

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50051747 (5-Amino-2-{4-[(2-amino-4-hydroxy-5,6,7,8-tetrahydr...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against mammalian Folyl-polyglutamate synthase | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50060962 (2-Amino-4-[1-({[3-(4-amino-butylamino)-propoxy]-hy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Glutathionylspermidine synthetase | J Med Chem 40: 3842-50 (1997) Article DOI: 10.1021/jm970414b BindingDB Entry DOI: 10.7270/Q2MP53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50060962 (2-Amino-4-[1-({[3-(4-amino-butylamino)-propoxy]-hy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Glutathionylspermidine synthetase from Escherichia coli, value taken from literature | J Med Chem 40: 3842-50 (1997) Article DOI: 10.1021/jm970414b BindingDB Entry DOI: 10.7270/Q2MP53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50060963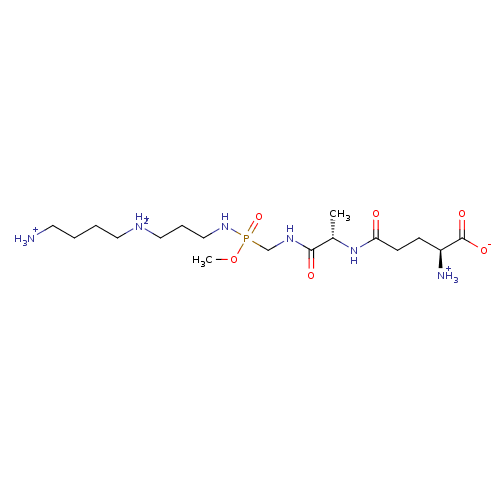 (O-Methyl-N-[3-[N-(4-aminobutyl)amino]propyl][[(glu...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Glutathionylspermidine synthetase. Values from different publications. | J Med Chem 40: 3842-50 (1997) Article DOI: 10.1021/jm970414b BindingDB Entry DOI: 10.7270/Q2MP53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50367029 (CHEMBL553016) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of Catechol O-methyltransferase using radiochemical assay | J Med Chem 24: 1271-7 (1982) BindingDB Entry DOI: 10.7270/Q26D5TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50367030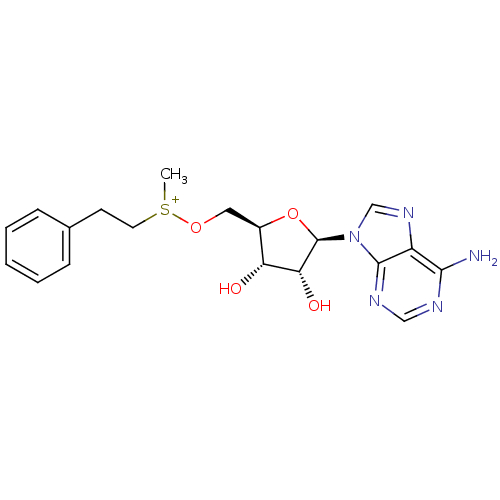 (CHEMBL539878) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of Catechol O-methyltransferase using radiochemical assay | J Med Chem 24: 1271-7 (1982) BindingDB Entry DOI: 10.7270/Q26D5TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50367031 (CHEMBL557014) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of Catechol O-methyltransferase using radiochemical assay | J Med Chem 24: 1271-7 (1982) BindingDB Entry DOI: 10.7270/Q26D5TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM22111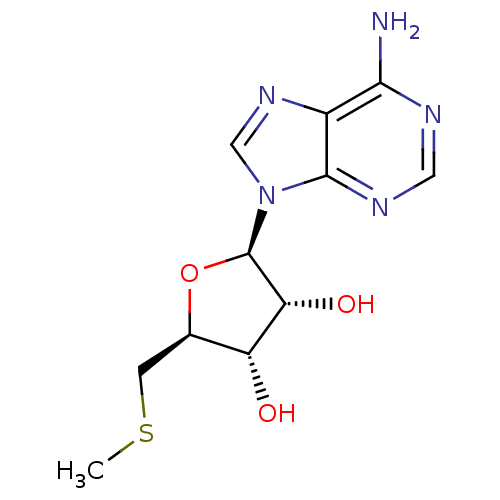 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | >5.