

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163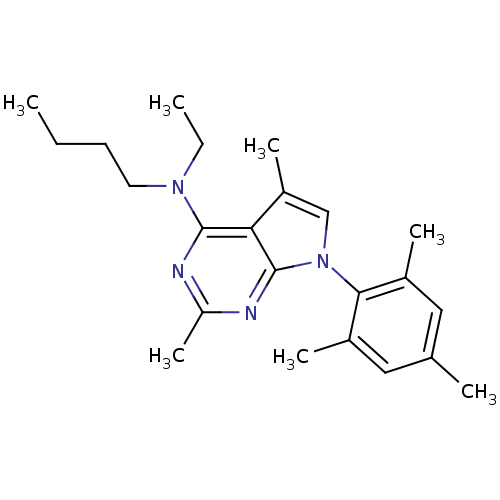 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against [125I]-Try0-o-Corticotropin-releasing Factor to Corticotropin releasing hormone receptor 1 from ovine | J Med Chem 40: 1749-54 (1997) Article DOI: 10.1021/jm960861b BindingDB Entry DOI: 10.7270/Q2SJ1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 2 (RAT) | BDBM50058163 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-Tyr0-sauvagine to Corticotropin releasing hormone receptor 2 (CRF2) | J Med Chem 40: 1749-54 (1997) Article DOI: 10.1021/jm960861b BindingDB Entry DOI: 10.7270/Q2SJ1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477060 (CHEMBL393761) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477067 (CHEMBL237875) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477038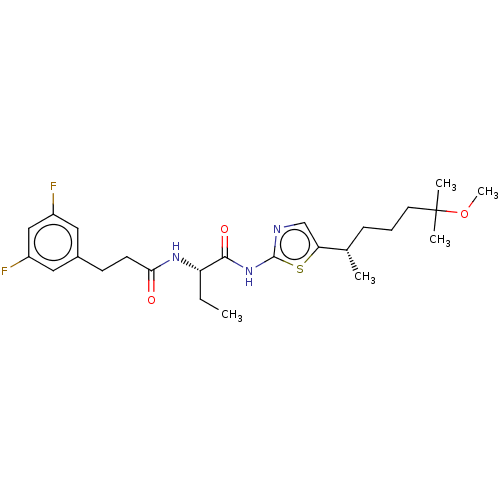 (CHEMBL237850) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477048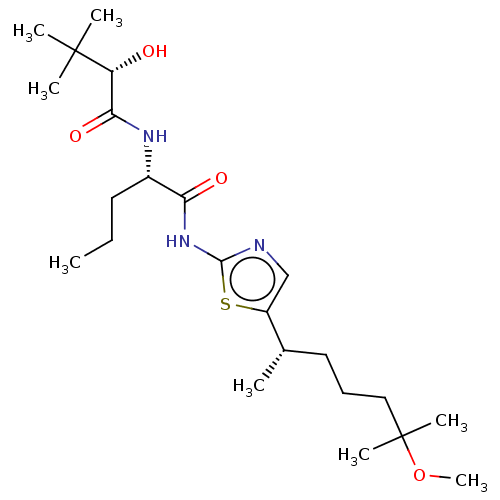 (CHEMBL236597) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477051 (CHEMBL237666) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477065 (CHEMBL236592) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477046 (CHEMBL236595) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477022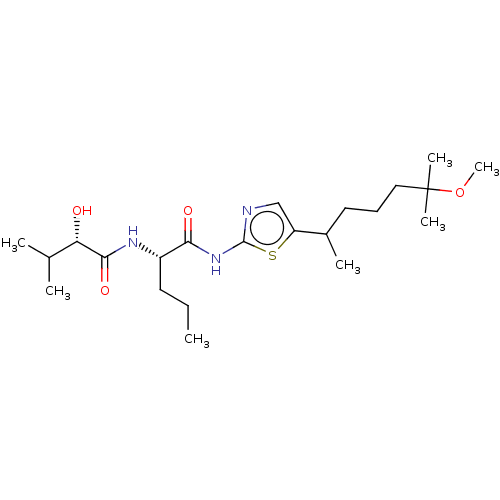 (CHEMBL236594) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477040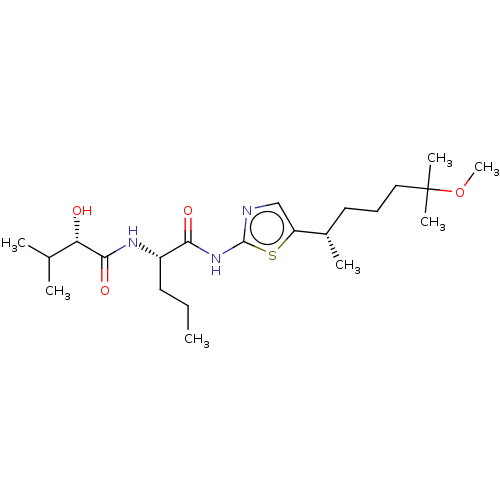 (CHEMBL237665) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477037 (CHEMBL237867) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31592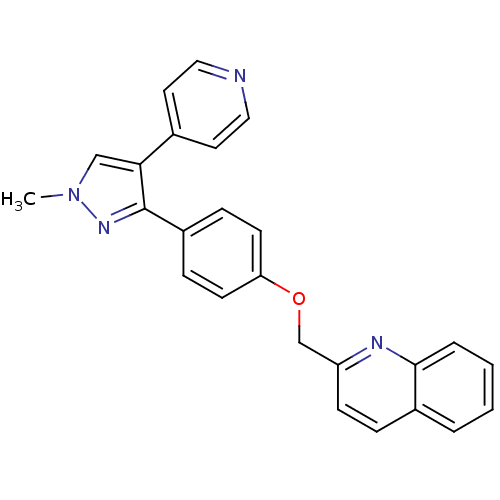 (PF-2545920 | US9138494, MP-10 | substituted pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM50300099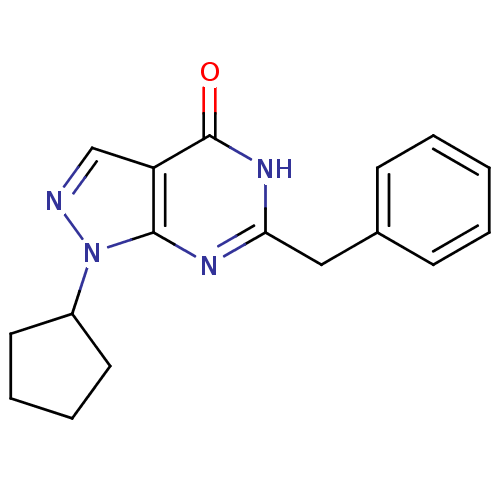 (6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE1C expressed in Sf9 cells using [3H]cAMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 55: 9045-54 (2012) Article DOI: 10.1021/jm3007799 BindingDB Entry DOI: 10.7270/Q24J0G79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31591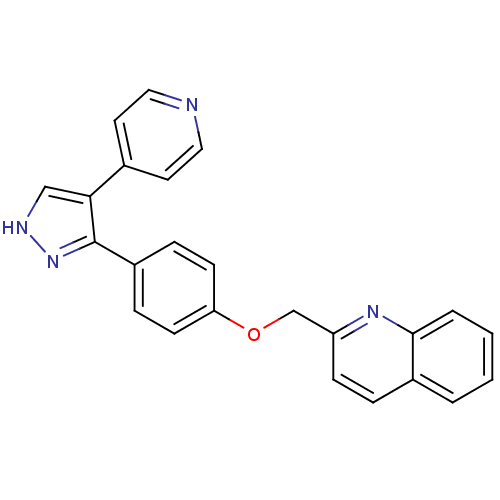 (pyrazole, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477021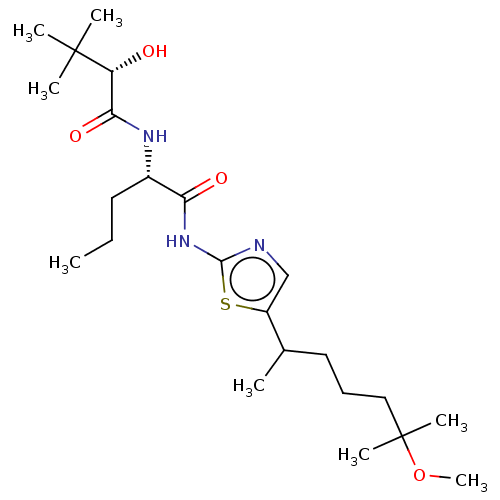 (CHEMBL236596) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477061 (CHEMBL393401) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31606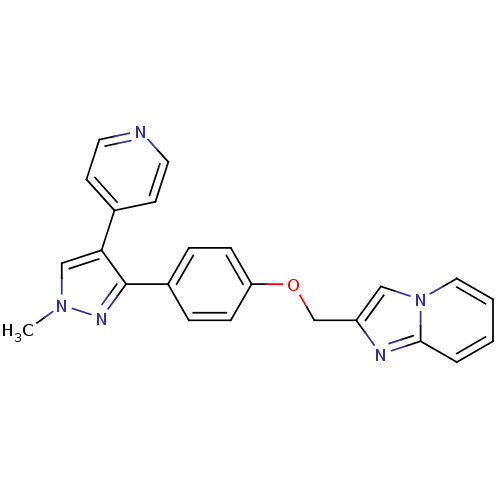 (methyl substituted pyrazole, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477041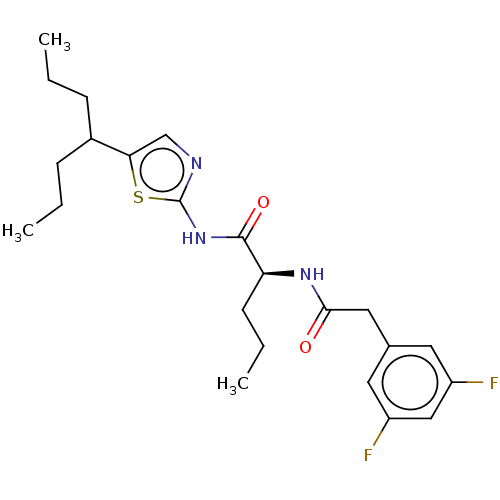 (CHEMBL393397) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50300099 (6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 55: 9045-54 (2012) Article DOI: 10.1021/jm3007799 BindingDB Entry DOI: 10.7270/Q24J0G79 