 Found 271 hits with Last Name = 'corless' and Initial = 'm'
Found 271 hits with Last Name = 'corless' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257637
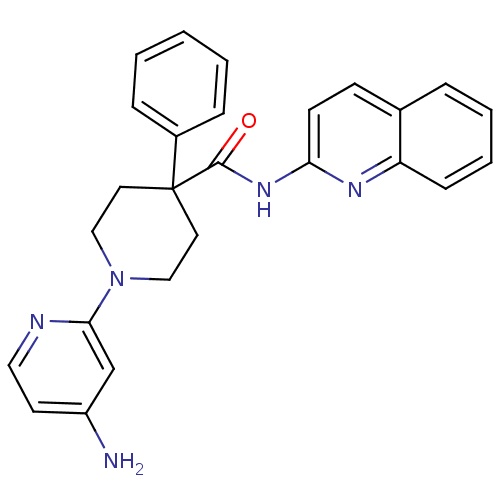
(1-(4-aminopyridin-2-yl)-4-phenyl-N-(quinolin-2-yl)...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)(C(=O)Nc1ccc2ccccc2n1)c1ccccc1 Show InChI InChI=1S/C26H25N5O/c27-21-12-15-28-24(18-21)31-16-13-26(14-17-31,20-7-2-1-3-8-20)25(32)30-23-11-10-19-6-4-5-9-22(19)29-23/h1-12,15,18H,13-14,16-17H2,(H2,27,28)(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257723

(2-(3-phenyl-3H-spiro[isobenzofuran-1,4'-piperidine...)Show SMILES Nc1ccnc(c1)N1CCC2(CC1)OC(c1ccccc21)c1ccccc1 Show InChI InChI=1S/C23H23N3O/c24-18-10-13-25-21(16-18)26-14-11-23(12-15-26)20-9-5-4-8-19(20)22(27-23)17-6-2-1-3-7-17/h1-10,13,16,22H,11-12,14-15H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257639
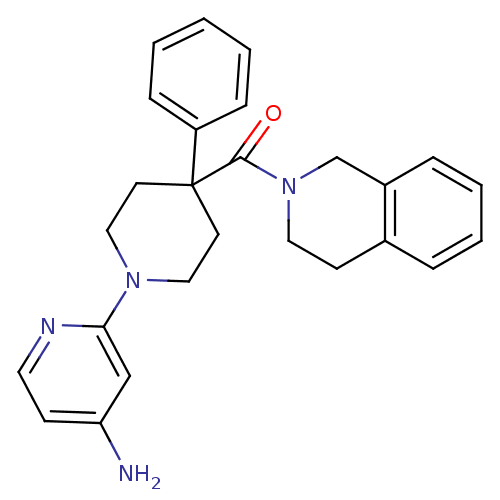
((1-(4-aminopyridin-2-yl)-4-phenylpiperidin-4-yl)(3...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)(C(=O)N1CCc2ccccc2C1)c1ccccc1 Show InChI InChI=1S/C26H28N4O/c27-23-10-14-28-24(18-23)29-16-12-26(13-17-29,22-8-2-1-3-9-22)25(31)30-15-11-20-6-4-5-7-21(20)19-30/h1-10,14,18H,11-13,15-17,19H2,(H2,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257684
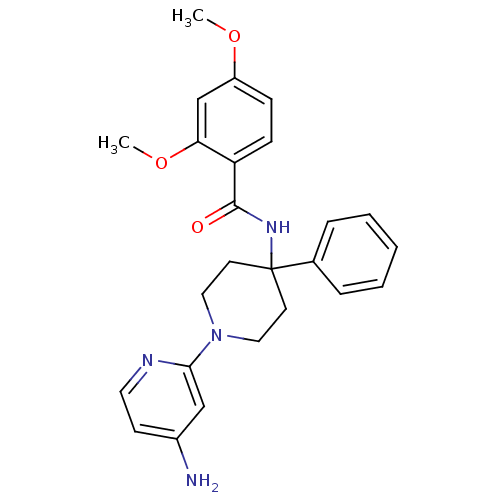
(CHEMBL495346 | N-(1-(4-aminopyridin-2-yl)-4-phenyl...)Show SMILES COc1ccc(C(=O)NC2(CCN(CC2)c2cc(N)ccn2)c2ccccc2)c(OC)c1 Show InChI InChI=1S/C25H28N4O3/c1-31-20-8-9-21(22(17-20)32-2)24(30)28-25(18-6-4-3-5-7-18)11-14-29(15-12-25)23-16-19(26)10-13-27-23/h3-10,13,16-17H,11-12,14-15H2,1-2H3,(H2,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257638
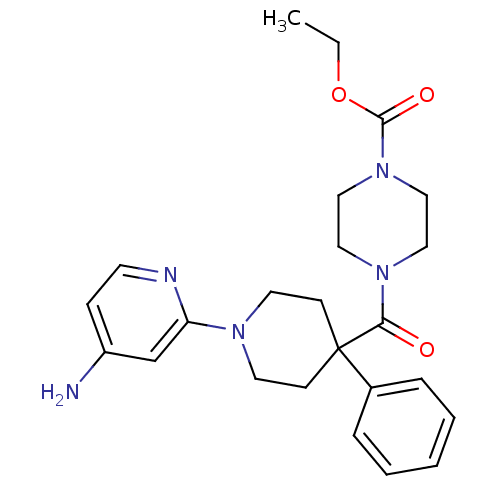
(CHEMBL493743 | ethyl 4-(1-(4-aminopyridin-2-yl)-4-...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)C1(CCN(CC1)c1cc(N)ccn1)c1ccccc1 Show InChI InChI=1S/C24H31N5O3/c1-2-32-23(31)29-16-14-28(15-17-29)22(30)24(19-6-4-3-5-7-19)9-12-27(13-10-24)21-18-20(25)8-11-26-21/h3-8,11,18H,2,9-10,12-17H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257683
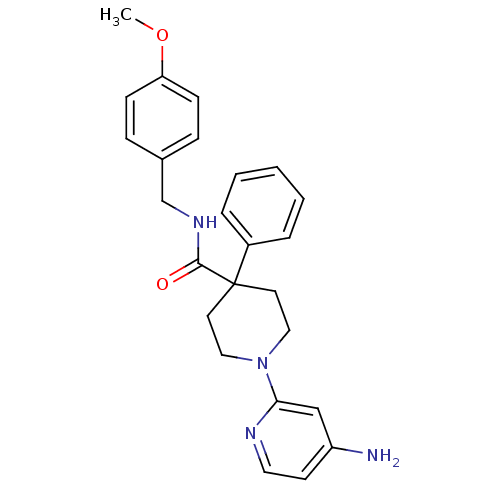
(1-(4-aminopyridin-2-yl)-N-(4-methoxybenzyl)-4-phen...)Show SMILES COc1ccc(CNC(=O)C2(CCN(CC2)c2cc(N)ccn2)c2ccccc2)cc1 Show InChI InChI=1S/C25H28N4O2/c1-31-22-9-7-19(8-10-22)18-28-24(30)25(20-5-3-2-4-6-20)12-15-29(16-13-25)23-17-21(26)11-14-27-23/h2-11,14,17H,12-13,15-16,18H2,1H3,(H2,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257685
