 Found 172 hits with Last Name = 'craig' and Initial = 'd'
Found 172 hits with Last Name = 'craig' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29098
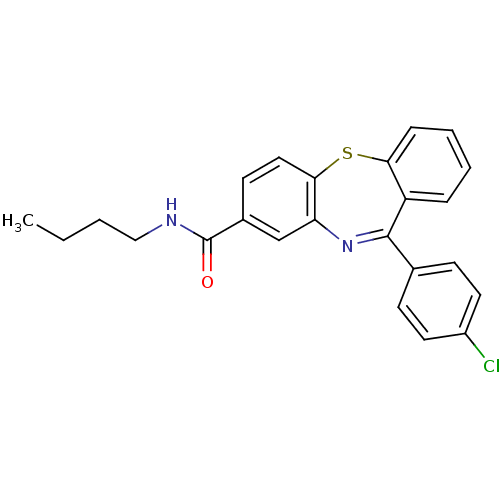
(dibenzothiazepine, 12e)Show SMILES CCCCNC(=O)c1ccc2Sc3ccccc3C(=Nc2c1)c1ccc(Cl)cc1 |c:19| Show InChI InChI=1S/C24H21ClN2OS/c1-2-3-14-26-24(28)17-10-13-22-20(15-17)27-23(16-8-11-18(25)12-9-16)19-6-4-5-7-21(19)29-22/h4-13,15H,2-3,14H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29100

(dibenzothiazepine, 12h)Show SMILES CCCCNC(=O)c1ccc2Sc3ccccc3C(=Nc2c1)c1ccc(F)c(Cl)c1 |c:19| Show InChI InChI=1S/C24H20ClFN2OS/c1-2-3-12-27-24(29)16-9-11-22-20(14-16)28-23(15-8-10-19(26)18(25)13-15)17-6-4-5-7-21(17)30-22/h4-11,13-14H,2-3,12H2,1H3,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50152456
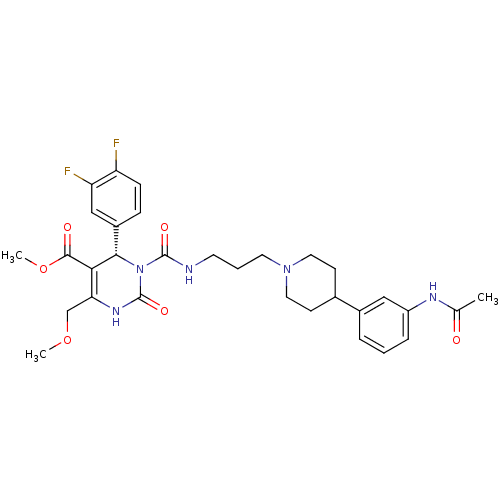
((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...)Show SMILES COCC1=C([C@@H](N(C(=O)NCCCN2CCC(CC2)c2cccc(NC(C)=O)c2)C(=O)N1)c1ccc(F)c(F)c1)C(=O)OC |t:3| Show InChI InChI=1S/C31H37F2N5O6/c1-19(39)35-23-7-4-6-21(16-23)20-10-14-37(15-11-20)13-5-12-34-30(41)38-28(22-8-9-24(32)25(33)17-22)27(29(40)44-3)26(18-43-2)36-31(38)42/h4,6-9,16-17,20,28H,5,10-15,18H2,1-3H3,(H,34,41)(H,35,39)(H,36,42)/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50152456

((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...)Show SMILES COCC1=C([C@@H](N(C(=O)NCCCN2CCC(CC2)c2cccc(NC(C)=O)c2)C(=O)N1)c1ccc(F)c(F)c1)C(=O)OC |t:3| Show InChI InChI=1S/C31H37F2N5O6/c1-19(39)35-23-7-4-6-21(16-23)20-10-14-37(15-11-20)13-5-12-34-30(41)38-28(22-8-9-24(32)25(33)17-22)27(29(40)44-3)26(18-43-2)36-31(38)42/h4,6-9,16-17,20,28H,5,10-15,18H2,1-3H3,(H,34,41)(H,35,39)(H,36,42)/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29096
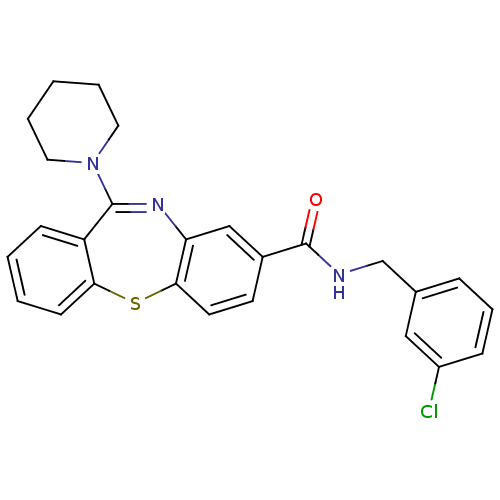
(dibenzothiazepine, 12b)Show SMILES Clc1cccc(CNC(=O)c2ccc3Sc4ccccc4C(=Nc3c2)N2CCCCC2)c1 |c:22| Show InChI InChI=1S/C26H24ClN3OS/c27-20-8-6-7-18(15-20)17-28-26(31)19-11-12-24-22(16-19)29-25(30-13-4-1-5-14-30)21-9-2-3-10-23(21)32-24/h2-3,6-12,15-16H,1,4-5,13-14,17H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50026917
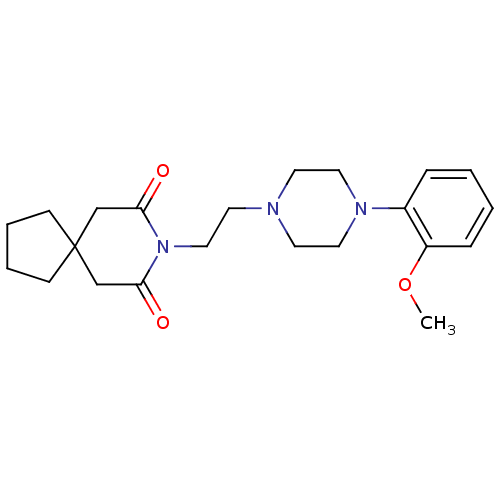
(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219036

