

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the absence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048608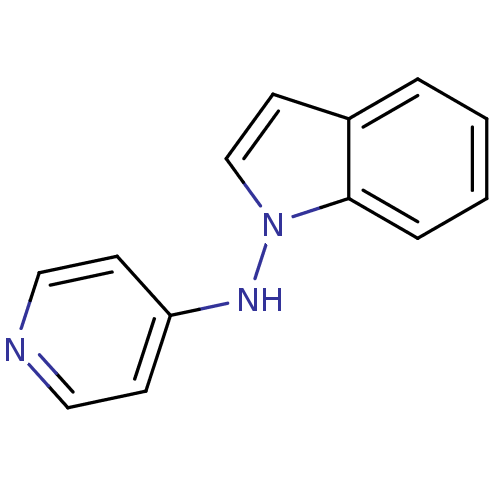 (CHEMBL154488 | Indol-1-yl-pyridin-4-yl-amine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9349 ((2Z)-but-2-enedioic acid; 9-amino-6-chloro-1,2,3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hoechst-Roussel Pharmaceuticals, Inc. | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. | J Med Chem 32: 1805-13 (1989) Article DOI: 10.1021/jm00128a024 BindingDB Entry DOI: 10.7270/Q24Q7S6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048589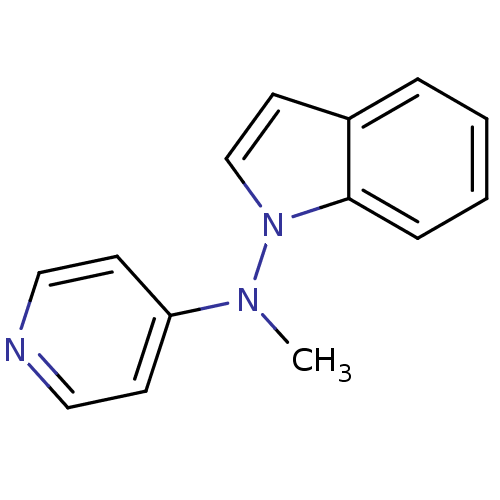 (CHEMBL152842 | Indol-1-yl-methyl-pyridin-4-yl-amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048600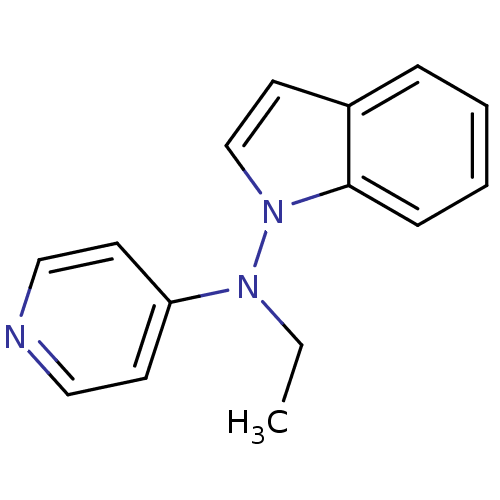 (CHEMBL154541 | Ethyl-indol-1-yl-pyridin-4-yl-amine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]idazoxan binding to Alpha-2 adrenergic receptor in rat cortex, in the presence of GPP | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]yohimbine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048600 (CHEMBL154541 | Ethyl-indol-1-yl-pyridin-4-yl-amine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9352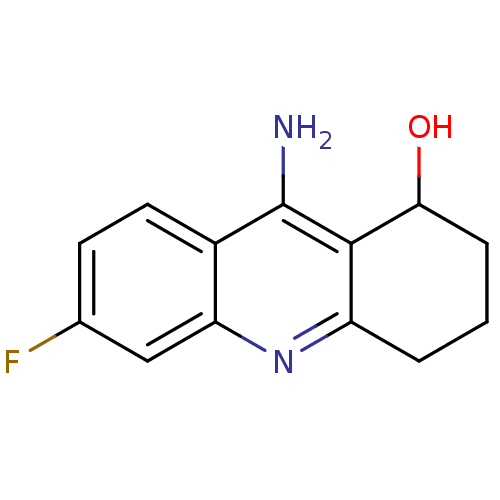 (9-Amino-1 ,2,3,4-tetrahydroacridin-1-ol deriv. 1f ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 292 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hoechst-Roussel Pharmaceuticals, Inc. | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. | J Med Chem 32: 1805-13 (1989) Article DOI: 10.1021/jm00128a024 BindingDB Entry DOI: 10.7270/Q24Q7S6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against acetylcholinesterase using rat striatal preparations | J Med Chem 31: 1278-9 (1988) BindingDB Entry DOI: 