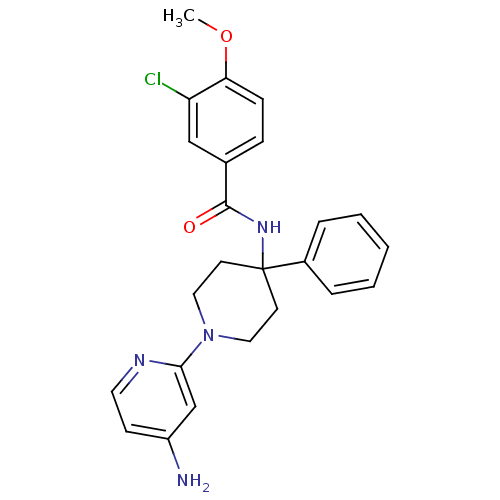
(CHEMBL492330 | N-(1-(4-aminopyridin-2-yl)-4-phenyl...)Show SMILES COc1ccc(cc1Cl)C(=O)NC1(CCN(CC1)c1cc(N)ccn1)c1ccccc1 Show InChI InChI=1S/C24H25ClN4O2/c1-31-21-8-7-17(15-20(21)25)23(30)28-24(18-5-3-2-4-6-18)10-13-29(14-11-24)22-16-19(26)9-12-27-22/h2-9,12,15-16H,10-11,13-14H2,1H3,(H2,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257833

(3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-benzyl...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)n1c2ncccc2n(Cc2ccccc2)c1=O Show InChI InChI=1S/C23H24N6O/c24-18-8-12-25-21(15-18)27-13-9-19(10-14-27)29-22-20(7-4-11-26-22)28(23(29)30)16-17-5-2-1-3-6-17/h1-8,11-12,15,19H,9-10,13-14,16H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257686
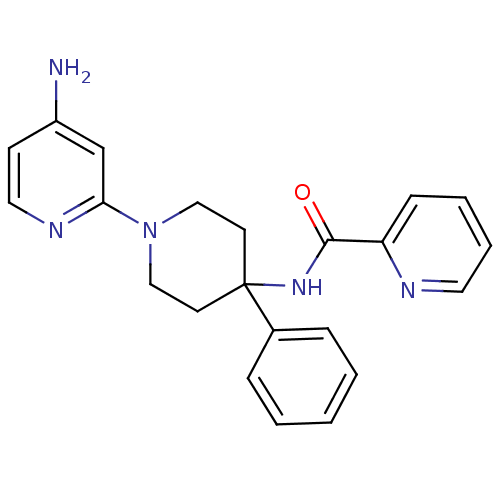
(CHEMBL522317 | N-(1-(4-aminopyridin-2-yl)-4-phenyl...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)(NC(=O)c1ccccn1)c1ccccc1 Show InChI InChI=1S/C22H23N5O/c23-18-9-13-25-20(16-18)27-14-10-22(11-15-27,17-6-2-1-3-7-17)26-21(28)19-8-4-5-12-24-19/h1-9,12-13,16H,10-11,14-15H2,(H2,23,25)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257834
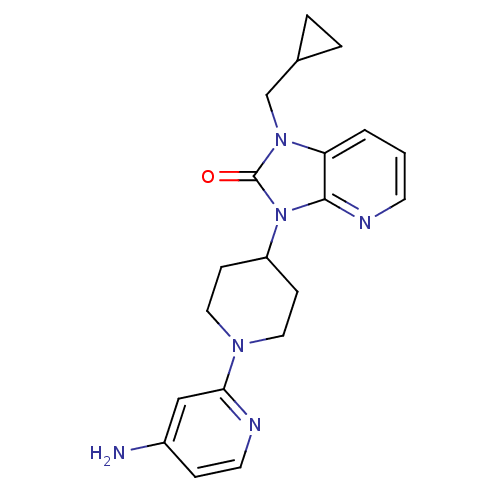
(3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-(cyclo...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)n1c2ncccc2n(CC2CC2)c1=O Show InChI InChI=1S/C20H24N6O/c21-15-5-9-22-18(12-15)24-10-6-16(7-11-24)26-19-17(2-1-8-23-19)25(20(26)27)13-14-3-4-14/h1-2,5,8-9,12,14,16H,3-4,6-7,10-11,13H2,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 746 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257831
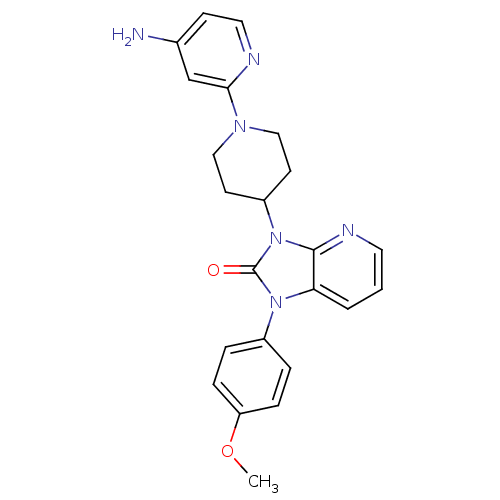
(3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-(4-met...)Show SMILES COc1ccc(cc1)-n1c2cccnc2n(C2CCN(CC2)c2cc(N)ccn2)c1=O Show InChI InChI=1S/C23H24N6O2/c1-31-19-6-4-17(5-7-19)28-20-3-2-11-26-22(20)29(23(28)30)18-9-13-27(14-10-18)21-15-16(24)8-12-25-21/h2-8,11-12,15,18H,9-10,13-14H2,1H3,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257832
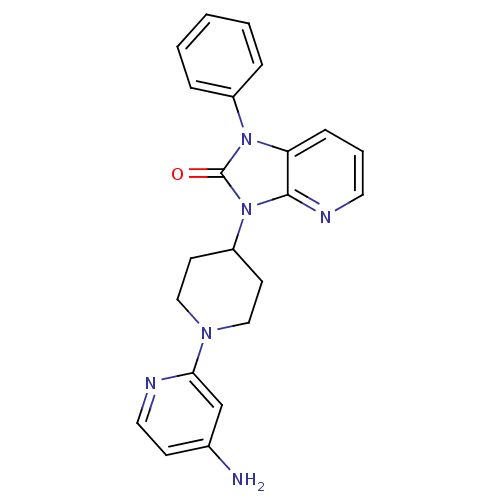
(3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-phenyl...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)n1c2ncccc2n(-c2ccccc2)c1=O Show InChI InChI=1S/C22H22N6O/c23-16-8-12-24-20(15-16)26-13-9-18(10-14-26)28-21-19(7-4-11-25-21)27(22(28)29)17-5-2-1-3-6-17/h1-8,11-12,15,18H,9-10,13-14H2,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 903 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257781