(CHEMBL388440 | N-{3-[1-(3-{[hydroxy(diphenyl)acety...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)C(O)(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C33H41N3O3/c1-24(2)31(37)35-29-16-15-25(3)30(23-29)26-17-21-36(22-18-26)20-10-19-34-32(38)33(39,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-16,23-24,26,39H,10,17-22H2,1-3H3,(H,34,38)(H,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219040

(CHEMBL429464 | N-{3-[1-(3-{[bis(4-fluorophenyl)ace...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)C(c2ccc(F)cc2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C33H39F2N3O2/c1-22(2)32(39)37-29-14-5-23(3)30(21-29)24-15-19-38(20-16-24)18-4-17-36-33(40)31(25-6-10-27(34)11-7-25)26-8-12-28(35)13-9-26/h5-14,21-22,24,31H,4,15-20H2,1-3H3,(H,36,40)(H,37,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29097
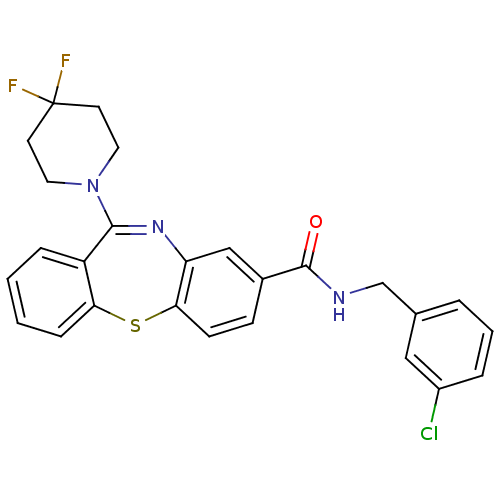
(dibenzothiazepine, 12c)Show SMILES FC1(F)CCN(CC1)C1=Nc2cc(ccc2Sc2ccccc12)C(=O)NCc1cccc(Cl)c1 |t:9| Show InChI InChI=1S/C26H22ClF2N3OS/c27-19-5-3-4-17(14-19)16-30-25(33)18-8-9-23-21(15-18)31-24(20-6-1-2-7-22(20)34-23)32-12-10-26(28,29)11-13-32/h1-9,14-15H,10-13,16H2,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.790 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50143674

(8-{2-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-ethyl}-8...)Show SMILES Fc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H28FN3O2/c22-17-5-1-2-6-18(17)24-12-9-23(10-13-24)11-14-25-19(26)15-21(16-20(25)27)7-3-4-8-21/h1-2,5-6H,3-4,7-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50143664
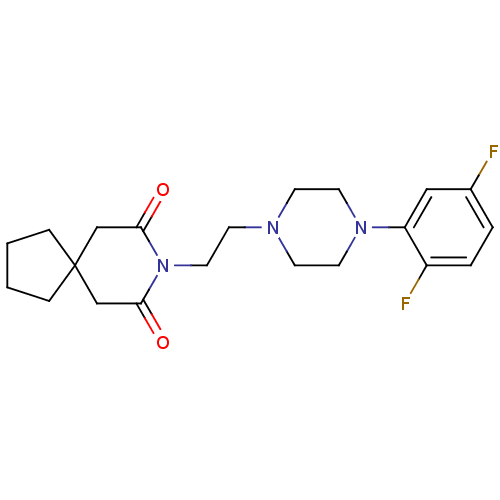
(8-{2-[4-(2,5-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(F)c(c1)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-17(23)18(13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29101
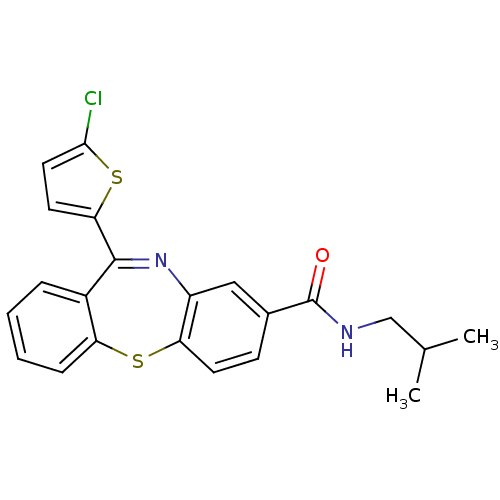
(dibenzothiazepine, 12j)Show SMILES CC(C)CNC(=O)c1ccc2Sc3ccccc3C(=Nc2c1)c1ccc(Cl)s1 |c:19| Show InChI InChI=1S/C22H19ClN2OS2/c1-13(2)12-24-22(26)14-7-8-18-16(11-14)25-21(19-9-10-20(23)28-19)15-5-3-4-6-17(15)27-18/h3-11,13H,12H2,1-2H3,(H,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50122803

