

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity to wild type human COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50491628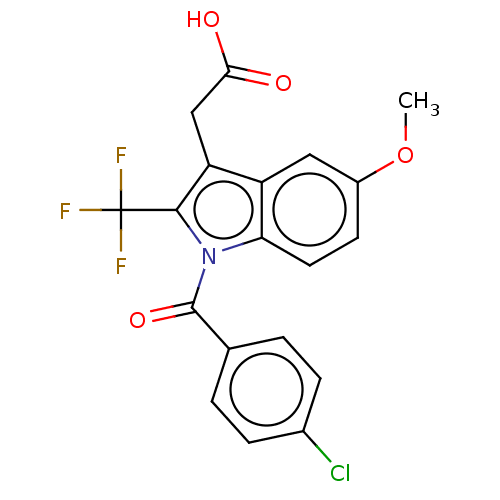 (CHEMBL2386352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity to wild type human COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50561580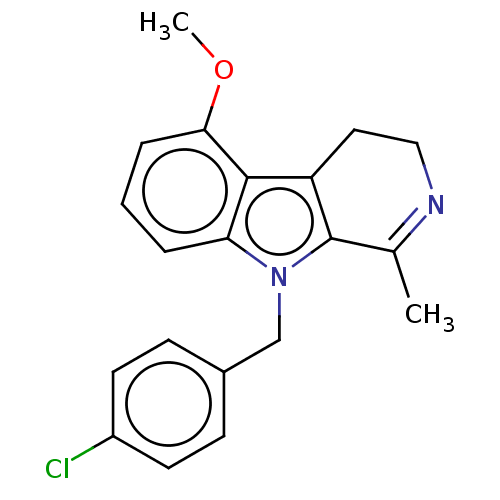 (CHEMBL4777544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse COX2 using 2-arachidonylglycerol as substrate preincubated for 3 mins followed by substrate addition and measured for 30 sec by L... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00555 BindingDB Entry DOI: 10.7270/Q2N58R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type ovine COX1 expressed in ram seminal vesicles using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC fol... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336971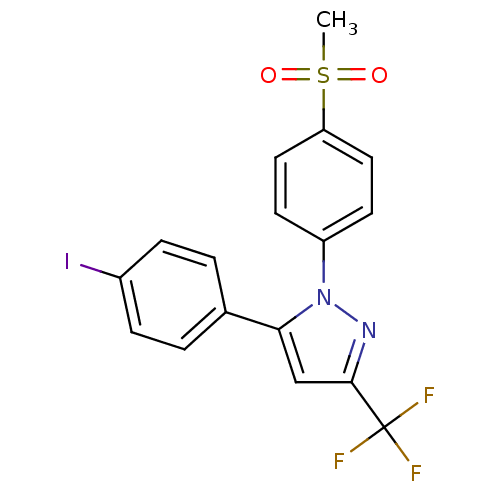 (5-(4-iodophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse LPS-stimulated RAW264.7 cells after 30 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22971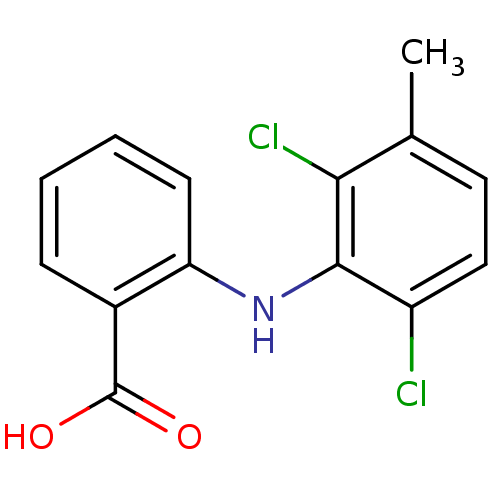 (2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of the human Prostaglandin G/H synthase 2 was determined by thin-layer chromatography assay | Bioorg Med Chem Lett 12: 521-4 (2002) BindingDB Entry DOI: 10.7270/Q2BP0247 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22955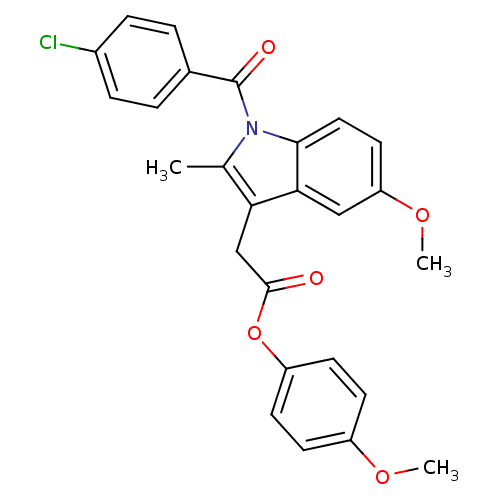 (4-methoxyphenyl 2-{1-[(4-chlorophenyl)carbonyl]-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090792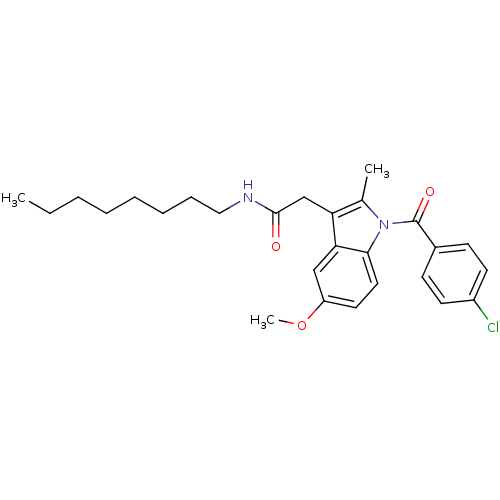 (2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090813 (2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX2 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090775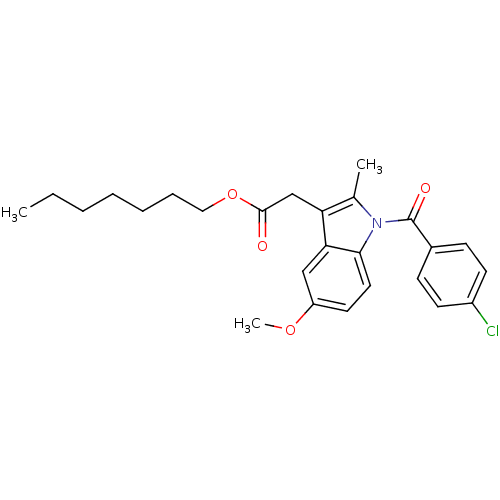 (CHEMBL330194 | [1-(4-Chloro-benzoyl)-5-methoxy-2-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM22971 (2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22967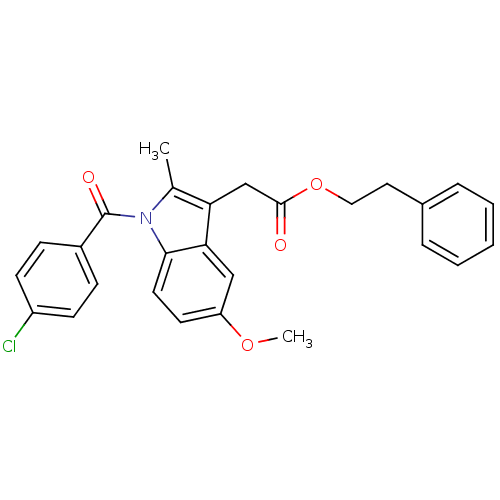 (2-phenylethyl 2-{1-[(4-chlorophenyl)carbonyl]-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336964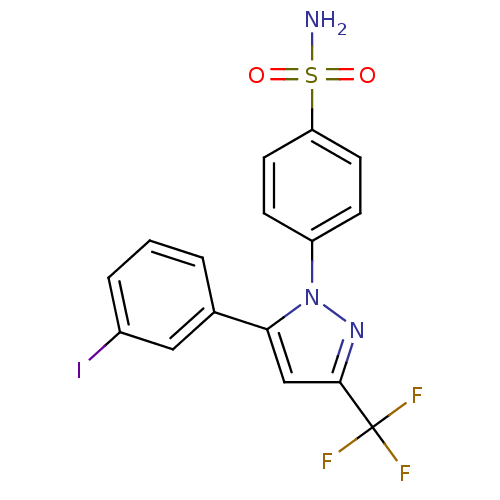 (4-[5-(3-Iodophenyl)-3-(trifluoromethyl)-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse LPS-stimulated RAW264.7 cells after 30 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22955 (4-methoxyphenyl 2-{1-[(4-chlorophenyl)carbonyl]-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50561529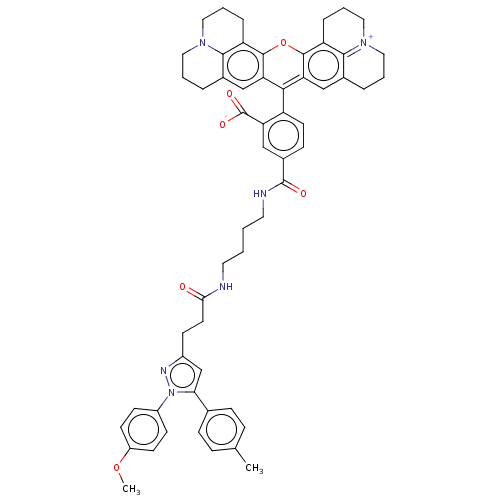 (CHEMBL4792985) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of COX-1 in human OVCAR3 cells assessed as [14C] arachidonic acid remaining using [14C] arachidonic acid as substrate preincubated for 30 ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00280 BindingDB Entry DOI: 10.7270/Q21C21J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090824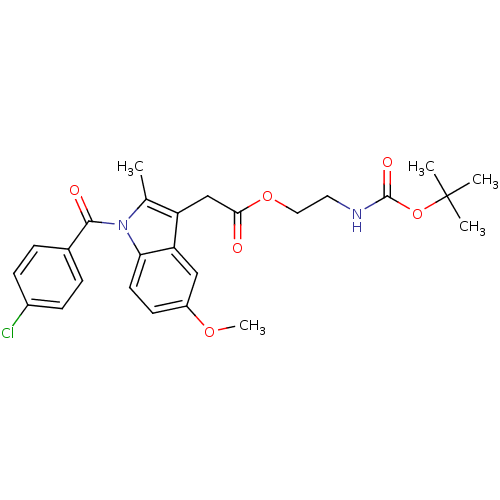 (CHEMBL433160 | [1-(4-Chloro-benzoyl)-5-methoxy-2-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090803 (2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22967 (2-phenylethyl 2-{1-[(4-chlorophenyl)carbonyl]-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22971 (2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM22955 (4-methoxyphenyl 