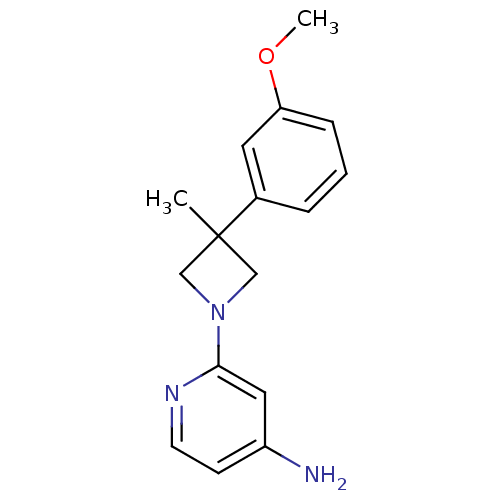
(2-(3-(3-methoxyphenyl)-3-methylazetidin-1-yl)pyrid...)Show InChI InChI=1S/C16H19N3O/c1-16(12-4-3-5-14(8-12)20-2)10-19(11-16)15-9-13(17)6-7-18-15/h3-9H,10-11H2,1-2H3,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257780
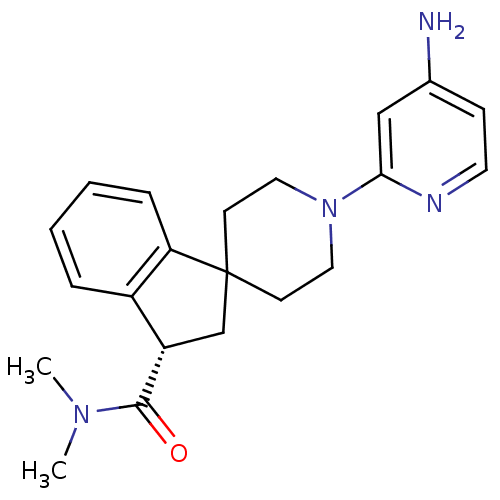
((S)-1'-(4-aminopyridin-2-yl)-N,N-dimethyl-2,3-dihy...)Show SMILES CN(C)C(=O)[C@H]1CC2(CCN(CC2)c2cc(N)ccn2)c2ccccc12 |r| Show InChI InChI=1S/C21H26N4O/c1-24(2)20(26)17-14-21(18-6-4-3-5-16(17)18)8-11-25(12-9-21)19-13-15(22)7-10-23-19/h3-7,10,13,17H,8-9,11-12,14H2,1-2H3,(H2,22,23)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257782

(CHEMBL493515 | N-(1-(4-aminopyridin-2-yl)piperidin...)Show SMILES COc1ccc(cc1)C(=O)N(C1CCN(CC1)c1cc(N)ccn1)c1cc(C)ccn1 Show InChI InChI=1S/C24H27N5O2/c1-17-7-11-27-23(15-17)29(24(30)18-3-5-21(31-2)6-4-18)20-9-13-28(14-10-20)22-16-19(25)8-12-26-22/h3-8,11-12,15-16,20H,9-10,13-14H2,1-2H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50170969

((S)-1-(1-Cyclopropyl-cyclopentyl)-5-(3,4-dichloro-...)Show SMILES Clc1ccc(cc1Cl)[C@@]1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(C1)C1(CCCC1)C1CC1 Show InChI InChI=1S/C28H39Cl2N3O2/c29-24-6-5-22(17-25(24)30)27(11-12-31-18-23(19-31)32-13-15-35-16-14-32)10-7-26(34)33(20-27)28(21-3-4-21)8-1-2-9-28/h5-6,17,21,23H,1-4,7-16,18-20H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ki for human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257784
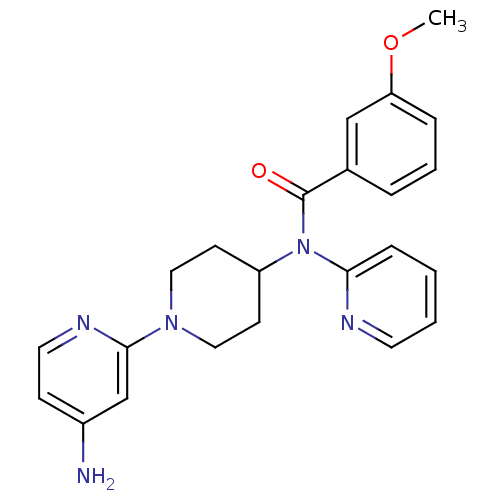
(CHEMBL493517 | N-(1-(4-aminopyridin-2-yl)piperidin...)Show SMILES COc1cccc(c1)C(=O)N(C1CCN(CC1)c1cc(N)ccn1)c1ccccn1 Show InChI InChI=1S/C23H25N5O2/c1-30-20-6-4-5-17(15-20)23(29)28(21-7-2-3-11-25-21)19-9-13-27(14-10-19)22-16-18(24)8-12-26-22/h2-8,11-12,15-16,19H,9-10,13-14H2,1H3,(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257722
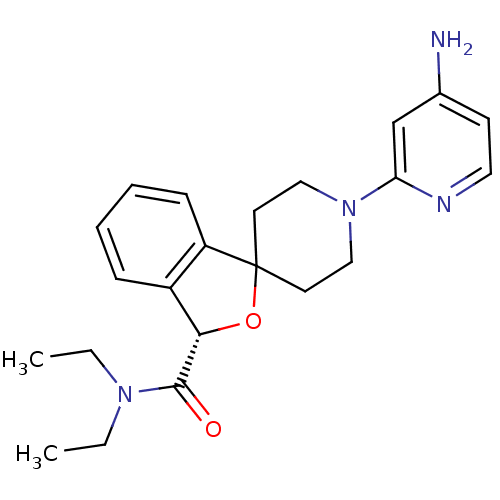
((S)-1'-(4-aminopyridin-2-yl)-N,N-diethyl-3H-spiro[...)Show SMILES CCN(CC)C(=O)[C@H]1OC2(CCN(CC2)c2cc(N)ccn2)c2ccccc12 |r| Show InChI InChI=1S/C22H28N4O2/c1-3-25(4-2)21(27)20-17-7-5-6-8-18(17)22(28-20)10-13-26(14-11-22)19-15-16(23)9-12-24-19/h5-9,12,15,20H,3-4,10-11,13-14H2,1-2H3,(H2,23,24)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257783
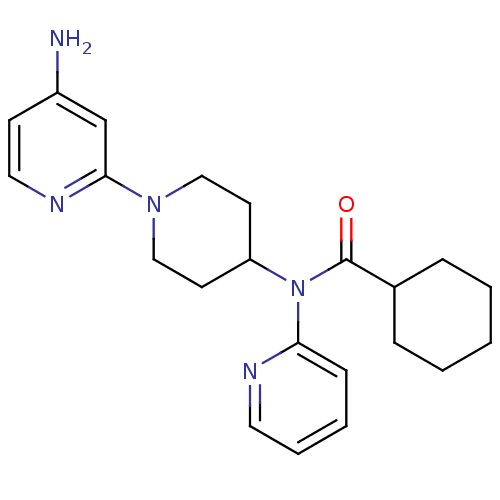