((SNAP-8719)[4-(4-Amino-6,7-dimethoxy-quinazolin-2-...)Show SMILES C[C@H](CN1CCN(CC1)c1cc(F)c(F)cc1F)N1C(=O)CC2(CCCC2)CC1=O Show InChI InChI=1S/C22H28F3N3O2/c1-15(28-20(29)12-22(13-21(28)30)4-2-3-5-22)14-26-6-8-27(9-7-26)19-11-17(24)16(23)10-18(19)25/h10-11,15H,2-9,12-14H2,1H3/t15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219026
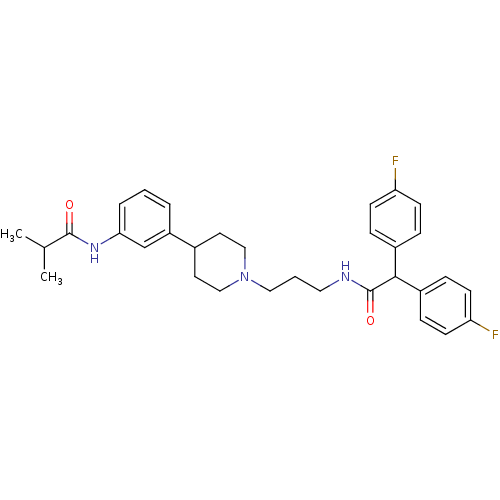
(CHEMBL243338 | N-{3-[1-(3-{[bis(4-fluorophenyl)ace...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCCNC(=O)C(c2ccc(F)cc2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C32H37F2N3O2/c1-22(2)31(38)36-29-6-3-5-26(21-29)23-15-19-37(20-16-23)18-4-17-35-32(39)30(24-7-11-27(33)12-8-24)25-9-13-28(34)14-10-25/h3,5-14,21-23,30H,4,15-20H2,1-2H3,(H,35,39)(H,36,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50026917

(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29102
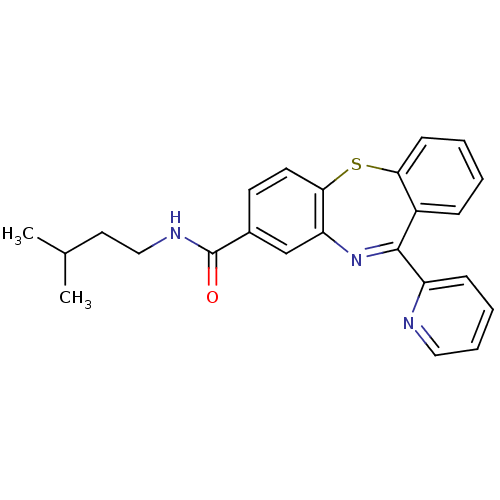
(dibenzothiazepine, 12k)Show SMILES CC(C)CCNC(=O)c1ccc2Sc3ccccc3C(=Nc2c1)c1ccccn1 |c:20| Show InChI InChI=1S/C24H23N3OS/c1-16(2)12-14-26-24(28)17-10-11-22-20(15-17)27-23(19-8-5-6-13-25-19)18-7-3-4-9-21(18)29-22/h3-11,13,15-16H,12,14H2,1-2H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219035

(CHEMBL243355 | N-{5-[1-(3-{[bis(4-fluorophenyl)ace...)Show SMILES CC(C)C(=O)Nc1cc(C2CCN(CCCNC(=O)C(c3ccc(F)cc3)c3ccc(F)cc3)CC2)c(F)cc1F Show InChI InChI=1S/C32H35F4N3O2/c1-20(2)31(40)38-29-18-26(27(35)19-28(29)36)21-12-16-39(17-13-21)15-3-14-37-32(41)30(22-4-8-24(33)9-5-22)23-6-10-25(34)11-7-23/h4-11,18-21,30H,3,12-17H2,1-2H3,(H,37,41)(H,38,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219047
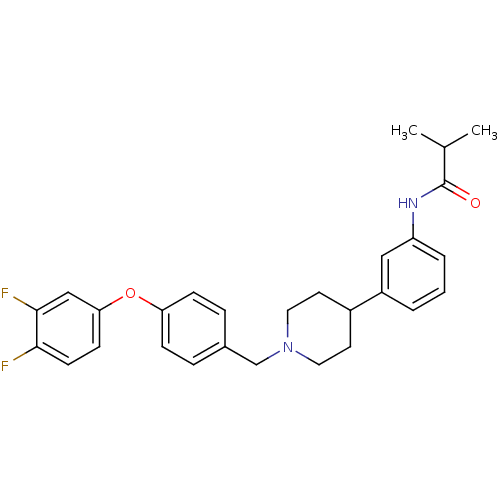
(CHEMBL245231 | N-(3-(1-(4-(3,4-difluorophenoxy)ben...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(Cc2ccc(Oc3ccc(F)c(F)c3)cc2)CC1 Show InChI InChI=1S/C28H30F2N2O2/c1-19(2)28(33)31-23-5-3-4-22(16-23)21-12-14-32(15-13-21)18-20-6-8-24(9-7-20)34-25-10-11-26(29)27(30)17-25/h3-11,16-17,19,21H,12-15,18H2,1-2H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219037
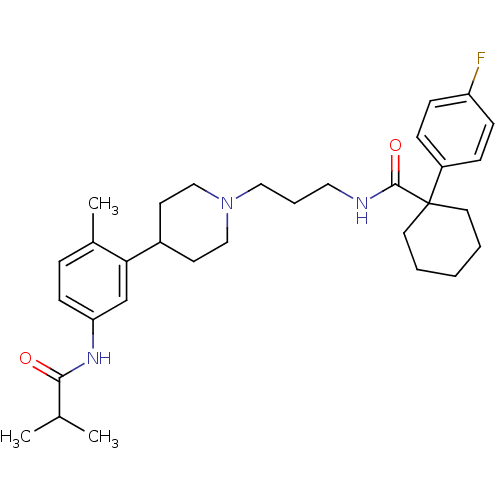
(1-(4-fluorophenyl)-N-(3-{4-[5-(isobutyrylamino)-2-...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)C2(CCCCC2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C32H44FN3O2/c1-23(2)30(37)35-28-13-8-24(3)29(22-28)25-14-20-36(21-15-25)19-7-18-34-31(38)32(16-5-4-6-17-32)26-9-11-27(33)12-10-26/h8-13,22-23,25H,4-7,14-21H2,1-3H3,(H,34,38)(H,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219023