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of Catechol O-methyltransferase using radiochemical assay | J Med Chem 24: 1271-7 (1982) BindingDB Entry DOI: 10.7270/Q26D5TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50367028 (CHEMBL553200) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of Catechol O-methyltransferase using radiochemical assay | J Med Chem 24: 1271-7 (1982) BindingDB Entry DOI: 10.7270/Q26D5TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50366942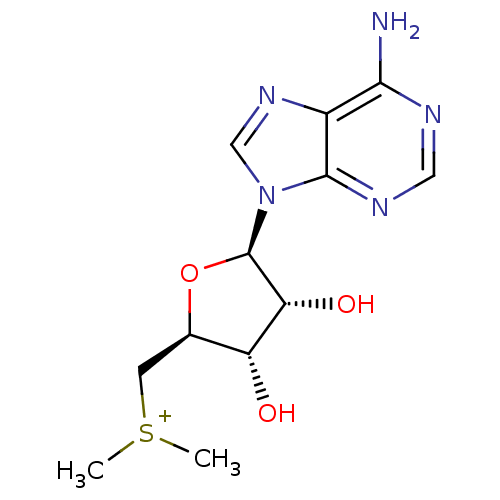 (CHEMBL540135) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of Catechol O-methyltransferase using radiochemical assay | J Med Chem 24: 1271-7 (1982) BindingDB Entry DOI: 10.7270/Q26D5TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against dihydrofolate reductase (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049156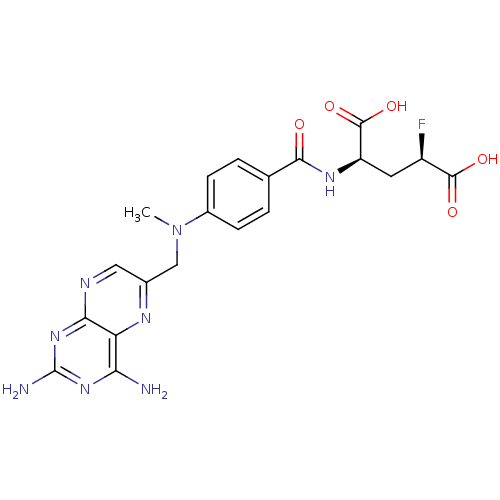 ((2R,4R)-2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 1 | J Med Chem 39: 56-65 (1996) Article DOI: 10.1021/jm950515e BindingDB Entry DOI: 10.7270/Q2Q81DR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50011886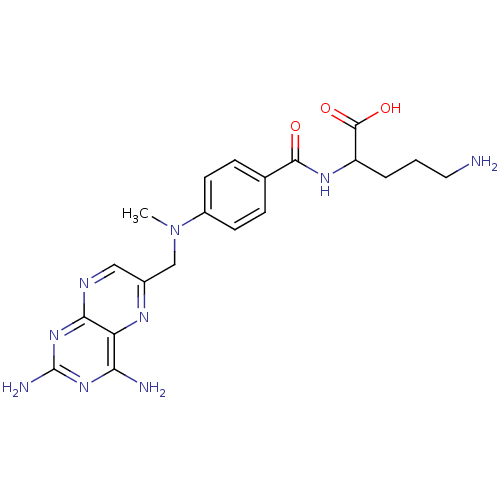 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. value mentioned is from li... | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 1 | J Med Chem 39: 56-65 (1996) Article DOI: 10.1021/jm950515e BindingDB Entry DOI: 10.7270/Q2Q81DR3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50051746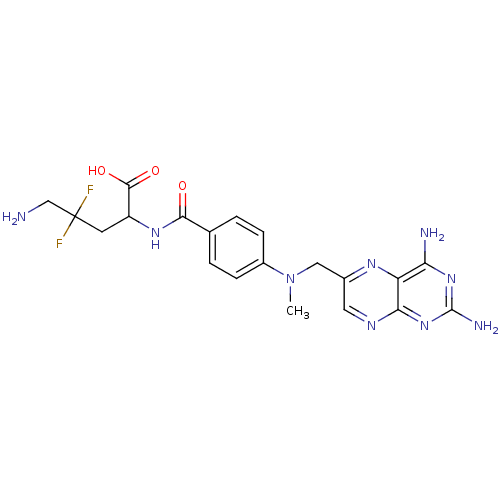 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049154 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 2 | J Med Chem 39: 56-65 (1996) Article DOI: 10.1021/jm950515e BindingDB Entry DOI: 10.7270/Q2Q81DR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 2 | J Med Chem 39: 56-65 (1996) Article DOI: 