(CHEMBL493516 | N-(1-(4-aminopyridin-2-yl)piperidin...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)N(C(=O)C1CCCCC1)c1ccccn1 Show InChI InChI=1S/C22H29N5O/c23-18-9-13-25-21(16-18)26-14-10-19(11-15-26)27(20-8-4-5-12-24-20)22(28)17-6-2-1-3-7-17/h4-5,8-9,12-13,16-17,19H,1-3,6-7,10-11,14-15H2,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257830
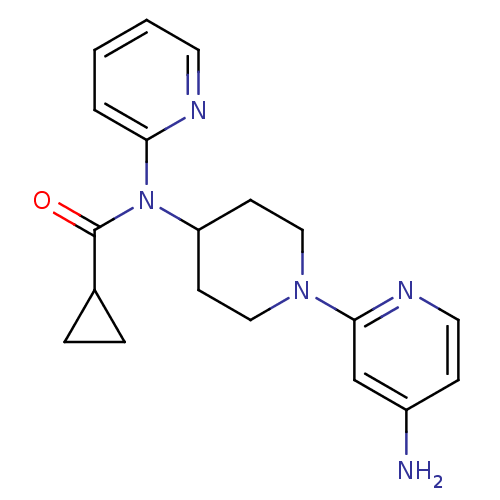
(CHEMBL493781 | N-(1-(4-aminopyridin-2-yl)piperidin...)Show InChI InChI=1S/C19H23N5O/c20-15-6-10-22-18(13-15)23-11-7-16(8-12-23)24(19(25)14-4-5-14)17-3-1-2-9-21-17/h1-3,6,9-10,13-14,16H,4-5,7-8,11-12H2,(H2,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50170967
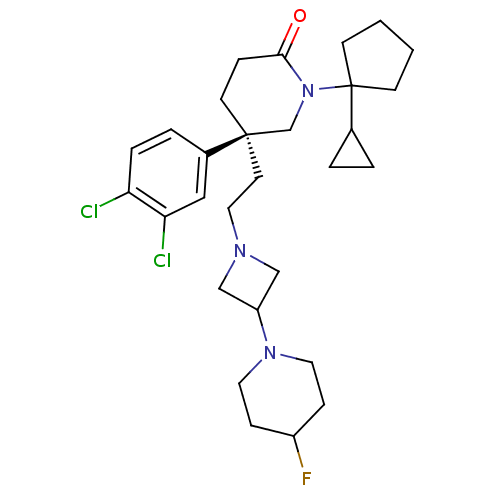
((S)-1-(1-Cyclopropyl-cyclopentyl)-5-(3,4-dichloro-...)Show SMILES FC1CCN(CC1)C1CN(CC[C@@]2(CCC(=O)N(C2)C2(CCCC2)C2CC2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C29H40Cl2FN3O/c30-25-6-5-22(17-26(25)31)28(13-16-33-18-24(19-33)34-14-8-23(32)9-15-34)12-7-27(36)35(20-28)29(21-3-4-21)10-1-2-11-29/h5-6,17,21,23-24H,1-4,7-16,18-20H2/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ki for human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257719

((S)-N-(1-(4-aminopyridin-2-yl)-4-phenylpiperidin-4...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)(NC(=O)[C@@H]1CCCO1)c1ccccc1 |r| Show InChI InChI=1S/C21H26N4O2/c22-17-8-11-23-19(15-17)25-12-9-21(10-13-25,16-5-2-1-3-6-16)24-20(26)18-7-4-14-27-18/h1-3,5-6,8,11,15,18H,4,7,9-10,12-14H2,(H2,22,23)(H,24,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359779
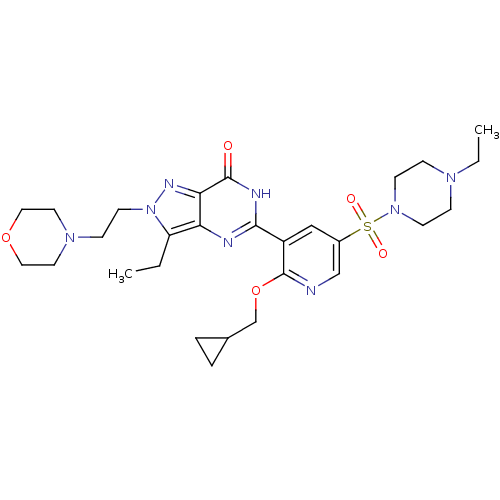
(CHEMBL1928271)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC2CC2)c(c1)-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C28H40N8O5S/c1-3-23-24-25(32-36(23)12-9-34-13-15-40-16-14-34)27(37)31-26(30-24)22-17-21(18-29-28(22)41-19-20-5-6-20)42(38,39)35-10-7-33(4-2)8-11-35/h17-18,20H,3-16,19H2,1-2H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359773
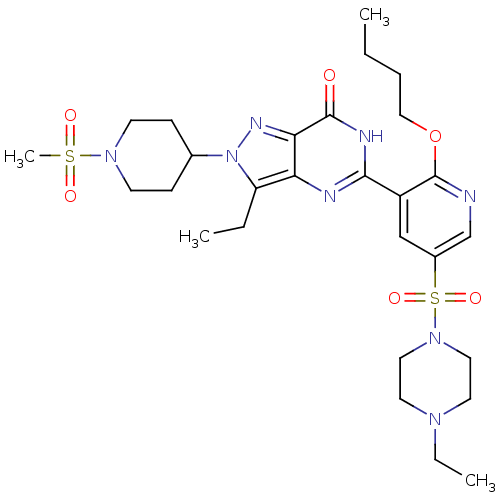
(CHEMBL1928265)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCN(CC1)S(C)(=O)=O)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C28H42N8O6S2/c1-5-8-17-42-28-22(18-21(19-29-28)44(40,41)35-15-13-33(7-3)14-16-35)26-30-24-23(6-2)36(32-25(24)27(37)31-26)20-9-11-34(12-10-20)43(4,38)39/h18-20H,5-17H2,1-4H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50170968