(CHEMBL243138 | N-{3-[1-(3-diphenylacetylamino-prop...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCCNC(=O)C(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C32H39N3O2/c1-24(2)31(36)34-29-16-9-15-28(23-29)25-17-21-35(22-18-25)20-10-19-33-32(37)30(26-11-5-3-6-12-26)27-13-7-4-8-14-27/h3-9,11-16,23-25,30H,10,17-22H2,1-2H3,(H,33,37)(H,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219029

(CHEMBL390679 | N-{3-[1-(2-diphenylacetylamino-ethy...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCNC(=O)C(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C31H37N3O2/c1-23(2)30(35)33-28-15-9-14-27(22-28)24-16-19-34(20-17-24)21-18-32-31(36)29(25-10-5-3-6-11-25)26-12-7-4-8-13-26/h3-15,22-24,29H,16-21H2,1-2H3,(H,32,36)(H,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50165417
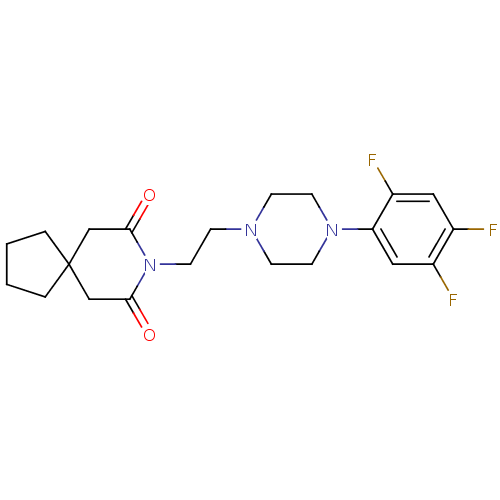
(8-{2-[4-(2,4,5-Trifluoro-phenyl)-piperazin-1-yl]-e...)Show SMILES Fc1cc(F)c(cc1F)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H26F3N3O2/c22-15-11-17(24)18(12-16(15)23)26-8-5-25(6-9-26)7-10-27-19(28)13-21(14-20(27)29)3-1-2-4-21/h11-12H,1-10,13-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219053
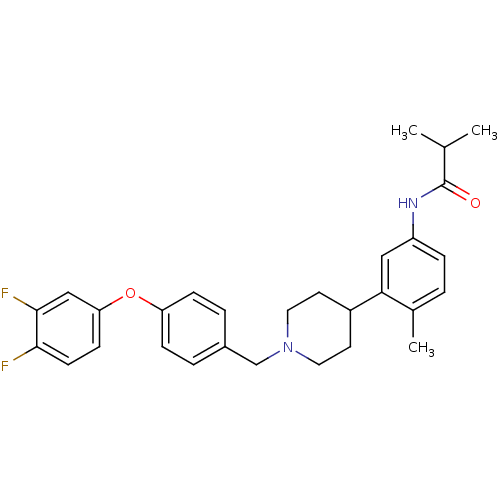
(CHEMBL242004 | N-(3-{1-4-(3,4-difluorophenoxy)benz...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(Cc2ccc(Oc3ccc(F)c(F)c3)cc2)CC1 Show InChI InChI=1S/C29H32F2N2O2/c1-19(2)29(34)32-23-7-4-20(3)26(16-23)22-12-14-33(15-13-22)18-21-5-8-24(9-6-21)35-25-10-11-27(30)28(31)17-25/h4-11,16-17,19,22H,12-15,18H2,1-3H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219044
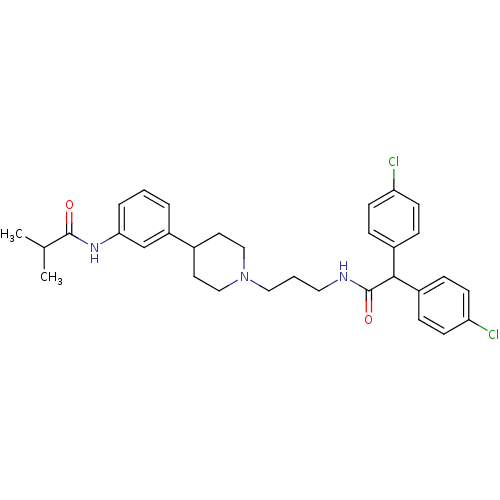
(CHEMBL243128 | N-{3-[1-(3-{[bis(4-chlorophenyl)ace...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCCNC(=O)C(c2ccc(Cl)cc2)c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C32H37Cl2N3O2/c1-22(2)31(38)36-29-6-3-5-26(21-29)23-15-19-37(20-16-23)18-4-17-35-32(39)30(24-7-11-27(33)12-8-24)25-9-13-28(34)14-10-25/h3,5-14,21-23,30H,4,15-20H2,1-2H3,(H,35,39)(H,36,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219025