2-{1-[(4-chlorophenyl)carbonyl]-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University School of Medicine | Assay Description Inhibition assays in triplicate were performed by preincubating enzyme (60-80 nM) and inhibitors (0-5 mM) for 20 min at 25°C followed by the add... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090827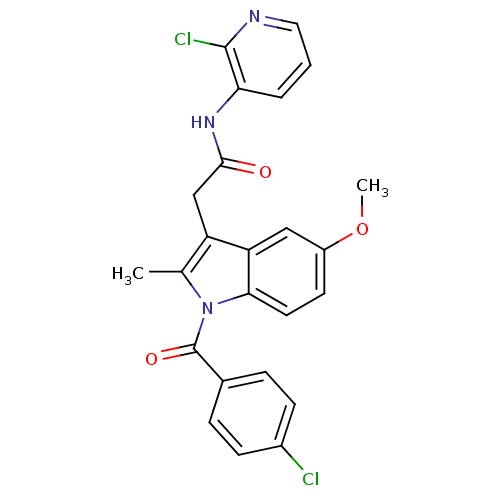 (2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22959 (CHEMBL96954 | Indomethacin derivative, 12 | pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090778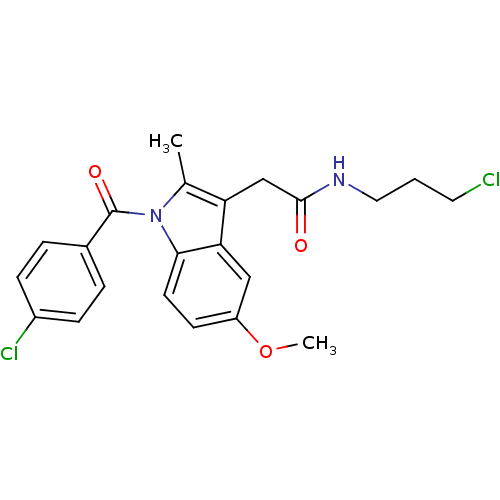 (2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX1 | Bioorg Med Chem Lett 20: 1787-91 (2010) Article DOI: 10.1016/j.bmcl.2010.01.009 BindingDB Entry DOI: 10.7270/Q2J966H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336971 (5-(4-iodophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM22971 (2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of the ovine Prostaglandin G/H synthase 1 was determined by thin-layer chromatography assay | Bioorg Med Chem Lett 12: 521-4 (2002) BindingDB Entry DOI: 10.7270/Q2BP0247 