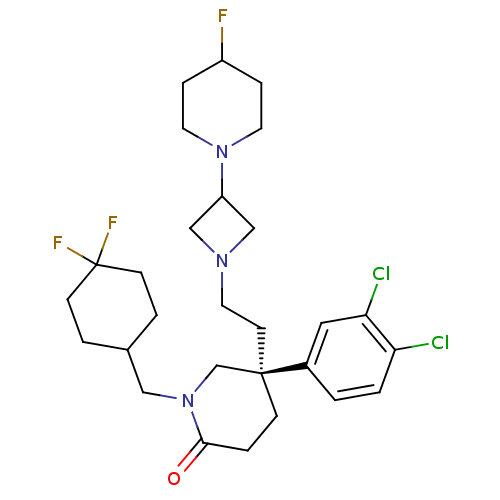
((S)-5-(3,4-Dichloro-phenyl)-1-(4,4-difluoro-cycloh...)Show SMILES FC1CCN(CC1)C1CN(CC[C@@]2(CCC(=O)N(CC3CCC(F)(F)CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C28H38Cl2F3N3O/c29-24-2-1-21(15-25(24)30)27(11-14-34-17-23(18-34)35-12-6-22(31)7-13-35)8-5-26(37)36(19-27)16-20-3-9-28(32,33)10-4-20/h1-2,15,20,22-23H,3-14,16-19H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50276291
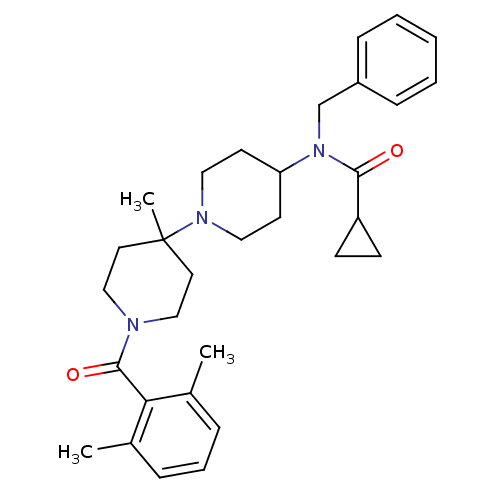
(CHEMBL472464 | N-benzyl-N-(1'-(2,6-dimethylbenzoyl...)Show SMILES Cc1cccc(C)c1C(=O)N1CCC(C)(CC1)N1CCC(CC1)N(Cc1ccccc1)C(=O)C1CC1 Show InChI InChI=1S/C31H41N3O2/c1-23-8-7-9-24(2)28(23)30(36)32-20-16-31(3,17-21-32)33-18-14-27(15-19-33)34(29(35)26-12-13-26)22-25-10-5-4-6-11-25/h4-11,26-27H,12-22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... |
Bioorg Med Chem Lett 19: 1084-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.012
BindingDB Entry DOI: 10.7270/Q2W095SG |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50197517
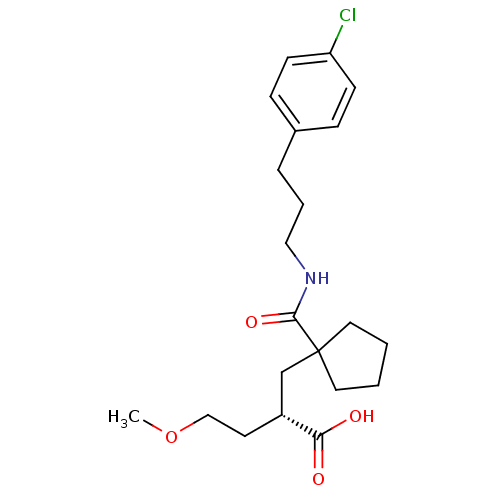
((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rat NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359795
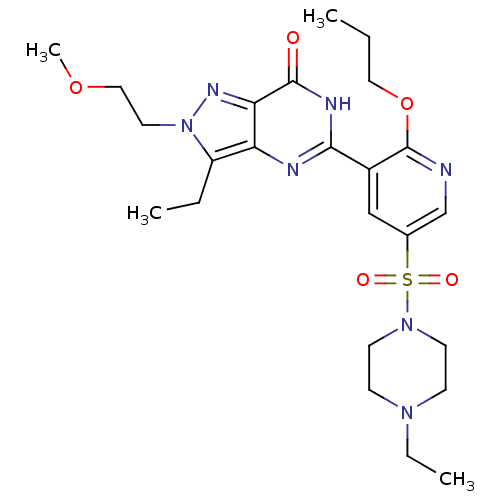
(CHEMBL1928258)Show SMILES CCCOc1ncc(cc1-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C24H35N7O5S/c1-5-13-36-24-18(15-17(16-25-24)37(33,34)30-10-8-29(7-3)9-11-30)22-26-20-19(6-2)31(12-14-35-4)28-21(20)23(32)27-22/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359767
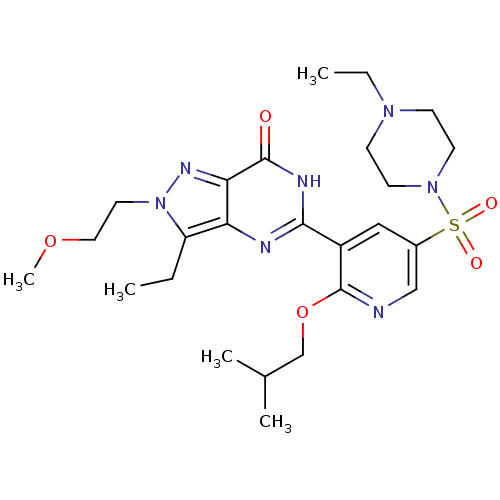
(CHEMBL1928259)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC(C)C)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H37N7O5S/c1-6-20-21-22(29-32(20)12-13-36-5)24(33)28-23(27-21)19-14-18(15-26-25(19)37-16-17(3)4)38(34,35)31-10-8-30(7-2)9-11-31/h14-15,17H,6-13,16H2,1-5H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359774

(CHEMBL1928266)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCN(C)CC1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C28H42N8O4S/c1-5-8-17-40-28-22(18-21(19-29-28)41(38,39)35-15-13-34(7-3)14-16-35)26-30-24-23(6-2)36(32-25(24)27(37)31-26)20-9-11-33(4)12-10-20/h18-20H,5-17H2,1-4H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359768
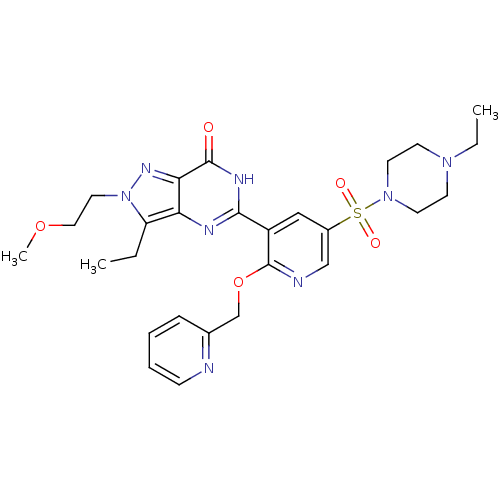