10.7270/Q25D8QTS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. | J Med Chem 32: 1805-13 (1989) Article DOI: 10.1021/jm00128a024 BindingDB Entry DOI: 10.7270/Q24Q7S6Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholinesterase in rat striatal preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048576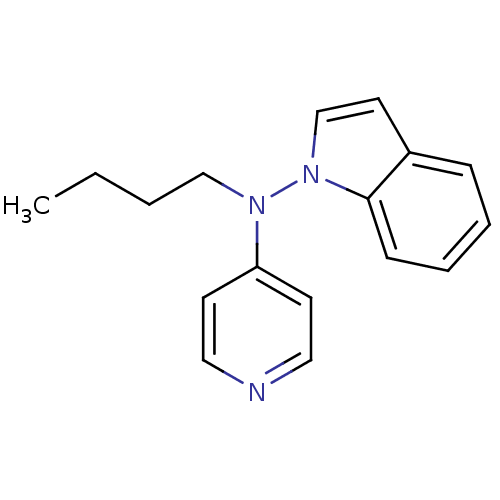 (Butyl-indol-1-yl-pyridin-4-yl-amine | CHEMBL155109) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]oxotremorine-M binding to muscarinic receptors in rat forebrain membrane | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-idazoxan binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048610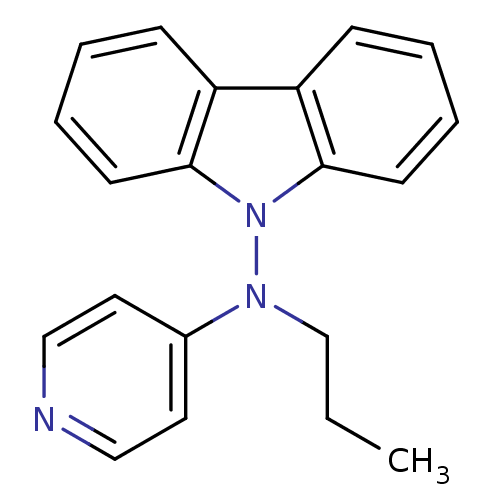 (CHEMBL435034 | Carbazol-9-yl-propyl-pyridin-4-yl-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048602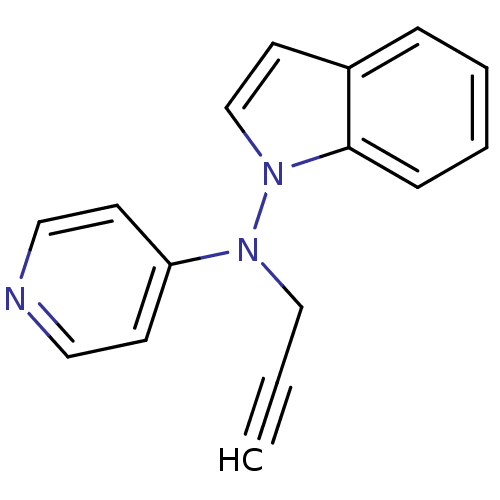 (CHEMBL348034 | Indol-1-yl-prop-2-ynyl-pyridin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50048608 (CHEMBL154488 | Indol-1-yl-pyridin-4-yl-amine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]WB-4101 binding to Alpha-1 adrenergic receptor in rat whole brain membrane | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048610 (CHEMBL435034 | Carbazol-9-yl-propyl-pyridin-4-yl-a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048575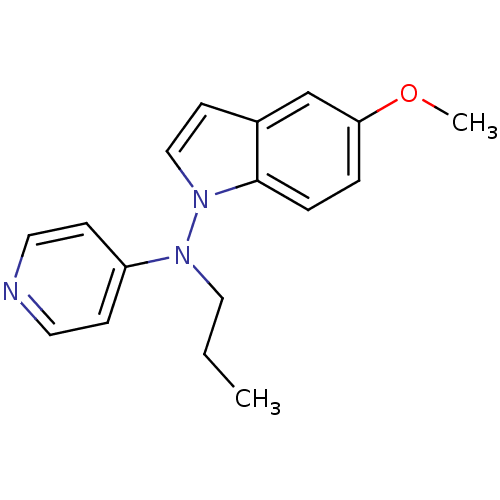 ((5-Methoxy-indol-1-yl)-propyl-pyridin-4-yl-amine |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048576 (Butyl-indol-1-yl-pyridin-4-yl-amine | CHEMBL155109) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048590 (CHEMBL154319 | Indol-1-yl-isopropyl-pyridin-4-yl-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]N-methyl-scopolamine in rat Muscarinic acetylcholine receptor M2 cerebellum in the presence GPP(NH)P | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50454302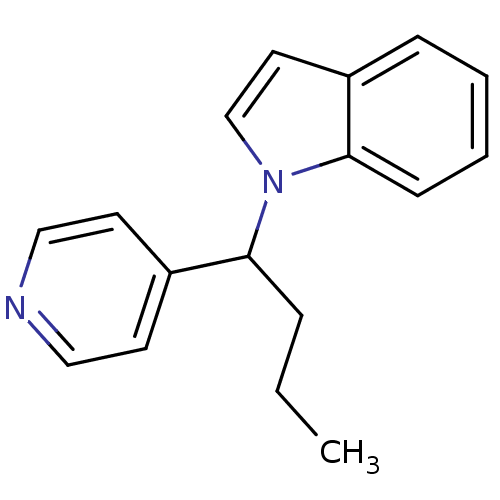 (CHEMBL347199) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048602 (CHEMBL348034 | Indol-1-yl-prop-2-ynyl-pyridin-4-yl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048611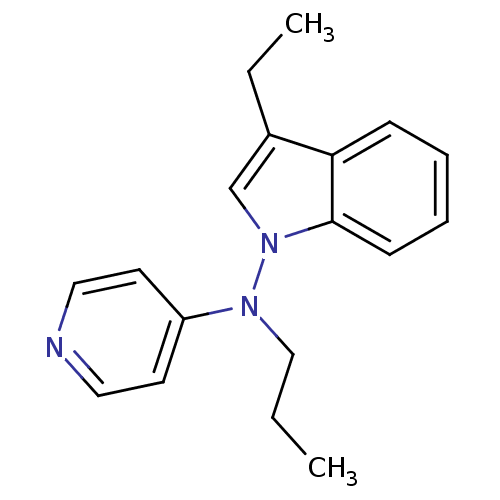 ((3-Ethyl-indol-1-yl)-propyl-pyridin-4-yl-amine | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048583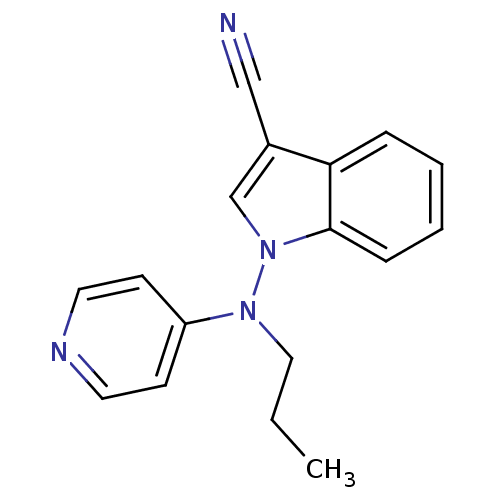 (1-(Propyl-pyridin-4-yl-amino)-1H-indole-3-carbonit...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9381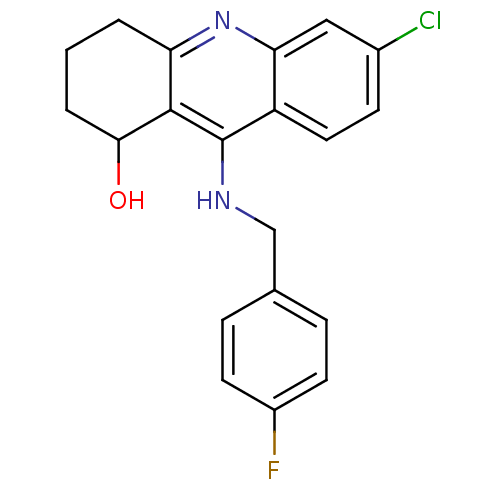 (6-chloro-9-{[(4-fluorophenyl)methyl]amino}-1,2,3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 823 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. | J Med Chem 32: 1805-13 (1989) Article DOI: 10.1021/jm00128a024 BindingDB Entry DOI: 10.7270/Q24Q7S6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048574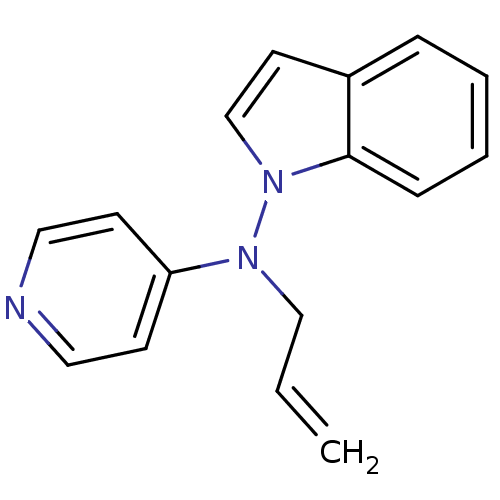 (Allyl-indol-1-yl-pyridin-4-yl-amine | CHEMBL347336) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-DHM binding to mu opioid receptor in rat whole brain | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against [3H]-N-methyl-scopalamine binding to Muscarinic acetylcholine receptor M2 in rat cerebellum | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048589 (CHEMBL152842 | Indol-1-yl-methyl-pyridin-4-yl-amin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048573 ((2-Methyl-indol-1-yl)-propyl-pyridin-4-yl-amine | ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50048589 (CHEMBL152842 | Indol-1-yl-methyl-pyridin-4-yl-amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]WB-4101 binding to Alpha-1 adrenergic receptor in rat whole brain membrane | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9380 (9-(benzylamino)-6-chloro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. | J