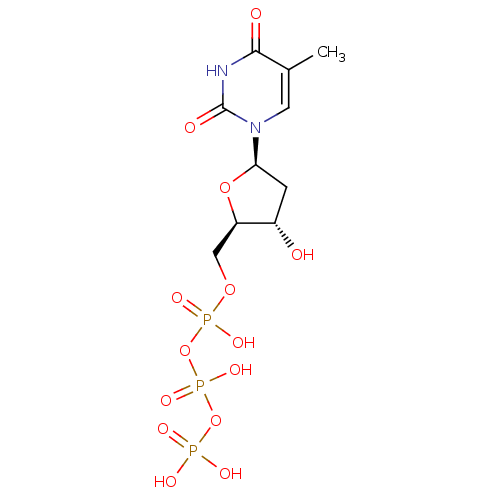
(2'-deoxythymidine triphosphate | 5'-TTP | CHEMBL36...)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H17N2O14P3/c1-5-3-12(10(15)11-9(5)14)8-2-6(13)7(24-8)4-23-28(19,20)26-29(21,22)25-27(16,17)18/h3,6-8,13H,2,4H2,1H3,(H,19,20)(H,21,22)(H,11,14,15)(H2,16,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164653
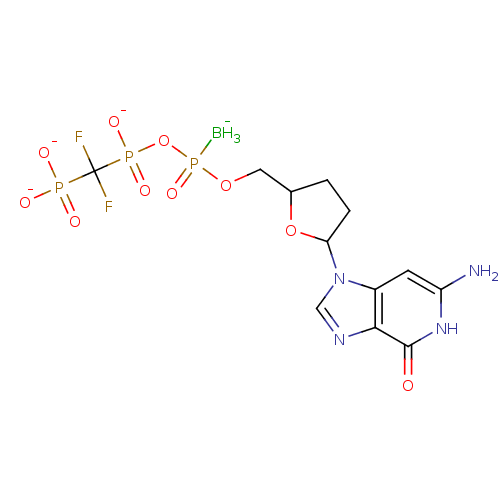
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1cnc2c1cc(N)[nH]c2=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C12H19BF2N4O10P3/c13-32(26,29-31(24,25)12(14,15)30(21,22)23)27-4-6-1-2-9(28-6)19-5-17-10-7(19)3-8(16)18-11(10)20/h3,5-6,9H,1-2,4H2,13H3,(H,24,25)(H3,16,18,20)(H2,21,22,23)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164650
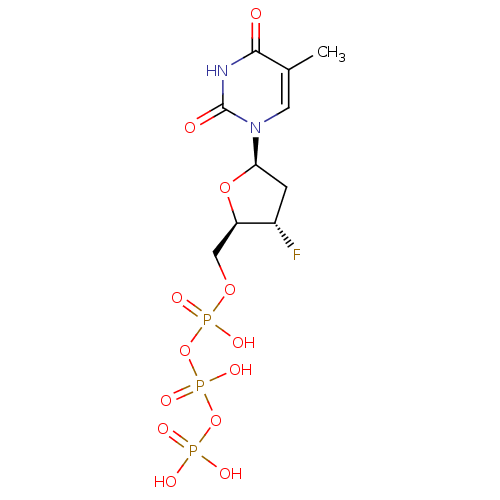
(CHEMBL191725 | [[[3-fluoro-5-(5-methyl-2,4-dioxo-1...)Show SMILES Cc1cn([C@H]2C[C@H](F)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C10H16FN2O13P3/c1-5-3-13(10(15)12-9(5)14)8-2-6(11)7(24-8)4-23-28(19,20)26-29(21,22)25-27(16,17)18/h3,6-8H,2,4H2,1H3,(H,19,20)(H,21,22)(H,12,14,15)(H2,16,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50370476