(CHEMBL1928260)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCc2ccccn2)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H34N8O5S/c1-4-22-23-24(32-35(22)14-15-39-3)26(36)31-25(30-23)21-16-20(41(37,38)34-12-10-33(5-2)11-13-34)17-29-27(21)40-18-19-8-6-7-9-28-19/h6-9,16-17H,4-5,10-15,18H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
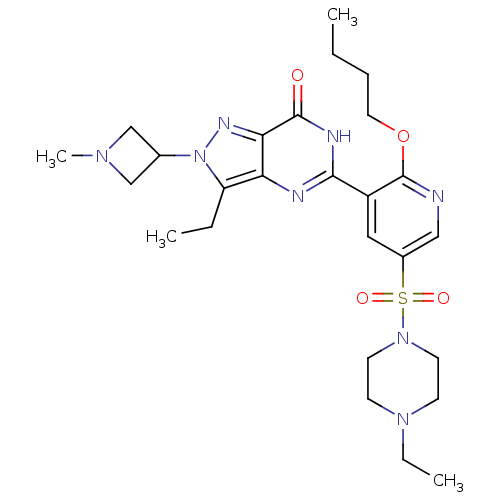
(Homo sapiens (Human)) | BDBM50359777
(CHEMBL1928269)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CN(C)C1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H38N8O4S/c1-5-8-13-38-26-20(14-19(15-27-26)39(36,37)33-11-9-32(7-3)10-12-33)24-28-22-21(6-2)34(18-16-31(4)17-18)30-23(22)25(35)29-24/h14-15,18H,5-13,16-17H2,1-4H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50409627
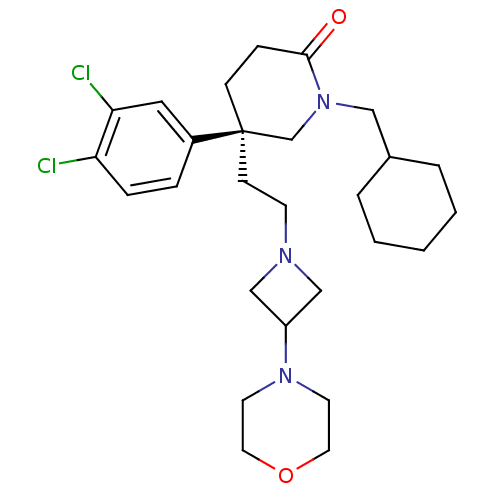
(CHEMBL185572)Show SMILES Clc1ccc(cc1Cl)[C@@]1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(CC2CCCCC2)C1 Show InChI InChI=1S/C27H39Cl2N3O2/c28-24-7-6-22(16-25(24)29)27(10-11-30-18-23(19-30)31-12-14-34-15-13-31)9-8-26(33)32(20-27)17-21-4-2-1-3-5-21/h6-7,16,21,23H,1-5,8-15,17-20H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359778
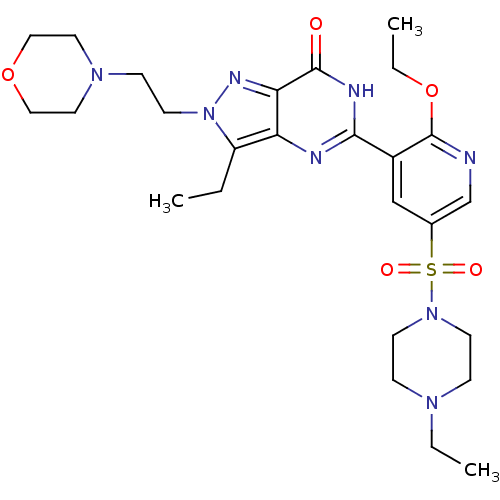
(CHEMBL1928270)Show SMILES CCOc1ncc(cc1-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H38N8O5S/c1-4-21-22-23(30-34(21)12-9-32-13-15-38-16-14-32)25(35)29-24(28-22)20-17-19(18-27-26(20)39-6-3)40(36,37)33-10-7-31(5-2)8-11-33/h17-18H,4-16H2,1-3H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359790

(CHEMBL1928253)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CC3CC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H35N7O5S/c1-4-20-21-22(29-32(20)16-17-6-7-17)24(33)28-23(27-21)19-14-18(15-26-25(19)37-13-12-36-3)38(34,35)31-10-8-30(5-2)9-11-31/h14-15,17H,4-13,16H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359789
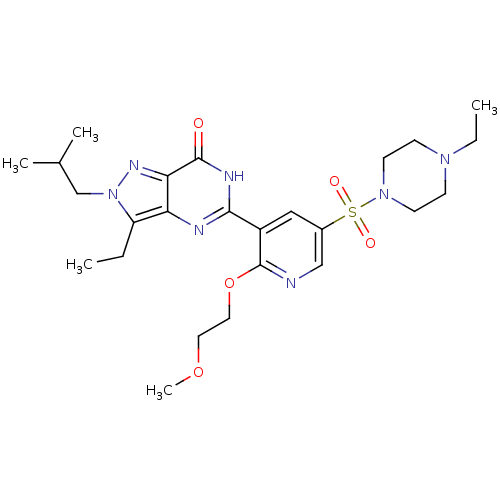
(CHEMBL1928252)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CC(C)C)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H37N7O5S/c1-6-20-21-22(29-32(20)16-17(3)4)24(33)28-23(27-21)19-14-18(15-26-25(19)37-13-12-36-5)38(34,35)31-10-8-30(7-2)9-11-31/h14-15,17H,6-13,16H2,1-5H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50276259

(CHEMBL481068 | exo-N-benzyl-N-((R)-1-(8-(2,6-dimet...)Show SMILES Cc1cccc(C)c1C(=O)N1[C@H]2CC[C@@H]1C[C@H](C2)N1CC[C@H](C1)N(Cc1ccccc1)C(=O)C1CC1 |r,THB:8:10:15.16.17:12.13| Show InChI InChI=1S/C31H39N3O2/c1-21-7-6-8-22(2)29(21)31(36)34-25-13-14-26(34)18-28(17-25)32-16-15-27(20-32)33(30(35)24-11-12-24)19-23-9-4-3-5-10-23/h3-10,24-28H,11-20H2,1-2H3/t25-,26+,27-,28-/m1/s1 | PDB
