 Found 1483 hits with Last Name = 'croston' and Initial = 'g'
Found 1483 hits with Last Name = 'croston' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
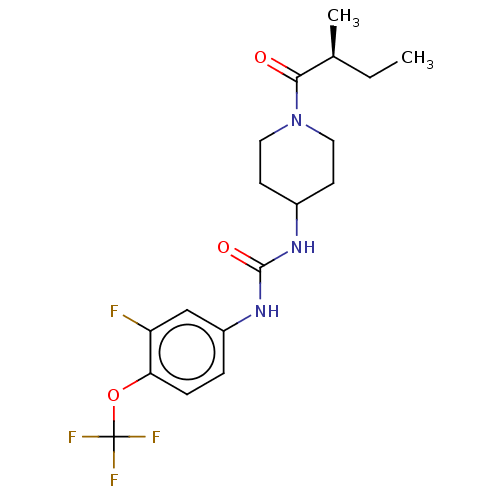
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409003
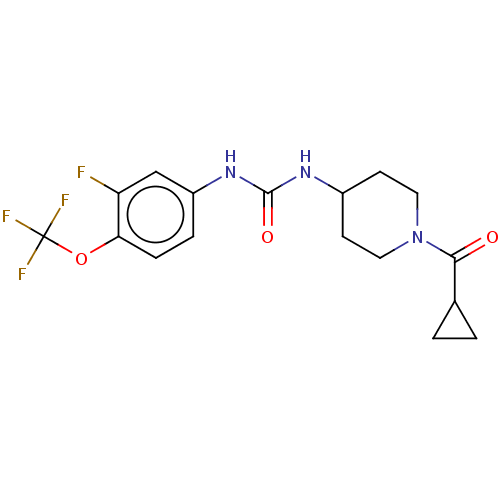
(US10377744, Compound No. 24 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2CC2)ccc1OC(F)(F)F Show InChI InChI=1S/C17H19F4N3O3/c18-13-9-12(3-4-14(13)27-17(19,20)21)23-16(26)22-11-5-7-24(8-6-11)15(25)10-1-2-10/h3-4,9-11H,1-2,5-8H2,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM408998
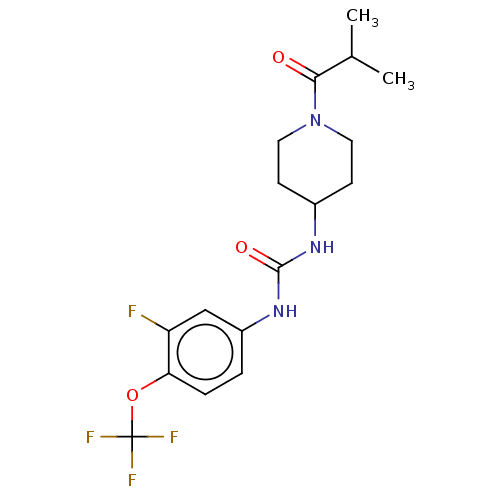
(US10377744, Compound No. 19 | US11123311, Compound...)Show SMILES CC(C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 Show InChI InChI=1S/C17H21F4N3O3/c1-10(2)15(25)24-7-5-11(6-8-24)22-16(26)23-12-3-4-14(13(18)9-12)27-17(19,20)21/h3-4,9-11H,5-8H2,1-2H3,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409014
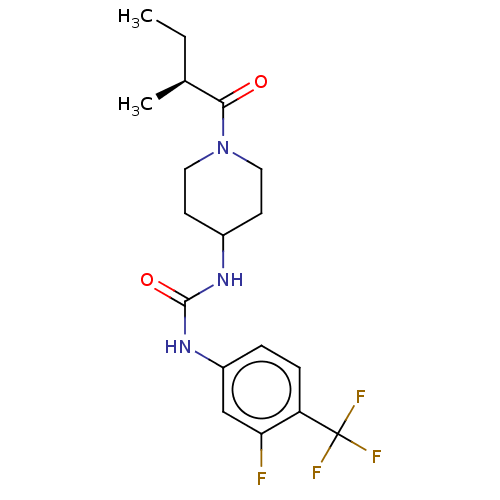
(US10377744, Compound No. 34 | US10377744, Syn34 | ...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(c(F)c1)C(F)(F)F |r| Show InChI InChI=1S/C18H23F4N3O2/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-14(15(19)10-13)18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409002
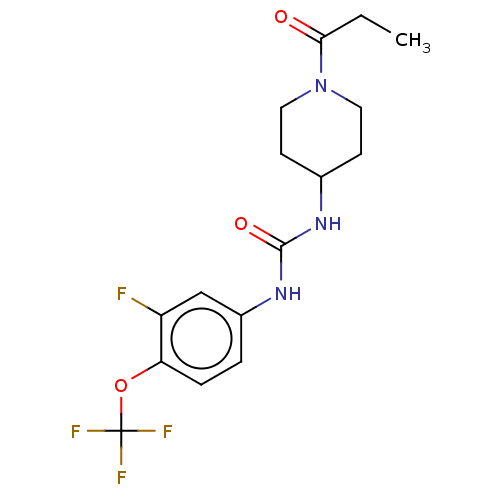
(US10377744, Compound No. 23 | US11123311, Compound...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 Show InChI InChI=1S/C16H19F4N3O3/c1-2-14(24)23-7-5-10(6-8-23)21-15(25)22-11-3-4-13(12(17)9-11)26-16(18,19)20/h3-4,9-10H,2,5-8H2,1H3,(H2,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327809
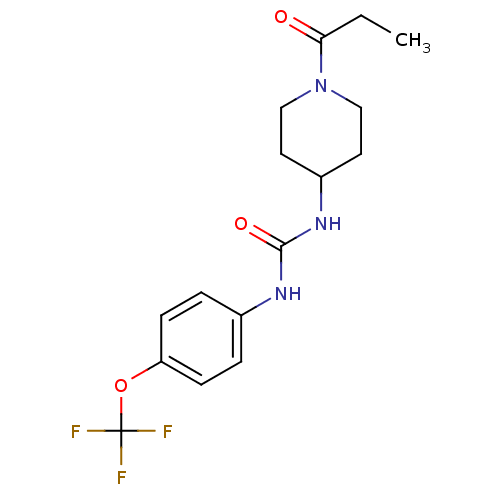