(Combivir | ZIDOVUDINE TRIPHOSPHATE)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO[P@@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16N5O13P3/c1-5-3-15(10(17)12-9(5)16)8-2-6(13-14-11)7(26-8)4-25-30(21,22)28-31(23,24)27-29(18,19)20/h3,6-8H,2,4H2,1H3,(H,21,22)(H,23,24)(H,12,16,17)(H2,18,19,20)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164640

(CHEMBL190609 | [[[[3-azido-5-(5-methyl-2,4-dioxo-1...)Show SMILES [BH3-]P(=O)(OCC1OC(CC1N=[N+]=[N-])n1cc(C)c(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C11H18BF2N5O11P3/c1-5-3-19(10(21)16-9(5)20)8-2-6(17-18-15)7(29-8)4-28-33(12,27)30-32(25,26)11(13,14)31(22,23)24/h3,6-8H,2,4H2,1,12H3,(H,25,26)(H,16,20,21)(H2,22,23,24)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50138406
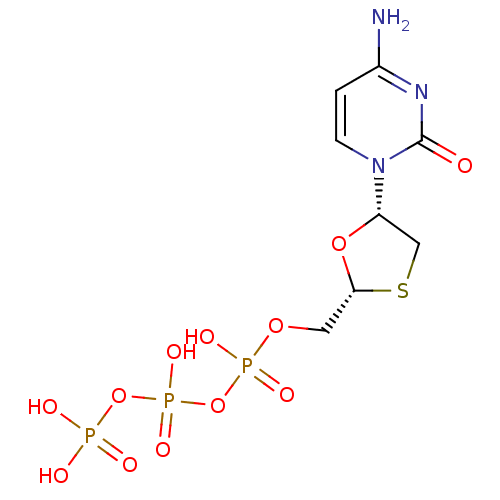
(3TC Triphosphate | CHEMBL1230 | LAMIVUDINE | Lamiv...)Show SMILES Nc1ccn([C@@H]2CS[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)n1 |r| Show InChI InChI=1S/C8H14N3O12P3S/c9-5-1-2-11(8(12)10-5)6-4-27-7(21-6)3-20-25(16,17)23-26(18,19)22-24(13,14)15/h1-2,6-7H,3-4H2,(H,16,17)(H,18,19)(H2,9,10,12)(H2,13,14,15)/t6-,7+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164655
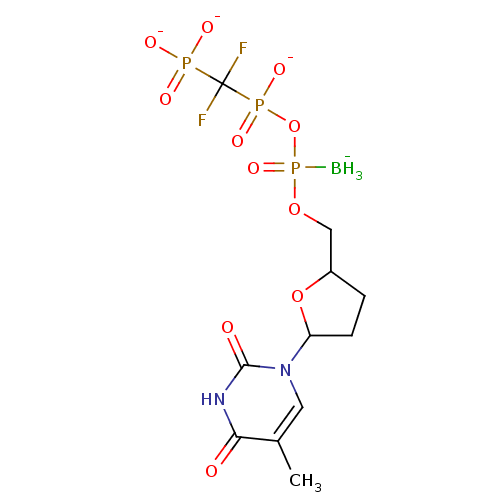
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1cc(C)c(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C11H19BF2N2O11P3/c1-6-4-16(10(18)15-9(6)17)8-3-2-7(26-8)5-25-30(12,24)27-29(22,23)11(13,14)28(19,20)21/h4,7-8H,2-3,5H2,1,12H3,(H,22,23)(H,15,17,18)(H2,19,20,21)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164651
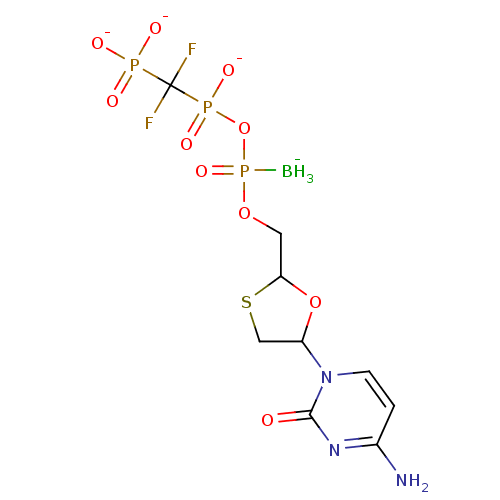
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1OC(CS1)n1ccc(N)nc1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C9H16BF2N3O10P3S/c10-28(22,25-27(20,21)9(11,12)26(17,18)19)23-3-7-24-6(4-29-7)15-2-1-5(13)14-8(15)16/h1-2,6-7H,3-4H2,10H3,(H,20,21)(H2,13,14,16)(H2,17,18,19)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164656