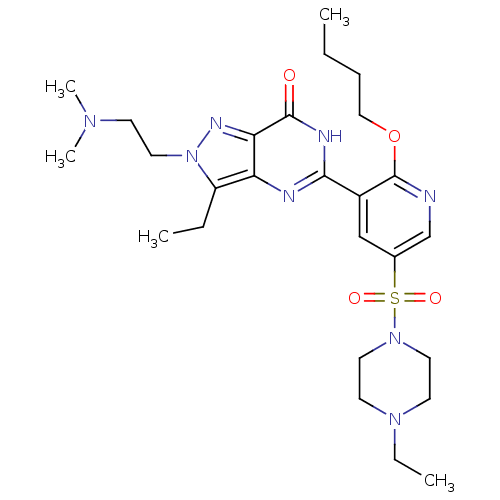
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... |
Bioorg Med Chem Lett 19: 1084-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.012
BindingDB Entry DOI: 10.7270/Q2W095SG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50276260
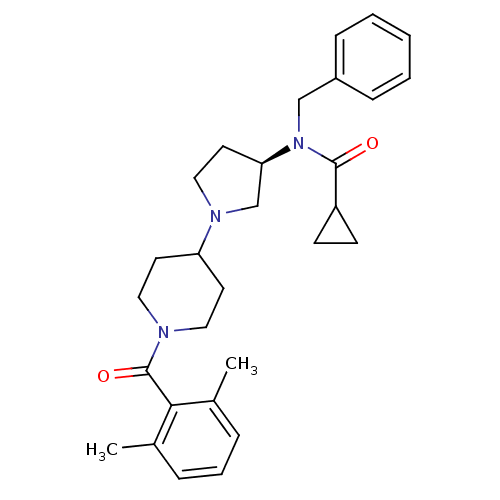
((R)-N-benzyl-N-(1-(1-(2,6-dimethylbenzoyl)piperidi...)Show SMILES Cc1cccc(C)c1C(=O)N1CCC(CC1)N1CC[C@H](C1)N(Cc1ccccc1)C(=O)C1CC1 |r| Show InChI InChI=1S/C29H37N3O2/c1-21-7-6-8-22(2)27(21)29(34)30-16-13-25(14-17-30)31-18-15-26(20-31)32(28(33)24-11-12-24)19-23-9-4-3-5-10-23/h3-10,24-26H,11-20H2,1-2H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... |
Bioorg Med Chem Lett 19: 1084-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.012
BindingDB Entry DOI: 10.7270/Q2W095SG |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359771
(CHEMBL1928263)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(CCN(C)C)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H40N8O4S/c1-6-9-16-38-26-20(17-19(18-27-26)39(36,37)33-13-11-32(8-3)12-14-33)24-28-22-21(7-2)34(15-10-31(4)5)30-23(22)25(35)29-24/h17-18H,6-16H2,1-5H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50410049
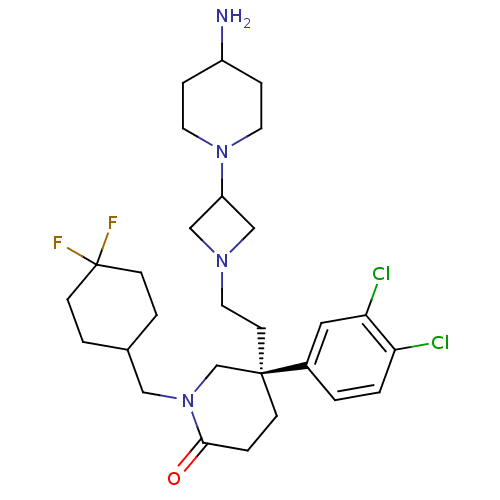
(CHEMBL434142)Show SMILES NC1CCN(CC1)C1CN(CC[C@@]2(CCC(=O)N(CC3CCC(F)(F)CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C28H40Cl2F2N4O/c29-24-2-1-21(15-25(24)30)27(11-14-34-17-23(18-34)35-12-6-22(33)7-13-35)8-5-26(37)36(19-27)16-20-3-9-28(31,32)10-4-20/h1-2,15,20,22-23H,3-14,16-19,33H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50246483
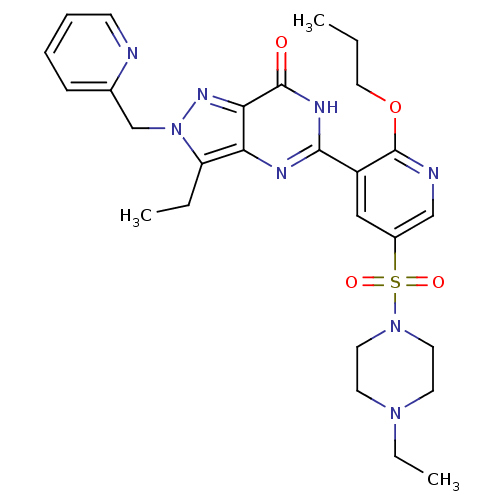
(3-ethyl-5-(5-(4-ethylpiperazin-1-ylsulfonyl)-2-pro...)Show SMILES CCCOc1ncc(cc1-c1nc2c(CC)n(Cc3ccccn3)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C27H34N8O4S/c1-4-15-39-27-21(16-20(17-29-27)40(37,38)34-13-11-33(6-3)12-14-34)25-30-23-22(5-2)35(32-24(23)26(36)31-25)18-19-9-7-8-10-28-19/h7-10,16-17H,4-6,11-15,18H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
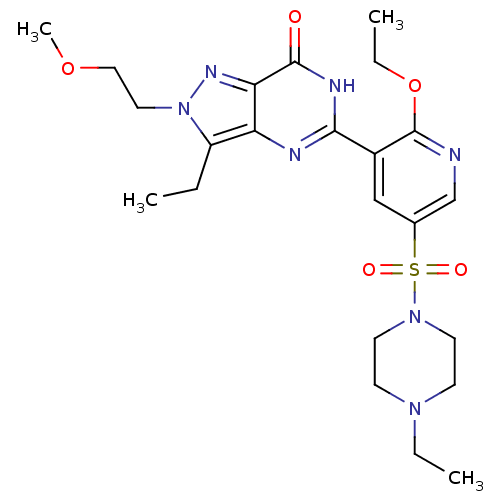
(Homo sapiens (Human)) | BDBM50359770
(CHEMBL1928262)Show SMILES CCOc1ncc(cc1-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H33N7O5S/c1-5-18-19-20(27-30(18)12-13-34-4)22(31)26-21(25-19)17-14-16(15-24-23(17)35-7-3)36(32,33)29-10-8-28(6-2)9-11-29/h14-15H,5-13H2,1-4H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50410051