Med Chem 32: 1805-13 (1989) Article DOI: 10.1021/jm00128a024 BindingDB Entry DOI: 10.7270/Q24Q7S6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]-pirenzepine binding against Muscarinic acetylcholine receptor M1 from rat cortical membranes using in vitr... | J Med Chem 39: 582-7 (1996) Article DOI: 10.1021/jm950644v BindingDB Entry DOI: 10.7270/Q27080HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against [3H]-pirenzepine binding to muscarinic M1 receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048587 ((3-Methyl-indol-1-yl)-propyl-pyridin-4-yl-amine | ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048599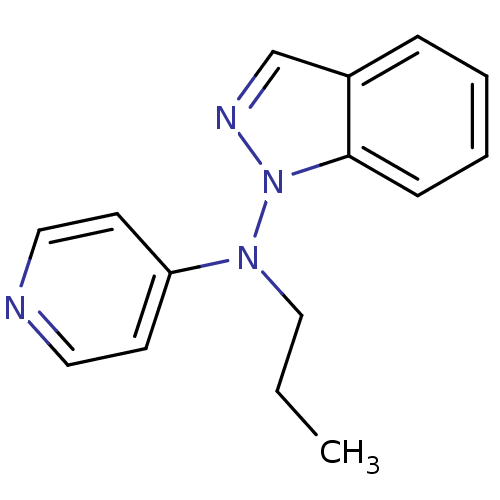 (CHEMBL346142 | Indazol-1-yl-propyl-pyridin-4-yl-am...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048579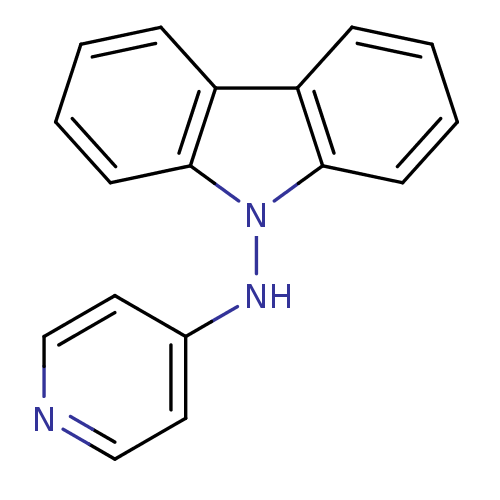 (CHEMBL345607 | Carbazol-9-yl-pyridin-4-yl-amine; c...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048573 ((2-Methyl-indol-1-yl)-propyl-pyridin-4-yl-amine | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50048609 (CHEMBL345251 | Indol-1-yl-propyl-pyrimidin-4-yl-am...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048576 (Butyl-indol-1-yl-pyridin-4-yl-amine | CHEMBL155109) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50048582 ((5-Bromo-indol-1-yl)-propyl-pyridin-4-yl-amine | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9354 (9-(methylamino)-1,2,3,4-tetrahydroacridin-1-ol | 9...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hoechst-Roussel Pharmaceuticals, Inc. | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. | J Med Chem 32: 1805-13 (1989) Article DOI: 10.1021/jm00128a024 BindingDB Entry DOI: 10.7270/Q24Q7S6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 240 total ) | Next | Last >> |