(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1ccc(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C10H17BF2N2O11P3/c11-29(23,26-28(21,22)10(12,13)27(18,19)20)24-5-6-1-2-8(25-6)15-4-3-7(16)14-9(15)17/h3-4,6,8H,1-2,5H2,11H3,(H,21,22)(H,14,16,17)(H2,18,19,20)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164645
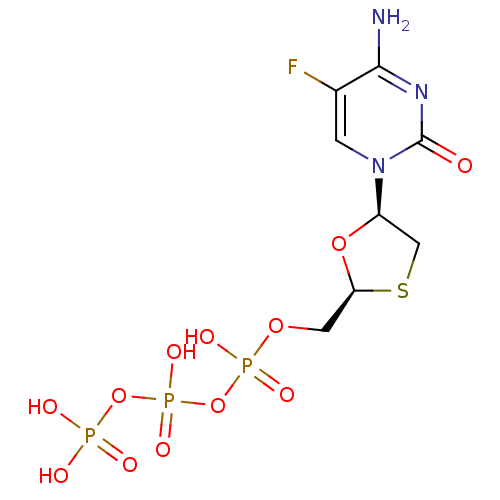
(CHEMBL192771 | [[[5-(4-amino-5-fluoro-2-oxo-1H-pyr...)Show SMILES Nc1nc(=O)n(cc1F)[C@H]1CS[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 Show InChI InChI=1S/C8H13FN3O12P3S/c9-4-1-12(8(13)11-7(4)10)5-3-28-6(22-5)2-21-26(17,18)24-27(19,20)23-25(14,15)16/h1,5-6H,2-3H2,(H,17,18)(H,19,20)(H2,10,11,13)(H2,14,15,16)/t5-,6+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164646

(CHEMBL192371 | [[[5-(2,4-dioxo-1H-pyrimidin-1-yl)t...)Show SMILES OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@@H]1CC[C@@H](O1)n1ccc(=O)[nH]c1=O Show InChI InChI=1S/C9H15N2O13P3/c12-7-3-4-11(9(13)10-7)8-2-1-6(22-8)5-21-26(17,18)24-27(19,20)23-25(14,15)16/h3-4,6,8H,1-2,5H2,(H,17,18)(H,19,20)(H,10,12,13)(H2,14,15,16)/t6-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164643

(({[({[5-(4-amino-5-fluoro-2-oxo-1,2-dihydropyrimid...)Show SMILES [BH3-]P(=O)(OCC1OC(CS1)n1cc(F)c(N)nc1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C9H15BF3N3O10P3S/c10-29(23,26-28(21,22)9(12,13)27(18,19)20)24-2-6-25-5(3-30-6)16-1-4(11)7(14)15-8(16)17/h1,5-6H,2-3H2,10H3,(H,21,22)(H2,14,15,17)(H2,18,19,20)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144942
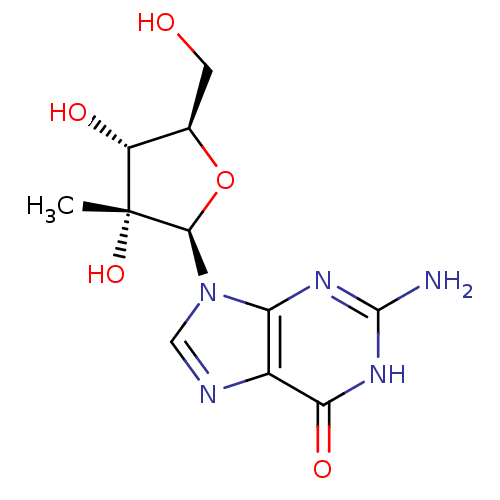
(2'-C-Me-guanosine | 2'-C-methyl-guanosine | 2'-C-m...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c1nc(N)[nH]c2=O Show InChI InChI=1S/C11H15N5O5/c1-11(20)6(18)4(2-17)21-9(11)16-3-13-5-7(16)14-10(12)15-8(5)19/h3-4,6,9,17-18,20H,2H2,1H3,(H3,12,14,15,19)/t4-,6-,9-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50022243