(CHEMBL185182)Show SMILES CS(=O)(=O)N1CCN(CC1)C1CN(CC[C@@]2(CCC(=O)N(CC3CCC(F)(F)CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C28H40Cl2F2N4O3S/c1-40(38,39)36-14-12-34(13-15-36)23-18-33(19-23)11-10-27(22-2-3-24(29)25(30)16-22)7-6-26(37)35(20-27)17-21-4-8-28(31,32)9-5-21/h2-3,16,21,23H,4-15,17-20H2,1H3/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50410048
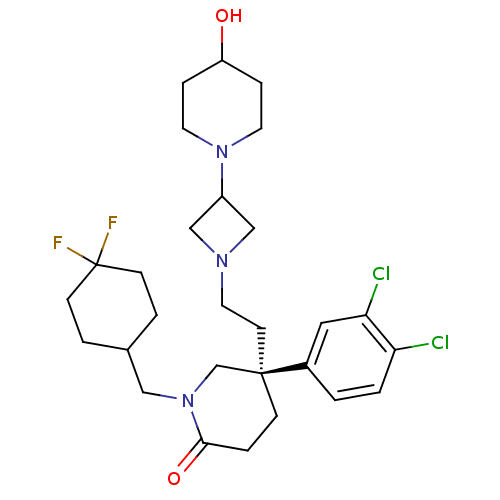
(CHEMBL434548)Show SMILES OC1CCN(CC1)C1CN(CC[C@@]2(CCC(=O)N(CC3CCC(F)(F)CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C28H39Cl2F2N3O2/c29-24-2-1-21(15-25(24)30)27(11-14-33-17-22(18-33)34-12-6-23(36)7-13-34)8-5-26(37)35(19-27)16-20-3-9-28(31,32)10-4-20/h1-2,15,20,22-23,36H,3-14,16-19H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
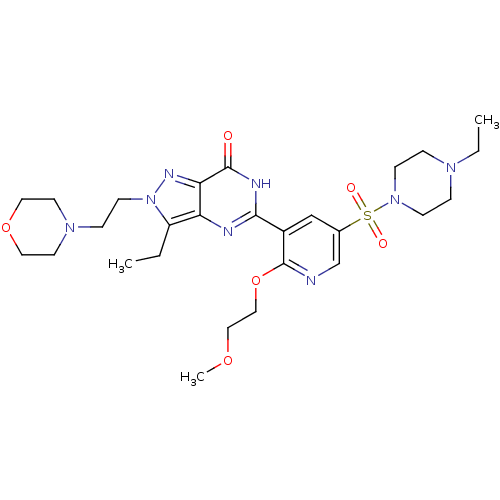
(Homo sapiens (Human)) | BDBM50359780
(CHEMBL1928272)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H40N8O6S/c1-4-22-23-24(31-35(22)11-8-33-12-14-40-15-13-33)26(36)30-25(29-23)21-18-20(19-28-27(21)41-17-16-39-3)42(37,38)34-9-6-32(5-2)7-10-34/h18-19H,4-17H2,1-3H3,(H,29,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359787
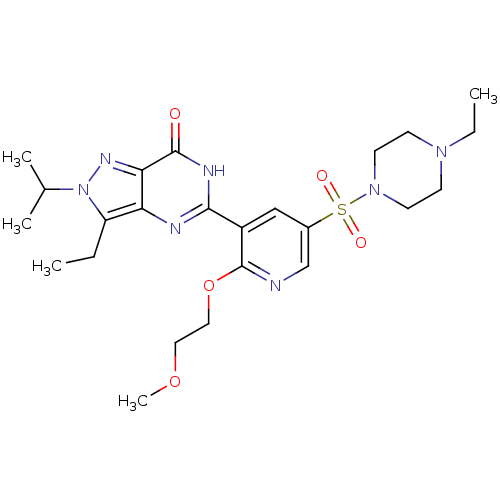
(CHEMBL1928250)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C(C)C Show InChI InChI=1S/C24H35N7O5S/c1-6-19-20-21(28-31(19)16(3)4)23(32)27-22(26-20)18-14-17(15-25-24(18)36-13-12-35-5)37(33,34)30-10-8-29(7-2)9-11-30/h14-16H,6-13H2,1-5H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359769

(CHEMBL1928261)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C24H35N7O6S/c1-5-19-20-21(28-31(19)11-12-35-3)23(32)27-22(26-20)18-15-17(16-25-24(18)37-14-13-36-4)38(33,34)30-9-7-29(6-2)8-10-30/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359791

(CHEMBL1928254)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C26H37N7O6S/c1-4-21-22-23(30-33(21)18-6-12-38-13-7-18)25(34)29-24(28-22)20-16-19(17-27-26(20)39-15-14-37-3)40(35,36)32-10-8-31(5-2)9-11-32/h16-18H,4-15H2,1-3H3,(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359786

(CHEMBL1928249)Show SMILES CCCn1nc2c(nc([nH]c2=O)-c2cc(cnc2OCCOC)S(=O)(=O)N2CCN(CC)CC2)c1CC Show InChI InChI=1S/C24H35N7O5S/c1-5-8-31-19(6-2)20-21(28-31)23(32)27-22(26-20)18-15-17(16-25-24(18)36-14-13-35-4)37(33,34)30-11-9-29(7-3)10-12-30/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data