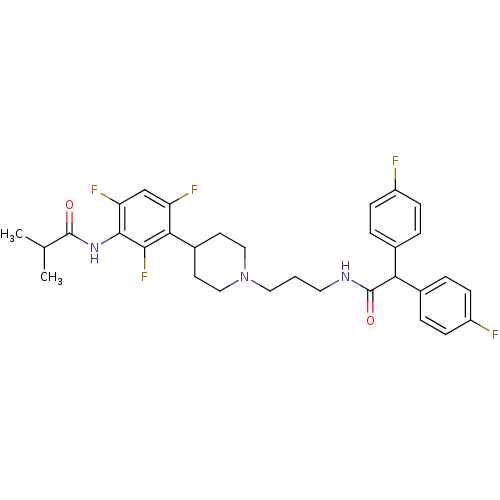
(CHEMBL243578 | N-{3-[1-(3-{[bis(4-fluorophenyl)ace...)Show SMILES CC(C)C(=O)Nc1c(F)cc(F)c(C2CCN(CCCNC(=O)C(c3ccc(F)cc3)c3ccc(F)cc3)CC2)c1F Show InChI InChI=1S/C32H34F5N3O2/c1-19(2)31(41)39-30-26(36)18-25(35)28(29(30)37)22-12-16-40(17-13-22)15-3-14-38-32(42)27(20-4-8-23(33)9-5-20)21-6-10-24(34)11-7-21/h4-11,18-19,22,27H,3,12-17H2,1-2H3,(H,38,42)(H,39,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219042
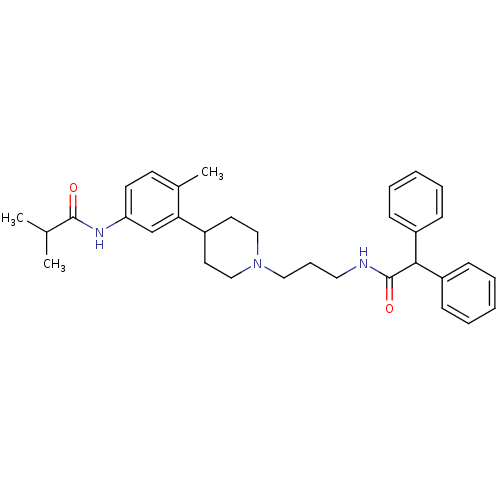
(CHEMBL242689 | N-[3-(1-{3-[(diphenylacetyl)amino]p...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)C(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C33H41N3O2/c1-24(2)32(37)35-29-16-15-25(3)30(23-29)26-17-21-36(22-18-26)20-10-19-34-33(38)31(27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-16,23-24,26,31H,10,17-22H2,1-3H3,(H,34,38)(H,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29095
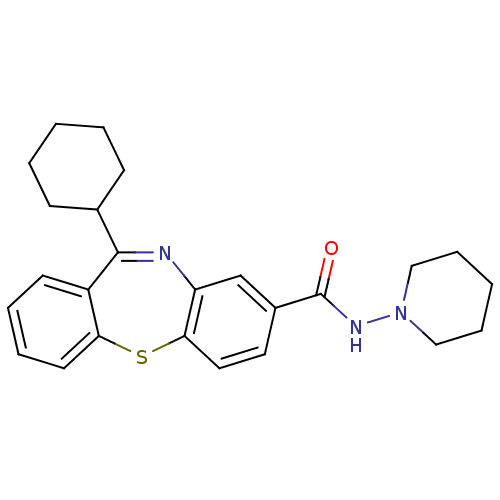
(dibenzothiazepine, 12a)Show SMILES O=C(NN1CCCCC1)c1ccc2Sc3ccccc3C(=Nc2c1)C1CCCCC1 |c:22| Show InChI InChI=1S/C25H29N3OS/c29-25(27-28-15-7-2-8-16-28)19-13-14-23-21(17-19)26-24(18-9-3-1-4-10-18)20-11-5-6-12-22(20)30-23/h5-6,11-14,17-18H,1-4,7-10,15-16H2,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29094
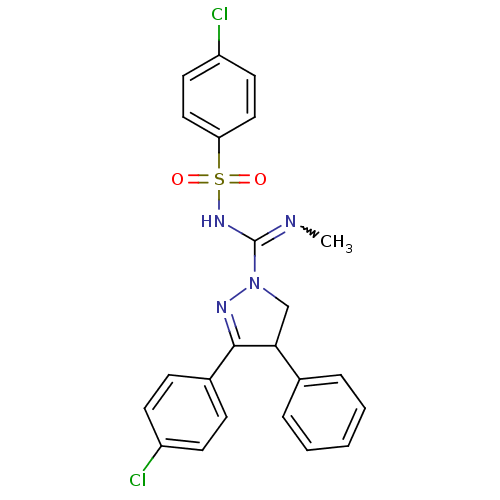
((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...)Show SMILES CN=C(NS(=O)(=O)c1ccc(Cl)cc1)N1CC(C(=N1)c1ccc(Cl)cc1)c1ccccc1 |w:1.0,c:18| Show InChI InChI=1S/C23H20Cl2N4O2S/c1-26-23(28-32(30,31)20-13-11-19(25)12-14-20)29-15-21(16-5-3-2-4-6-16)22(27-29)17-7-9-18(24)10-8-17/h2-14,21H,15H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50026917