(2,8-Diamino-1,9-dihydro-purin-6-one | 8-AMINOGUANI...)Show InChI InChI=1S/C5H6N6O/c6-4-8-1-2(9-4)10-5(7)11-3(1)12/h(H6,6,7,8,9,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 29: 2034-7 (1986)
BindingDB Entry DOI: 10.7270/Q29P326Z |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144937
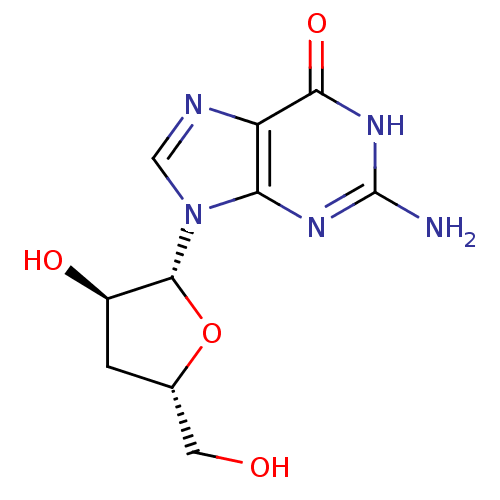
(2-Amino-9-((2R,3R,5S)-3-hydroxy-5-hydroxymethyl-te...)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](CO)C[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-10-13-7-6(8(18)14-10)12-3-15(7)9-5(17)1-4(2-16)19-9/h3-5,9,16-17H,1-2H2,(H3,11,13,14,18)/t4-,5+,9+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028
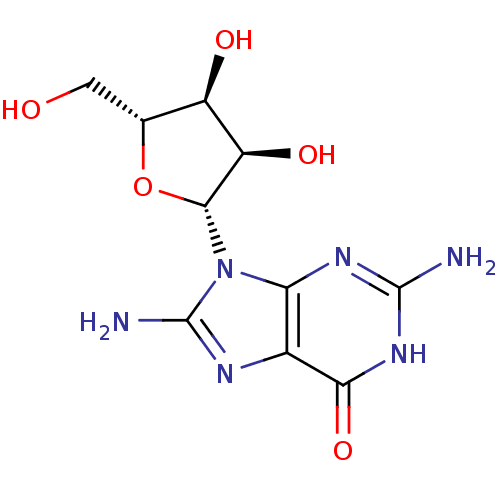
(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 29: 2034-7 (1986)
BindingDB Entry DOI: 10.7270/Q29P326Z |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144947