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50109676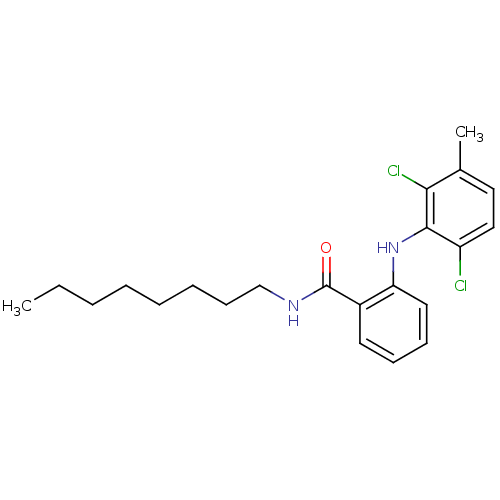 (2-(2,6-Dichloro-3-methyl-phenylamino)-N-octyl-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of the ovine Prostaglandin G/H synthase 1 was determined by thin-layer chromatography assay | Bioorg Med Chem Lett 12: 521-4 (2002) BindingDB Entry DOI: 10.7270/Q2BP0247 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against ovine Prostaglandin G/H synthase 1 (44 nM) using [14C]AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090796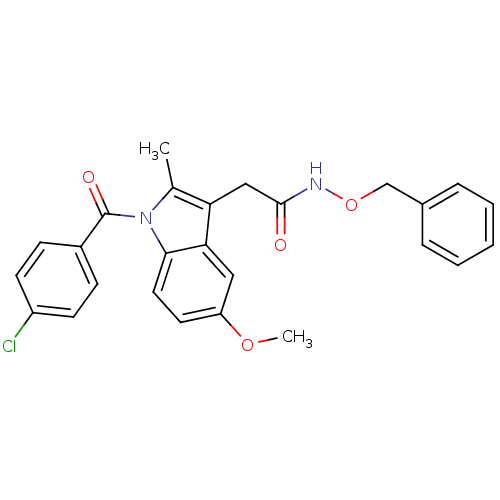 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090810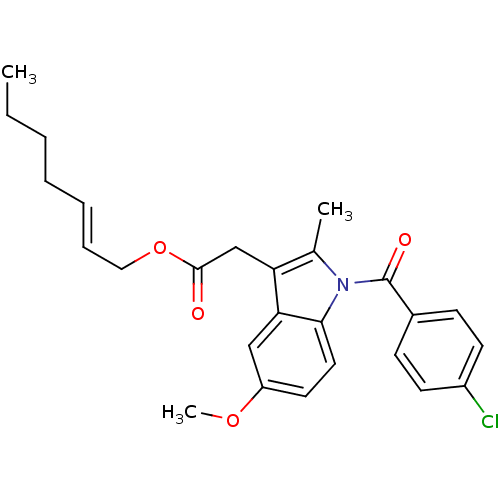 (CHEMBL95989 | [1-(4-Chloro-benzoyl)-5-methoxy-2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090812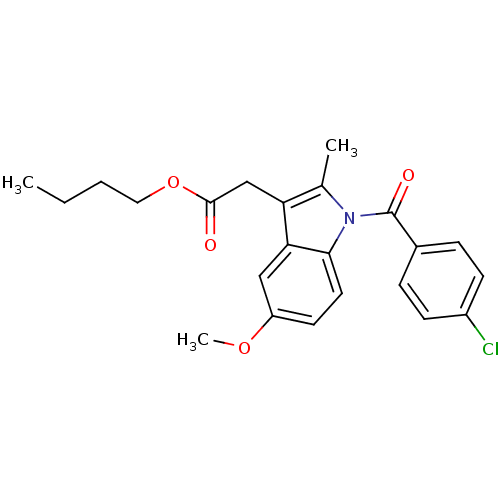 (CHEMBL97179 | [1-(4-Chloro-benzoyl)-5-methoxy-2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090819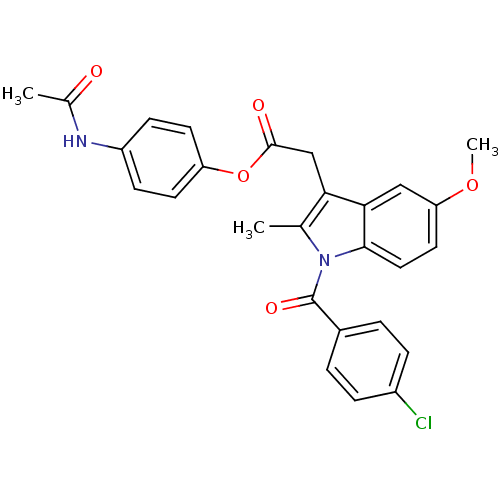 (CHEMBL97562 | [1-(4-Chloro-benzoyl)-5-methoxy-2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090799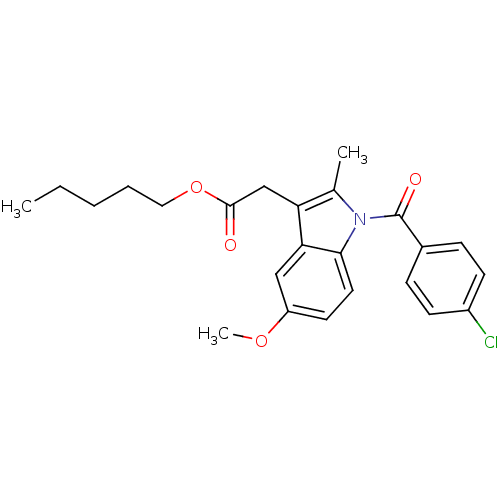 (CHEMBL99199 | [1-(4-Chloro-benzoyl)-5-methoxy-2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22959 (CHEMBL96954 | Indomethacin derivative, 12 | pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22962 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22962 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-2 in human HNSCC 1483 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured a... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090793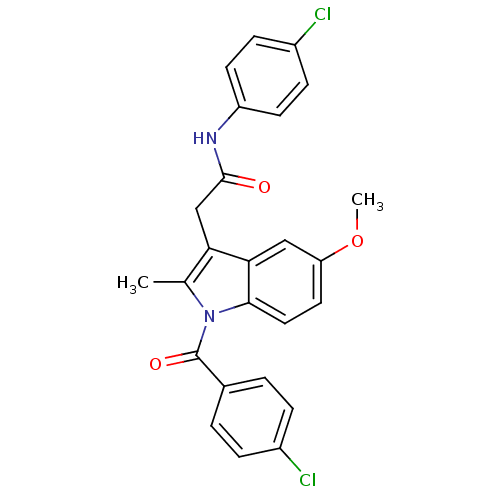 (2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090809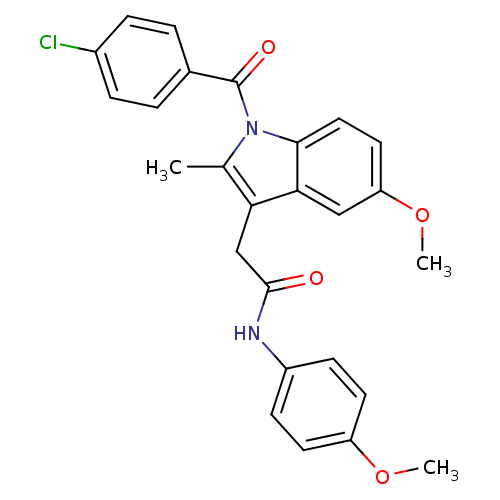 (2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090787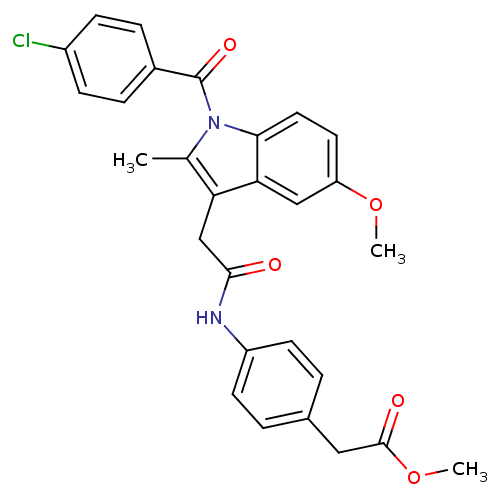 ((4-{2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50090774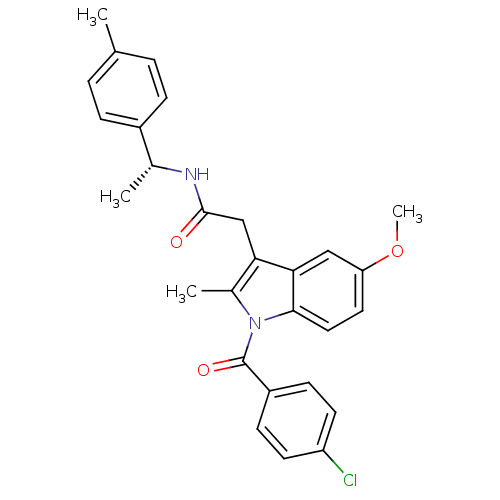 (2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22961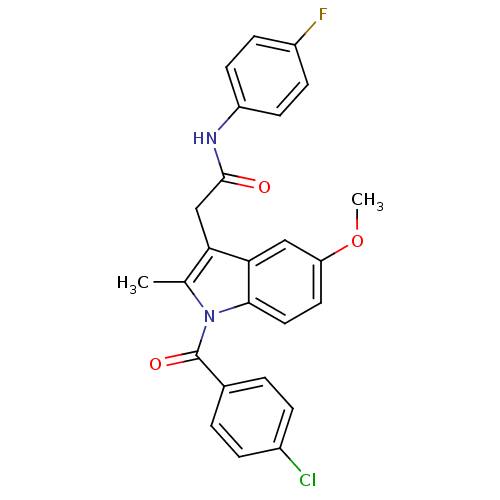 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22973 (CHEMBL149213 | Meclofenamic acid derivative, 25 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 676 total ) | Next | Last >> |