10.1021/jm950515e BindingDB Entry DOI: 10.7270/Q2Q81DR3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049155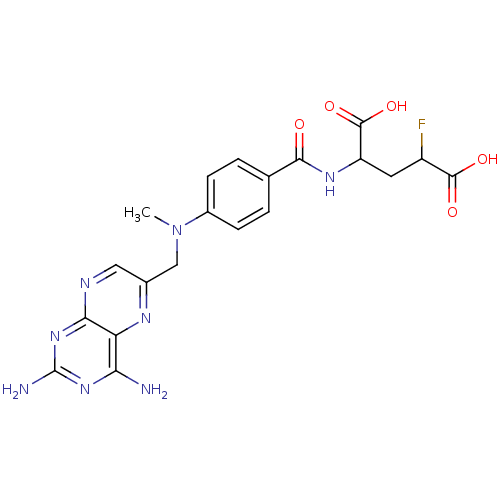 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 1 | J Med Chem 39: 56-65 (1996) Article DOI: 10.1021/jm950515e BindingDB Entry DOI: 10.7270/Q2Q81DR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 2 | J Med Chem 39: 56-65 (1996) Article DOI: 10.1021/jm950515e BindingDB Entry DOI: 10.7270/Q2Q81DR3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50011886 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116251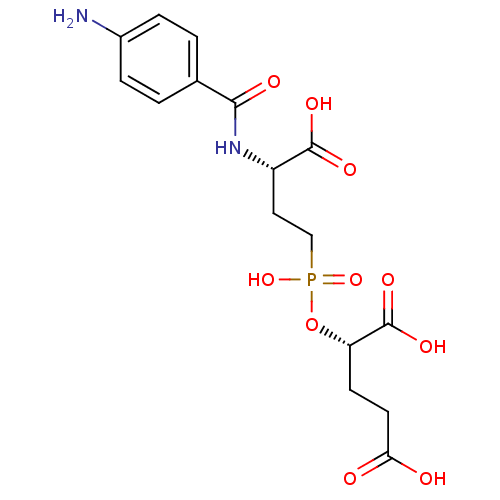 (2-{[3-(4-Amino-benzoylamino)-3-carboxy-propyl]-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Homo sapiens (Human)) | BDBM50369035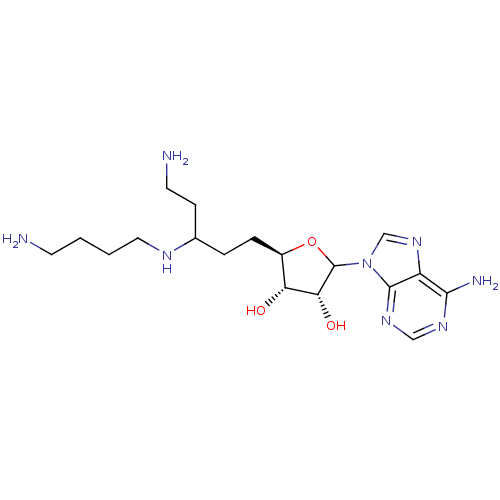 (CHEMBL608868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of prokaryotic Escherichia coli Putrescine aminopropyltransferase was determined | J Med Chem 38: 2714-27 (1995) BindingDB Entry DOI: 10.7270/Q23T9HW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116250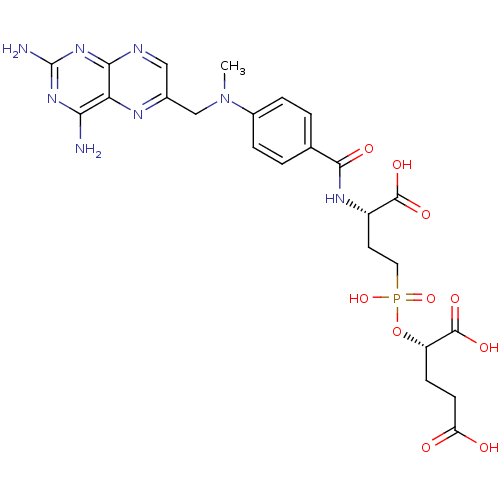 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116253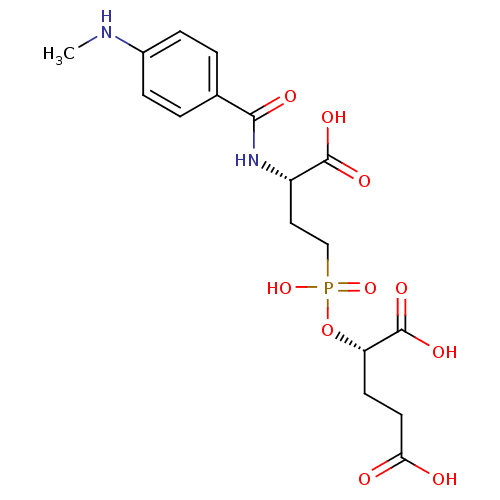 (2-{[3-Carboxy-3-(4-methylamino-benzoylamino)-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116252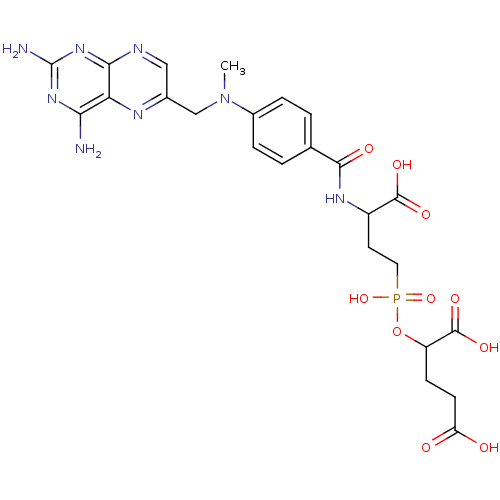 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Homo sapiens (Human)) | BDBM50369035 (CHEMBL608868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Putrescine aminopropyl transferase (PAPT) in rat liver | J Med Chem 38: 2714-27 (1995) BindingDB Entry DOI: 10.7270/Q23T9HW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50116252 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Folylpoly-gamma-glutamyl synthetase | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50095449 (2-[1-(2-Amino-ethyl)-6-(3-amino-propylamino)-hexyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Spermidine aminopropyltransferase (SAPT) in rat