(2-Amino-9-((2R,3R,4R,5R)-4-hydroxy-5-hydroxymethyl...)Show SMILES CO[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c1nc(N)[nH]c2=O Show InChI InChI=1S/C11H15N5O5/c1-20-7-6(18)4(2-17)21-10(7)16-3-13-5-8(16)14-11(12)15-9(5)19/h3-4,6-7,10,17-18H,2H2,1H3,(H3,12,14,15,19)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144938
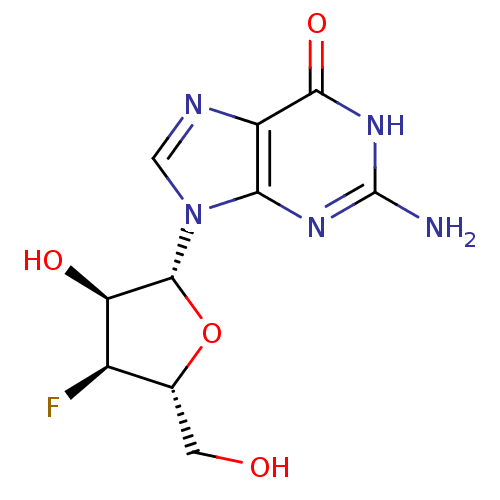
(2-Amino-9-((2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-hydr...)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](CO)[C@@H](F)[C@H]1O Show InChI InChI=1S/C10H12FN5O4/c11-4-3(1-17)20-9(6(4)18)16-2-13-5-7(16)14-10(12)15-8(5)19/h2-4,6,9,17-18H,1H2,(H3,12,14,15,19)/t3-,4-,6-,9-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144948

((2R,3R,4R,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H15N5O4/c1-11(19)7(18)5(2-17)20-10(11)16-4-15-6-8(12)13-3-14-9(6)16/h3-5,7,10,17-19H,2H2,1H3,(H2,12,13,14)/t5-,7-,10-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144949
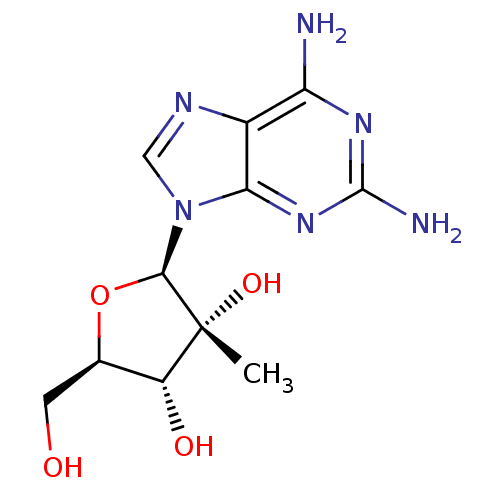
((2R,3R,4R,5R)-2-(2,6-diamino-9H-purin-9-yl)-5-(hyd...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c(N)nc(N)nc12 Show InChI InChI=1S/C11H16N6O4/c1-11(20)6(19)4(2-18)21-9(11)17-3-14-5-7(12)15-10(13)16-8(5)17/h3-4,6,9,18-20H,2H2,1H3,(H4,12,13,15,16)/t4-,6-,9-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144934

((2R,3R,4R,5R)-2-(6-hydroxy-9H-purin-9-yl)-5-(hydro...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C11H14N4O5/c1-11(19)7(17)5(2-16)20-10(11)15-4-14-6-8(15)12-3-13-9(6)18/h3-5,7,10,16-17,19H,2H2,1H3,(H,12,13,18)/t5-,7-,10-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50200094

(2-amino-1,9-dihydro-6H-purin-6-one | CHEMBL219568 ...)Show InChI InChI=1S/C5H5N5O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H4,6,7,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 29: 2034-7 (1986)
BindingDB Entry DOI: 10.7270/Q29P326Z |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50022253