(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat Dopamine receptor D3 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219031
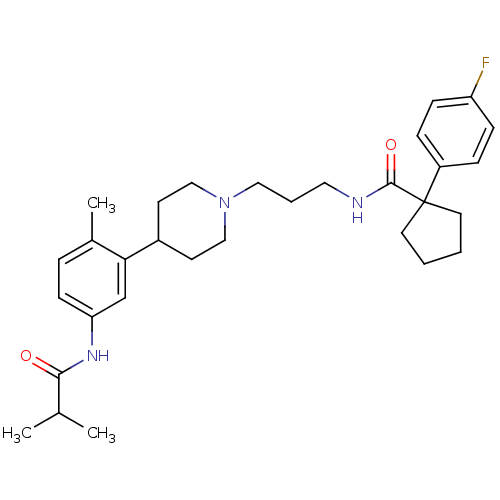
(1-(4-fluorophenyl)-N-(3-{4-[5-(isobutylamino)-2-me...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)C2(CCCC2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C31H42FN3O2/c1-22(2)29(36)34-27-12-7-23(3)28(21-27)24-13-19-35(20-14-24)18-6-17-33-30(37)31(15-4-5-16-31)25-8-10-26(32)11-9-25/h7-12,21-22,24H,4-6,13-20H2,1-3H3,(H,33,37)(H,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219062

(CHEMBL394569 | N-(3-{1-[4-(4-chlorophenyl)-4-oxobu...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCCC(=O)c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C25H31ClN2O2/c1-18(2)25(30)27-23-6-3-5-21(17-23)19-12-15-28(16-13-19)14-4-7-24(29)20-8-10-22(26)11-9-20/h3,5-6,8-11,17-19H,4,7,12-16H2,1-2H3,(H,27,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50143674

(8-{2-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-ethyl}-8...)Show SMILES Fc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H28FN3O2/c22-17-5-1-2-6-18(17)24-12-9-23(10-13-24)11-14-25-19(26)15-21(16-20(25)27)7-3-4-8-21/h1-2,5-6H,3-4,7-16H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat Dopamine receptor D3 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219064
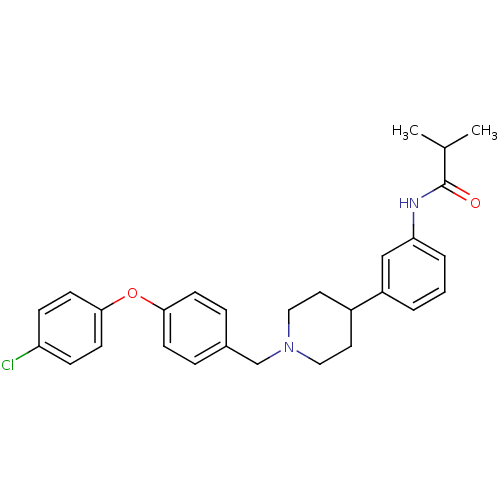
(CHEMBL245010 | N-(3-{1-[4-(4-chlorophenoxy)benzyl]...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(Cc2ccc(Oc3ccc(Cl)cc3)cc2)CC1 Show InChI InChI=1S/C28H31ClN2O2/c1-20(2)28(32)30-25-5-3-4-23(18-25)22-14-16-31(17-15-22)19-21-6-10-26(11-7-21)33-27-12-8-24(29)9-13-27/h3-13,18,20,22H,14-17,19H2,1-2H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50165418
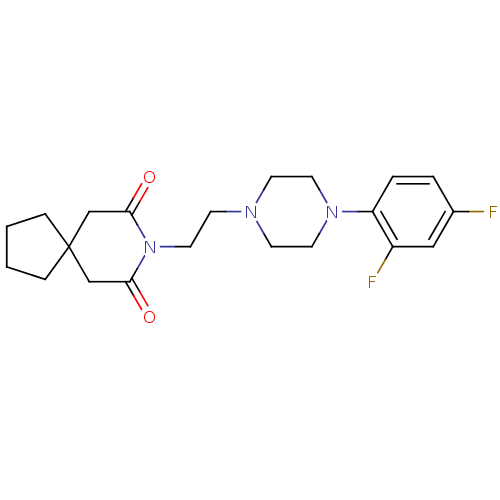
(8-{2-[4-(2,4-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(N2CCN(CCN3C(=O)CC4(CCCC4)CC3=O)CC2)c(F)c1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-18(17(23)13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219032
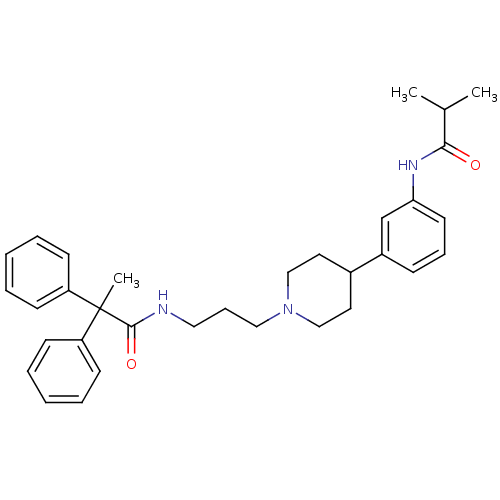
(CHEMBL242902 | N-(3-{4-[3-(isobutyrylamino)phenyl]...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCCNC(=O)C(C)(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C33H41N3O2/c1-25(2)31(37)35-30-17-10-12-27(24-30)26-18-22-36(23-19-26)21-11-20-34-32(38)33(3,28-13-6-4-7-14-28)29-15-8-5-9-16-29/h4-10,12-17,24-26H,11,18-23H2,1-3H3,(H,34,38)(H,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50143674