liver. | J Med Chem 38: 2714-27 (1995) BindingDB Entry DOI: 10.7270/Q23T9HW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50095450 (2-[6-Amino-1-(2-amino-ethyl)-hexylsulfanylmethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Putrescine aminopropyl transferase (PAPT) in rat liver | J Med Chem 38: 2714-27 (1995) BindingDB Entry DOI: 10.7270/Q23T9HW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50116250 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Folylpoly-gamma-glutamyl synthetase | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Homo sapiens (Human)) | BDBM50095450 (2-[6-Amino-1-(2-amino-ethyl)-hexylsulfanylmethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of prokaryotic Escherichia coli Putrescine aminopropyltransferase was determined | J Med Chem 38: 2714-27 (1995) BindingDB Entry DOI: 10.7270/Q23T9HW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50095450 (2-[6-Amino-1-(2-amino-ethyl)-hexylsulfanylmethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against spermidine synthase | J Med Chem 24: 1277-84 (1982) BindingDB Entry DOI: 10.7270/Q2XP763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against purified human dihydrofolate reductase (DHFR) in human leukemia cells (CCRF-CEM) | J Med Chem 39: 66-72 (1996) Article DOI: 10.1021/jm950514m BindingDB Entry DOI: 10.7270/Q21835KH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50369035 (CHEMBL608868) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Spermidine aminopropyltransferase (SAPT) in rat liver. | J Med Chem 38: 2714-27 (1995) BindingDB Entry DOI: 10.7270/Q23T9HW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049157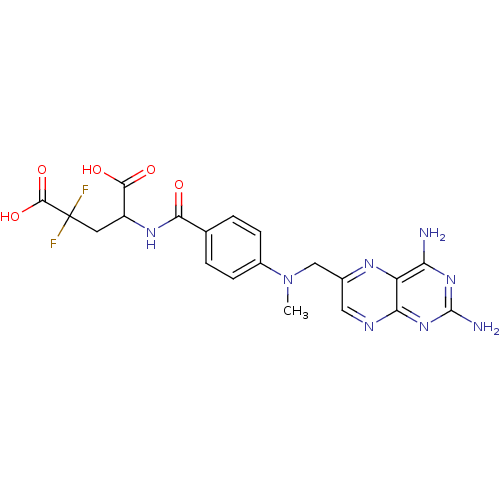 (4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against purified human dihydrofolate reductase (DHFR) in human leukemia cells (CCRF-CEM) | J Med Chem 39: 66-72 (1996) Article DOI: 10.1021/jm950514m BindingDB Entry DOI: 10.7270/Q21835KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50116253 (2-{[3-Carboxy-3-(4-methylamino-benzoylamino)-propy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Folylpoly-gamma-glutamyl synthetase | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50116251 (2-{[3-(4-Amino-benzoylamino)-3-carboxy-propyl]-hyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Folylpoly-gamma-glutamyl synthetase | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50095449 (2-[1-(2-Amino-ethyl)-6-(3-amino-propylamino)-hexyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Putrescine aminopropyltransferase (PAPT) in rat liver. | J Med Chem 38: 2714-27 (1995) BindingDB Entry DOI: 10.7270/Q23T9HW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50404650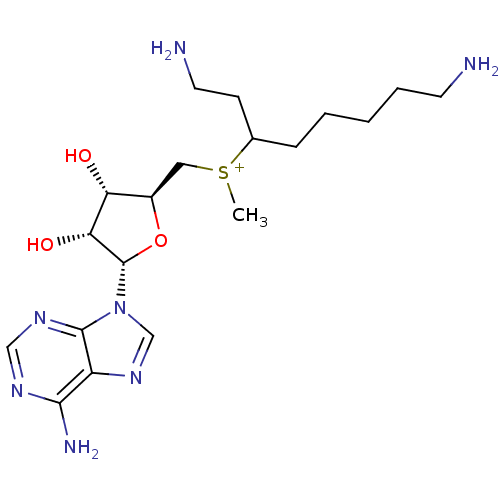 (CHEMBL557606) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against spermidine synthase | J Med Chem 24: 1277-84 (1982) BindingDB Entry DOI: 10.7270/Q2XP763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50011886 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against human Folyl-polyglutamate synthase isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50404650 (CHEMBL557606) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against spermine synthase | J Med Chem 24: 1277-84 (1982) BindingDB Entry DOI: 10.7270/Q2XP763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50095450 (2-[6-Amino-1-(2-amino-ethyl)-hexylsulfanylmethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Spermidine aminopropyltransferase (SAPT) in rat liver. | J Med Chem 38: 2714-27 (1995) BindingDB Entry DOI: 10.7270/Q23T9HW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Rattus norvegicus) | BDBM50404649 (CHEMBL178068) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against spermidine synthase | J Med Chem 24: 1277-84 (1982) BindingDB Entry DOI: 10.7270/Q2XP763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50051746 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against human Folyl-polyglutamate synthase isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||