(2,6-Diamino-1,5-dihydro-imidazo[4,5-c]pyridin-4-on...)Show InChI InChI=1S/C6H7N5O/c7-3-1-2-4(5(12)10-3)11-6(8)9-2/h1H,(H3,7,10,12)(H3,8,9,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 29: 2034-7 (1986)
BindingDB Entry DOI: 10.7270/Q29P326Z |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50409930
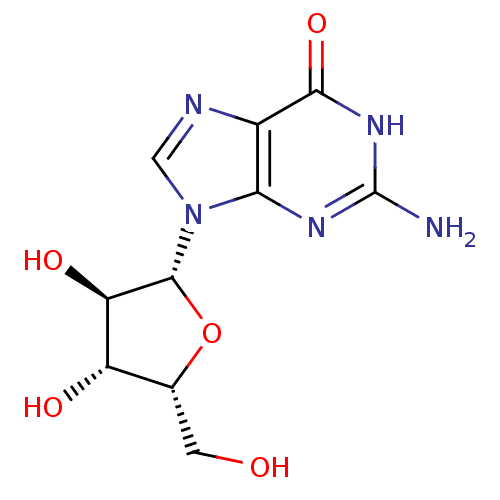
(CHEMBL2021379)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](CO)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H13N5O5/c11-10-13-7-4(8(19)14-10)12-2-15(7)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19)/t3-,5+,6-,9-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144950

(3'-deoxyadenosine | 9-(beta-D-3'-Deoxyribofuranosy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)C[C@H]1O |r| Show InChI InChI=1S/C10H13N5O3/c11-8-7-9(13-3-12-8)15(4-14-7)10-6(17)1-5(2-16)18-10/h3-6,10,16-17H,1-2H2,(H2,11,12,13)/t5-,6+,10+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
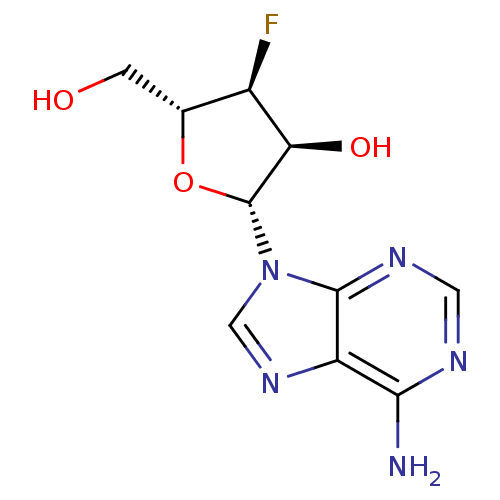
(Hepatitis C virus) | BDBM50144933
((2R,3S,4S,5R)-4-Fluoro-5-hydroxymethyl-2-(6-methyl...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](F)[C@H]1O Show InChI InChI=1S/C10H12FN5O3/c11-5-4(1-17)19-10(7(5)18)16-3-15-6-8(12)13-2-14-9(6)16/h2-5,7,10,17-18H,1H2,(H2,12,13,14)/t4-,5-,7-,10-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144939