(8-{2-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-ethyl}-8...)Show SMILES Fc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H28FN3O2/c22-17-5-1-2-6-18(17)24-12-9-23(10-13-24)11-14-25-19(26)15-21(16-20(25)27)7-3-4-8-21/h1-2,5-6H,3-4,7-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50165419
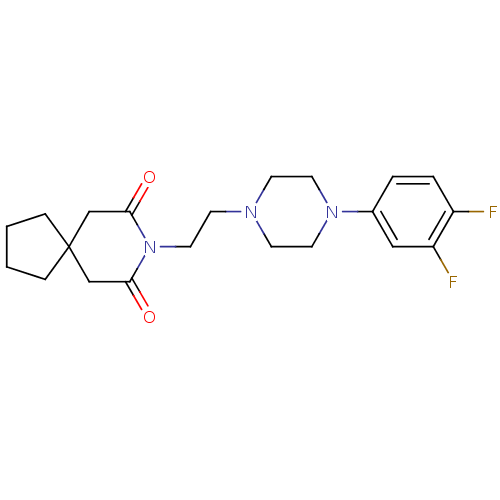
(8-{2-[4-(3,4-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(cc1F)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H27F2N3O2/c22-17-4-3-16(13-18(17)23)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50143664

(8-{2-[4-(2,5-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(F)c(c1)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-17(23)18(13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat Dopamine receptor D3 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219050
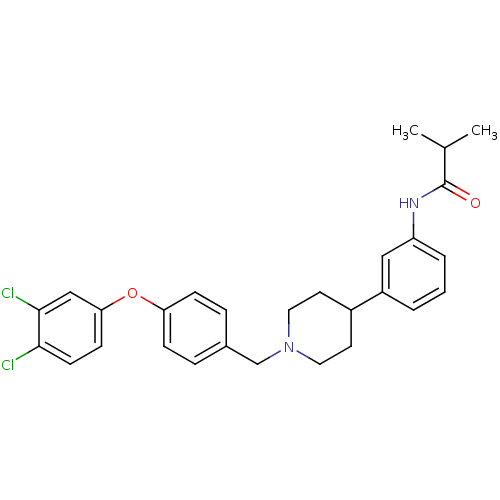
(CHEMBL389129 | N-(3-{1-[4-(3,4-dichlorophenoxy)ben...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(Cc2ccc(Oc3ccc(Cl)c(Cl)c3)cc2)CC1 Show InChI InChI=1S/C28H30Cl2N2O2/c1-19(2)28(33)31-23-5-3-4-22(16-23)21-12-14-32(15-13-21)18-20-6-8-24(9-7-20)34-25-10-11-26(29)27(30)17-25/h3-11,16-17,19,21H,12-15,18H2,1-2H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219038
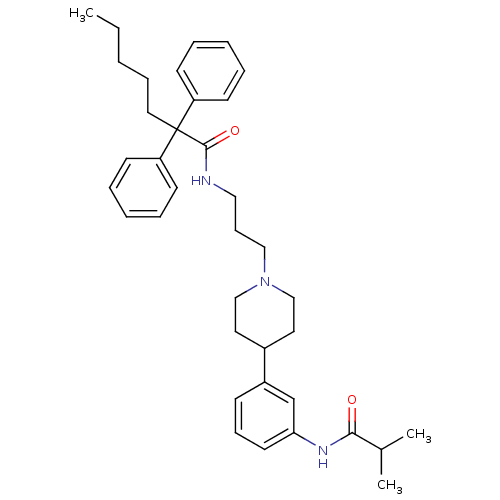
(CHEMBL243507 | N-(3-{4-[3-(isobutylrylamino)phenyl...)Show SMILES CCCCCC(C(=O)NCCCN1CCC(CC1)c1cccc(NC(=O)C(C)C)c1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C37H49N3O2/c1-4-5-12-23-37(32-16-8-6-9-17-32,33-18-10-7-11-19-33)36(42)38-24-14-25-40-26-21-30(22-27-40)31-15-13-20-34(28-31)39-35(41)29(2)3/h6-11,13,15-20,28-30H,4-5,12,14,21-27H2,1-3H3,(H,38,42)(H,39,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219054
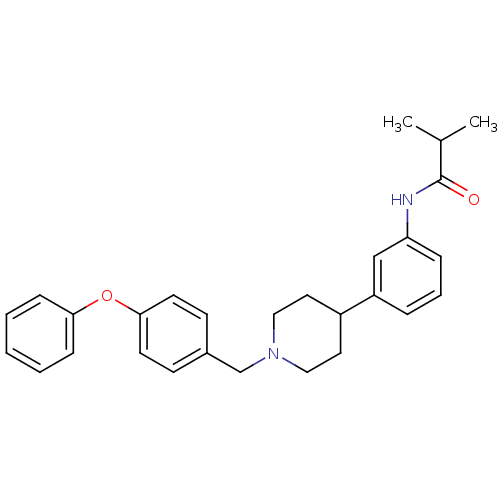
(2-methyl-N-{3-[1-(4-phenoxybenzyl)-4-piperidinyl]p...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C28H32N2O2/c1-21(2)28(31)29-25-8-6-7-24(19-25)23-15-17-30(18-16-23)20-22-11-13-27(14-12-22)32-26-9-4-3-5-10-26/h3-14,19,21,23H,15-18,20H2,1-2H3,(H,29,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219043