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50397844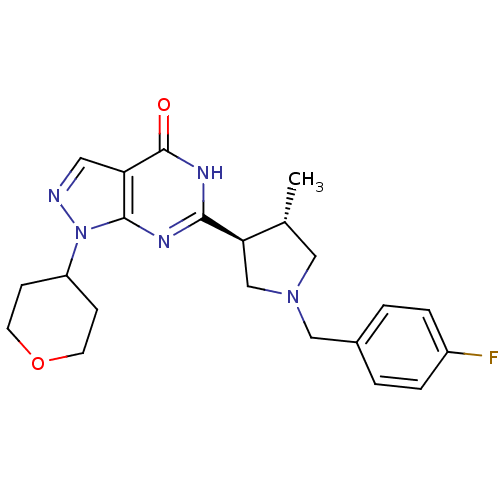 (CHEMBL2179099) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 55: 9045-54 (2012) Article DOI: 10.1021/jm3007799 BindingDB Entry DOI: 10.7270/Q24J0G79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31594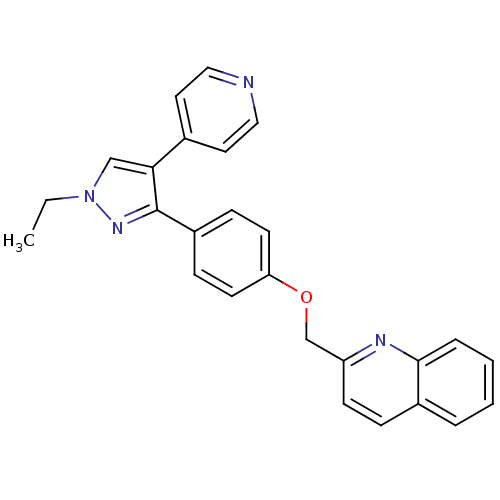 (substituted pyrazole, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477050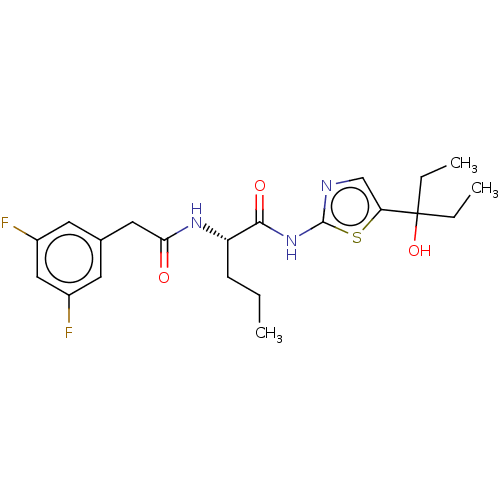 (CHEMBL236775) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase C100Flag cleavaging activity in HeLa cell membranes assessed as M-amyloid beta-40 production by cell free assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50397850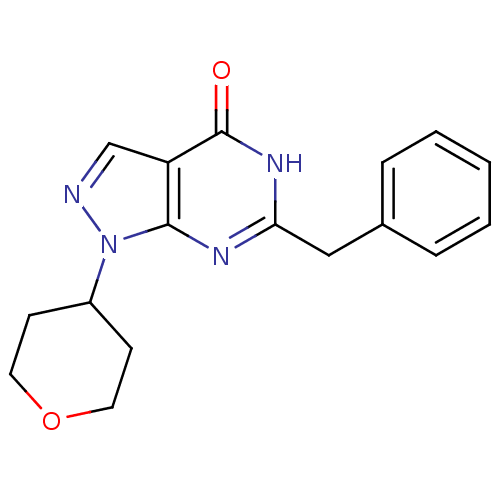 (CHEMBL2179093) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 55: 9045-54 (2012) Article DOI: 10.1021/jm3007799 BindingDB Entry DOI: 10.7270/Q24J0G79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477056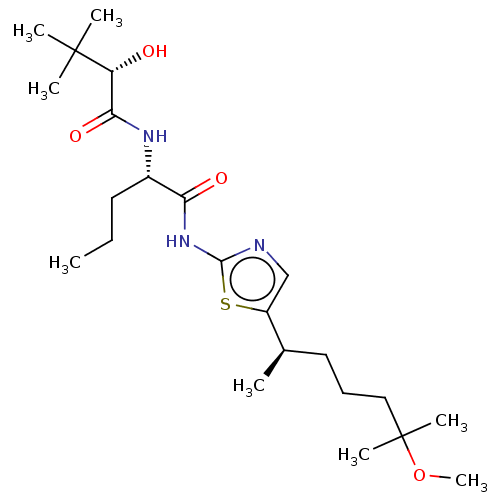 (CHEMBL236773) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50397845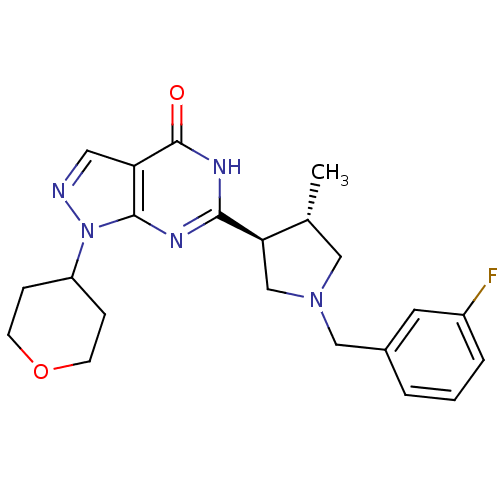 (CHEMBL2177125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 55: 9045-54 (2012) Article DOI: 10.1021/jm3007799 BindingDB Entry DOI: 10.7270/Q24J0G79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477047 (CHEMBL240700) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477050 (CHEMBL236775) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31605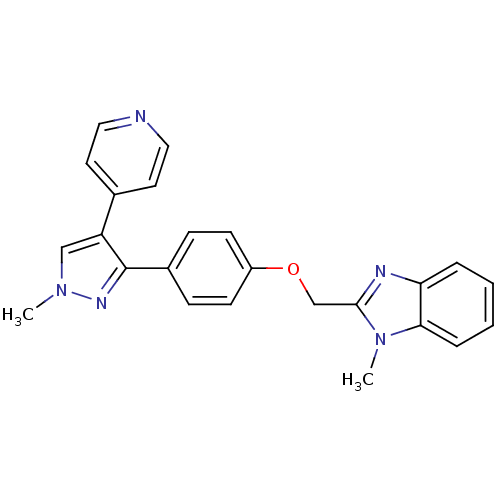 (methyl substituted pyrazole, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477069 (CHEMBL240068) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31596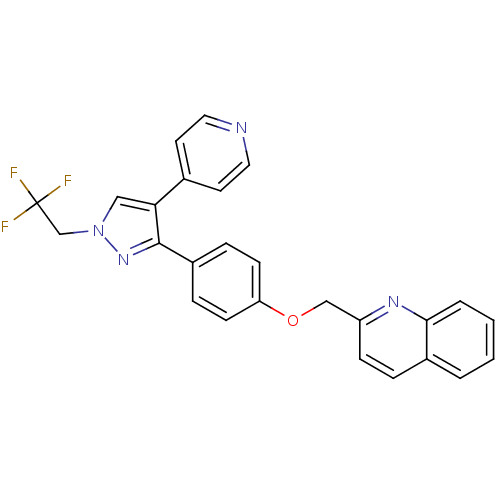 (substituted pyrazole, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31593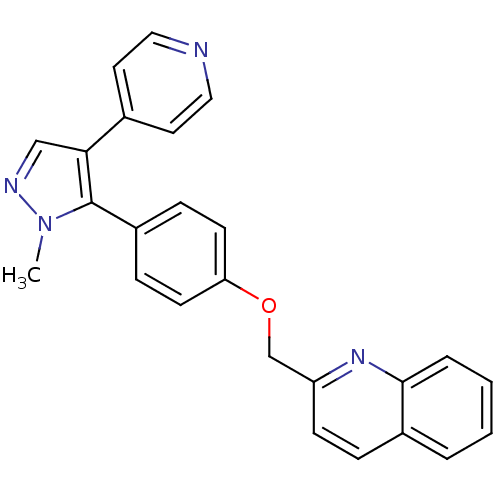 (substituted pyrazole, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477057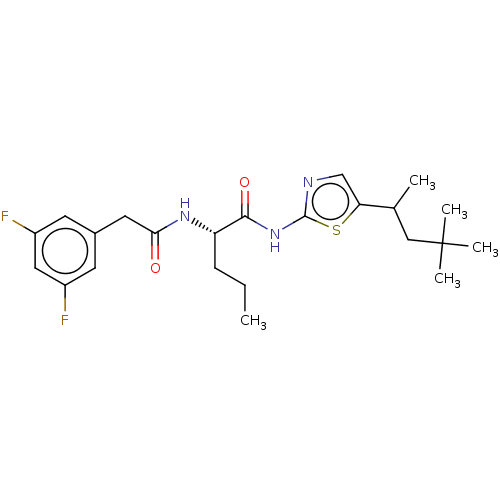 (CHEMBL237669) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50397848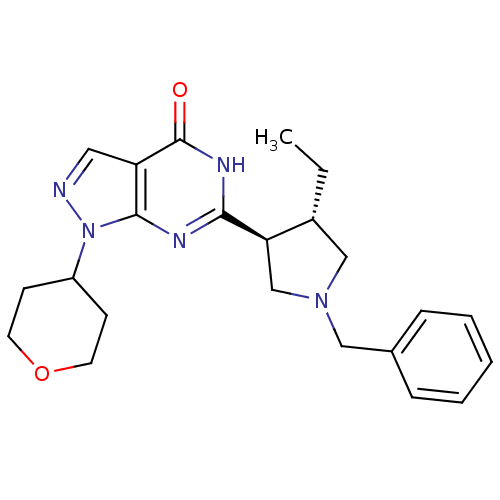 (CHEMBL2179096) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 55: 9045-54 (2012) Article DOI: 10.1021/jm3007799 BindingDB Entry DOI: 10.7270/Q24J0G79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50300113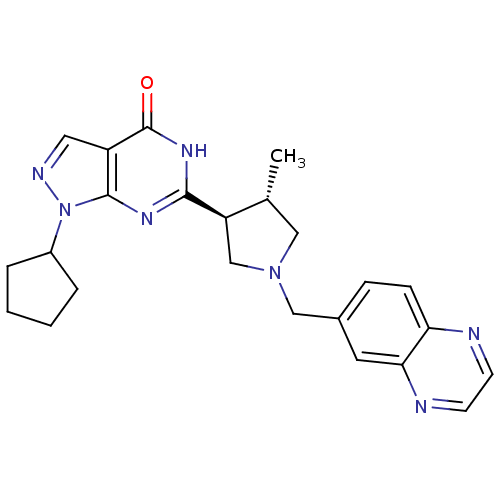 (1-cyclopentyl-6-[(3S,4S)-4-methyl-1-(quinoxalin-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA | J Med Chem 52: 7946-9 (2009) Article DOI: 10.1021/jm9015334 BindingDB Entry DOI: 10.7270/Q2RV0NRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50300113 (1-cyclopentyl-6-[(3S,4S)-4-methyl-1-(quinoxalin-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA | J Med Chem 52: 7946-9 (2009) Article DOI: 10.1021/jm9015334 BindingDB Entry DOI: 10.7270/Q2RV0NRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50300112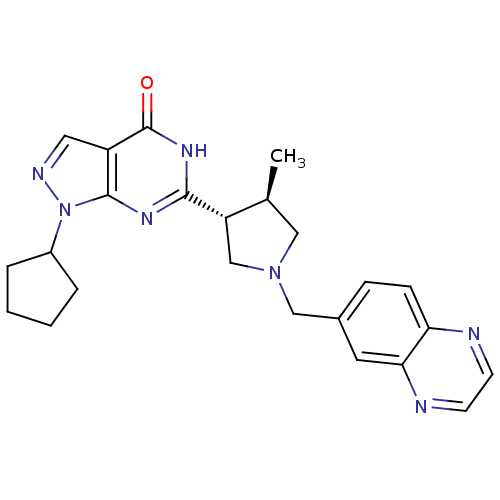 (CHEMBL565667 | trans-1-cyclopentyl-6-(4-methyl-1-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA | J Med Chem 52: 7946-9 (2009) Article DOI: 10.1021/jm9015334 BindingDB Entry DOI: 10.7270/Q2RV0NRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50300112 (CHEMBL565667 | trans-1-cyclopentyl-6-(4-methyl-1-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA | J Med Chem 52: 7946-9 (2009) Article DOI: 10.1021/jm9015334 BindingDB Entry DOI: 10.7270/Q2RV0NRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477027 (CHEMBL392759) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477052 (CHEMBL240066) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM50300099 (6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE1C expressed in Sf9 cells by SPA | J Med Chem 52: 7946-9 (2009) Article DOI: 10.1021/jm9015334 BindingDB Entry DOI: 10.7270/Q2RV0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM50300099 (6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE1C expressed in Sf9 cells by SPA | J Med Chem 52: 7946-9 (2009) Article DOI: 10.1021/jm9015334 BindingDB Entry DOI: 10.7270/Q2RV0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50300099 (6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA | J Med Chem 52: 7946-9 (2009) Article DOI: 10.1021/jm9015334 BindingDB Entry DOI: 10.7270/Q2RV0NRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50300099 (6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA | J Med Chem 52: 7946-9 (2009) Article DOI: 10.1021/jm9015334 BindingDB Entry DOI: 10.7270/Q2RV0NRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477068 (CHEMBL236790) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477042 (CHEMBL236774) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477055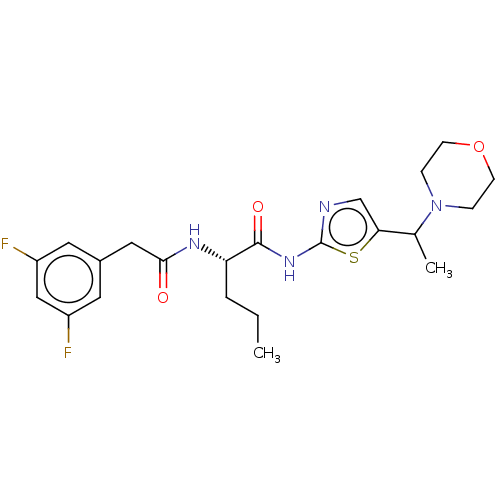 (CHEMBL241350) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477063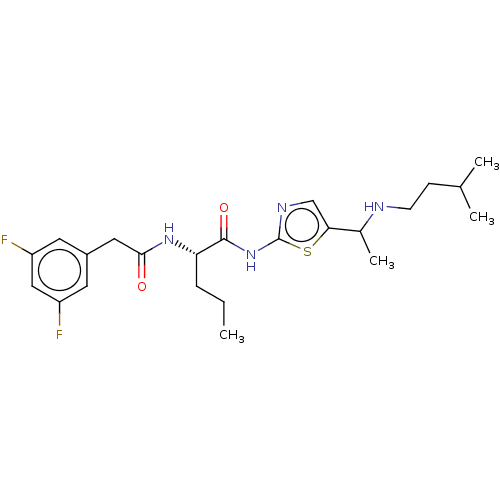 (CHEMBL393756) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase activity in human H4 cells transfected with APP695 mutant assessed as beta amyloid(1-40) production by whole cell assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477063 (CHEMBL393756) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase C100Flag cleavaging activity in HeLa cell membranes assessed as M-amyloid beta-40 production by cell free assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477047 (CHEMBL240700) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase C100Flag cleavaging activity in HeLa cell membranes assessed as M-amyloid beta-40 production by cell free assay | Bioorg Med Chem Lett 17: 5518-22 (2007) Article DOI: 10.1016/j.bmcl.2007.08.035 BindingDB Entry DOI: 10.7270/Q2XG9TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 230 total ) | Next | Last >> |