((2R,3R,4R,5R)-2-(2-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2cnc(N)nc12 Show InChI InChI=1S/C11H15N5O4/c1-11(19)7(18)6(3-17)20-9(11)16-4-14-5-2-13-10(12)15-8(5)16/h2,4,6-7,9,17-19H,3H2,1H3,(H2,12,13,15)/t6-,7-,9-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144945
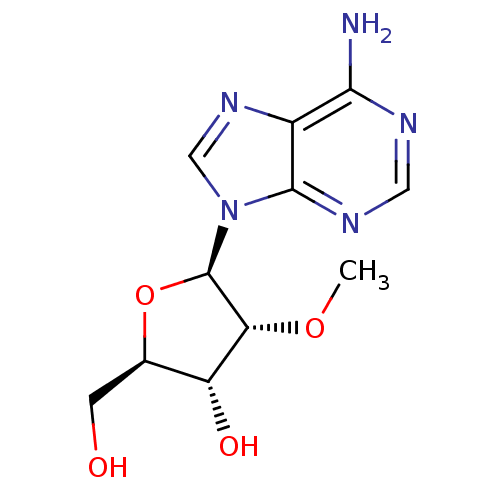
((2R,3R,4R,5R)-2-Hydroxymethyl-4-methoxy-5-(6-methy...)Show SMILES CO[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c(N)ncnc12 Show InChI InChI=1S/C11H15N5O4/c1-19-8-7(18)5(2-17)20-11(8)16-4-15-6-9(12)13-3-14-10(6)16/h3-5,7-8,11,17-18H,2H2,1H3,(H2,12,13,14)/t5-,7-,8-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
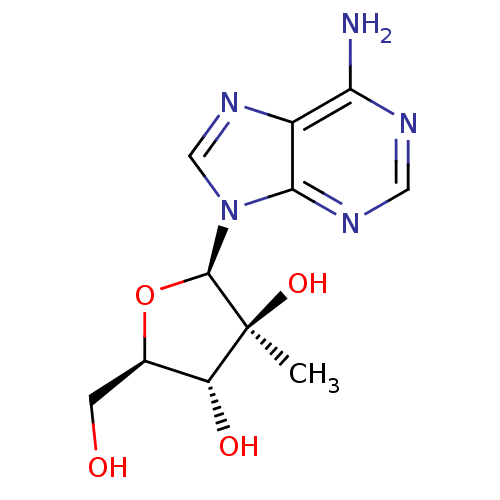
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144940
((2R,3S,4R,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES C[C@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c(N)ncnc12 Show InChI InChI=1S/C11H15N5O4/c1-11(19)7(18)5(2-17)20-10(11)16-4-15-6-8(12)13-3-14-9(6)16/h3-5,7,10,17-19H,2H2,1H3,(H2,12,13,14)/t5-,7-,10-,11+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144936
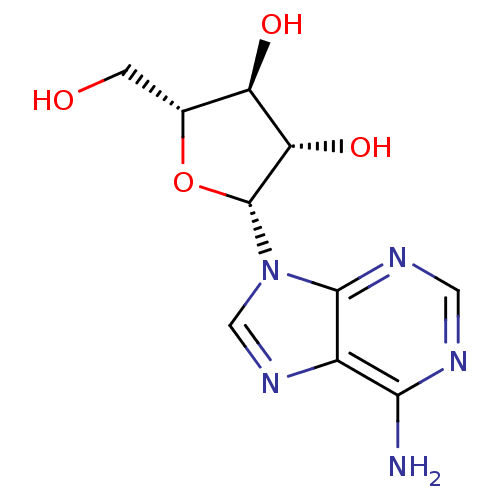
(CHEMBL1090 | VIDARABINE | adenine arabinoside)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7+,10-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144941

((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-(hydroxy...)Show SMILES CO[C@]1(C)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H17N5O4/c1-12(20-2)8(19)6(3-18)21-11(12)17-5-16-7-9(13)14-4-15-10(7)17/h4-6,8,11,18-19H,3H2,1-2H3,(H2,13,14,15)/t6-,8-,11-,12-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144935

((2R,3R,4R,5R)-2-(6-amino-9H-purin-9-yl)-3-ethyl-5-...)Show SMILES CC[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H17N5O4/c1-2-12(20)8(19)6(3-18)21-11(12)17-5-16-7-9(13)14-4-15-10(7)17/h4-6,8,11,18-20H,2-3H2,1H3,(H2,13,14,15)/t6-,8-,11-,12-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144932

((2R,3R,4R,5R)-4-Fluoro-2-hydroxymethyl-5-(6-methyl...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1F Show InChI InChI=1S/C10H12FN5O3/c11-5-7(18)4(1-17)19-10(5)16-3-15-6-8(12)13-2-14-9(6)16/h2-5,7,10,17-18H,1H2,(H2,12,13,14)/t4-,5-,7-,10-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50422565