((2S)-2-(4-fluorophenyl)-N-(3-{4-[5-(isobutyrylamin...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)[C@@H](C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C28H38FN3O2/c1-19(2)27(33)31-25-11-6-20(3)26(18-25)23-12-16-32(17-13-23)15-5-14-30-28(34)21(4)22-7-9-24(29)10-8-22/h6-11,18-19,21,23H,5,12-17H2,1-4H3,(H,30,34)(H,31,33)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50143661

(8-{2-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-ethyl}-8...)Show SMILES Fc1ccc(cc1)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H28FN3O2/c22-17-3-5-18(6-4-17)24-12-9-23(10-13-24)11-14-25-19(26)15-21(16-20(25)27)7-1-2-8-21/h3-6H,1-2,7-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219041
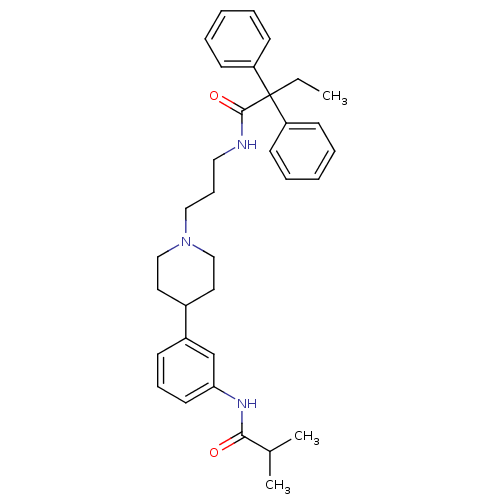
(CHEMBL242901 | N-{3-[4-(3-isobutyrylamino-phenyl)-...)Show SMILES CCC(C(=O)NCCCN1CCC(CC1)c1cccc(NC(=O)C(C)C)c1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C34H43N3O2/c1-4-34(29-14-7-5-8-15-29,30-16-9-6-10-17-30)33(39)35-21-12-22-37-23-19-27(20-24-37)28-13-11-18-31(25-28)36-32(38)26(2)3/h5-11,13-18,25-27H,4,12,19-24H2,1-3H3,(H,35,39)(H,36,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50026917

(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Dopamine receptor D2 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219055
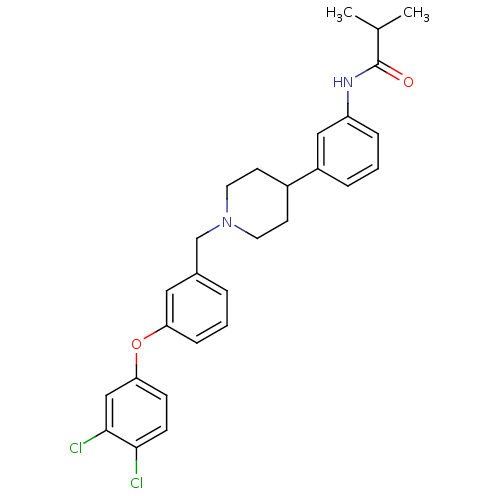
(CHEMBL244791 | N-(3-{1-[3-(3,4-dichlorophenoxy)ben...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(Cc2cccc(Oc3ccc(Cl)c(Cl)c3)c2)CC1 Show InChI InChI=1S/C28H30Cl2N2O2/c1-19(2)28(33)31-23-7-4-6-22(16-23)21-11-13-32(14-12-21)18-20-5-3-8-24(15-20)34-25-9-10-26(29)27(30)17-25/h3-10,15-17,19,21H,11-14,18H2,1-2H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50165417

(8-{2-[4-(2,4,5-Trifluoro-phenyl)-piperazin-1-yl]-e...)Show SMILES Fc1cc(F)c(cc1F)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H26F3N3O2/c22-15-11-17(24)18(12-16(15)23)26-8-5-25(6-9-26)7-10-27-19(28)13-21(14-20(27)29)3-1-2-4-21/h11-12H,1-10,13-14H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat Dopamine receptor D3 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
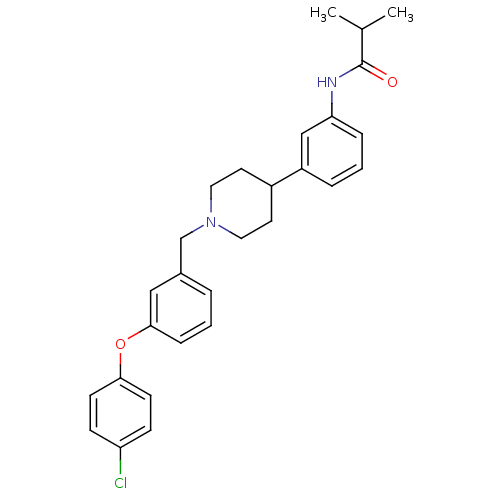
(RAT) | BDBM50219066
(CHEMBL244578 | N-(3-{1-[3-(4-chlorophenoxy)benzyl]...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(Cc2cccc(Oc3ccc(Cl)cc3)c2)CC1 Show InChI InChI=1S/C28H31ClN2O2/c1-20(2)28(32)30-25-7-4-6-23(18-25)22-13-15-31(16-14-22)19-21-5-3-8-27(17-21)33-26-11-9-24(29)10-12-26/h3-12,17-18,20,22H,13-16,19H2,1-2H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50165418

(8-{2-[4-(2,4-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(N2CCN(CCN3C(=O)CC4(CCCC4)CC3=O)CC2)c(F)c1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-18(17(23)13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data