(CHEMBL2311161)Show SMILES C[C@]1(O)[C@@H](CO)O[C@H]([C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C11H15N5O4/c1-11(19)5(2-17)20-10(7(11)18)16-4-15-6-8(12)13-3-14-9(6)16/h3-5,7,10,17-19H,2H2,1H3,(H2,12,13,14)/t5-,7+,10-,11+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50408410
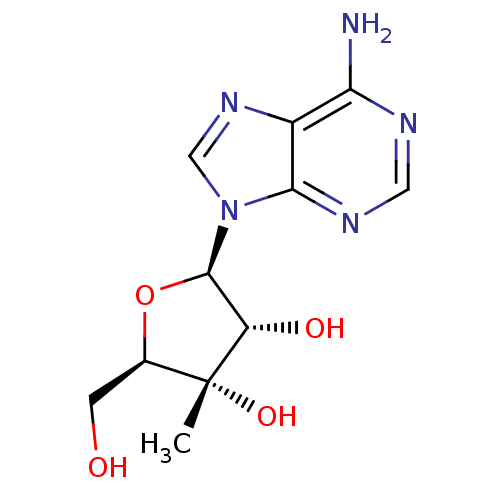
(CHEMBL480143)Show SMILES C[C@@]1(O)[C@@H](CO)O[C@H]([C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H15N5O4/c1-11(19)5(2-17)20-10(7(11)18)16-4-15-6-8(12)13-3-14-9(6)16/h3-5,7,10,17-19H,2H2,1H3,(H2,12,13,14)/t5-,7+,10-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50022256

(6-Amino-1,5-dihydro-imidazo[4,5-c]pyridin-4-one | ...)Show InChI InChI=1S/C6H6N4O/c7-4-1-3-5(6(11)10-4)9-2-8-3/h1-2H,(H,8,9)(H3,7,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 29: 2034-7 (1986)
BindingDB Entry DOI: 10.7270/Q29P326Z |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50225889

(CHEMBL3144008)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)C1=NNC2C1=NC=NC2=O |r,c:17,t:10,15| Show InChI InChI=1S/C10H12N4O5/c15-1-3-7(16)8(17)9(19-3)5-4-6(14-13-5)10(18)12-2-11-4/h2-3,6-9,14-17H,1H2/t3-,6?,7-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 29: 2034-7 (1986)
BindingDB Entry DOI: 10.7270/Q29P326Z |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50367286

(CHEMBL3245950 | CHEMBL605683)Show SMILES Nc1cc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H14N4O5/c12-6-1-4-7(10(19)14-6)13-3-15(4)11-9(18)8(17)5(2-16)20-11/h1,3,5,8-9,11,16-18H,2H2,(H3,12,14,19)/t5-,8-,9-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 29: 2034-7 (1986)
BindingDB Entry DOI: 10.7270/Q29P326Z |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144932

((2R,3R,4R,5R)-4-Fluoro-2-hydroxymethyl-5-(6-methyl...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1F Show InChI InChI=1S/C10H12FN5O3/c11-5-7(18)4(1-17)19-10(5)16-3-15-6-8(12)13-2-14-9(6)16/h2-5,7,10,17-18H,1H2,(H2,12,13,14)/t4-,5-,7-,10-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV RNA replication |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144930
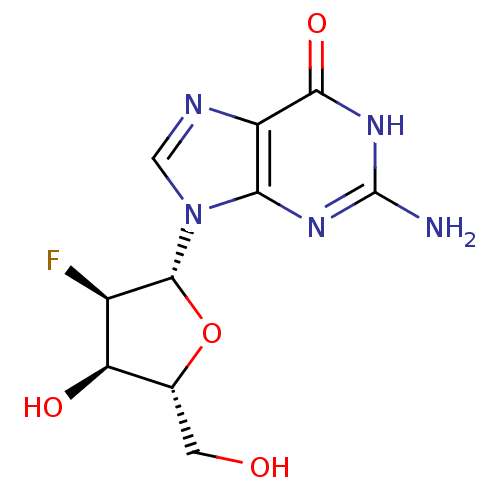
(2-Amino-9-((2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-hydr...)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1F Show InChI InChI=1S/C10H12FN5O4/c11-4-6(18)3(1-17)20-9(4)16-2-13-5-7(16)14-10(12)15-8(5)19/h2-4,6,9,17-18H,1H2,(H3,12,14,15,19)/t3-,4-,6-,9-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV RNA replication |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data