(1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H20F3N3O3/c1-2-14(23)22-9-7-12(8-10-22)21-15(24)20-11-3-5-13(6-4-11)25-16(17,18)19/h3-6,12H,2,7-10H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50052588
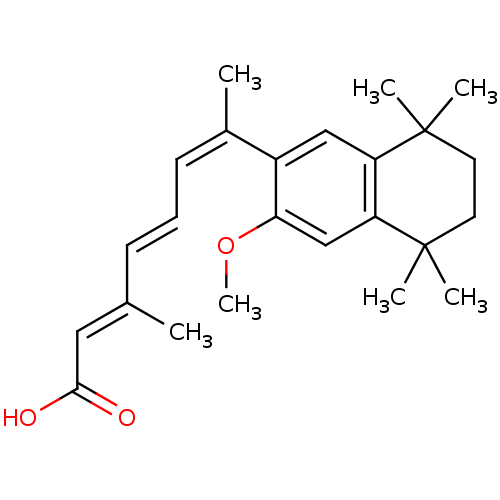
((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...)Show SMILES COc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H32O3/c1-16(13-22(25)26)9-8-10-17(2)18-14-19-20(15-21(18)27-7)24(5,6)12-11-23(19,3)4/h8-10,13-15H,11-12H2,1-7H3,(H,25,26)/b9-8+,16-13+,17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50052588

((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...)Show SMILES COc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H32O3/c1-16(13-22(25)26)9-8-10-17(2)18-14-19-20(15-21(18)27-7)24(5,6)12-11-23(19,3)4/h8-10,13-15H,11-12H2,1-7H3,(H,25,26)/b9-8+,16-13+,17-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290187
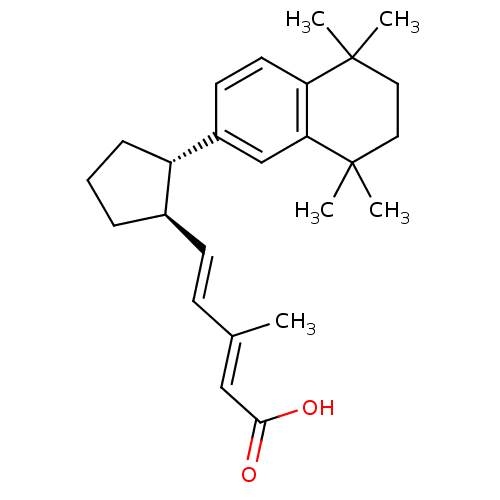
((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...)Show SMILES C\C(\C=C\[C@H]1CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C25H34O2/c1-17(15-23(26)27)9-10-18-7-6-8-20(18)19-11-12-21-22(16-19)25(4,5)14-13-24(21,2)3/h9-12,15-16,18,20H,6-8,13-14H2,1-5H3,(H,26,27)/b10-9+,17-15+/t18-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290659
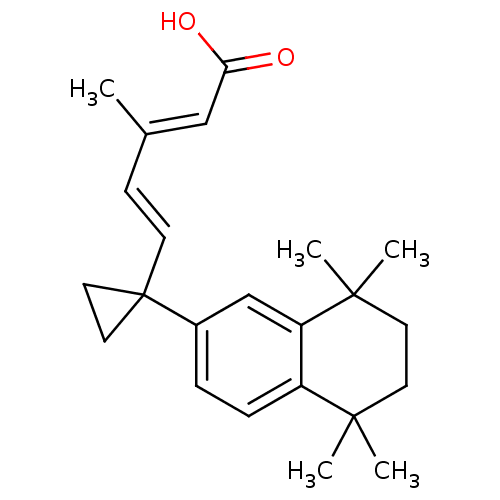
((2E,4E)-3-Methyl-5-[1-(5,5,8,8-tetramethyl-5,6,7,8...)Show SMILES C\C(\C=C\C1(CC1)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C23H30O2/c1-16(14-20(24)25)8-9-23(12-13-23)17-6-7-18-19(15-17)22(4,5)11-10-21(18,2)3/h6-9,14-15H,10-13H2,1-5H3,(H,24,25)/b9-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR gamma |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290192
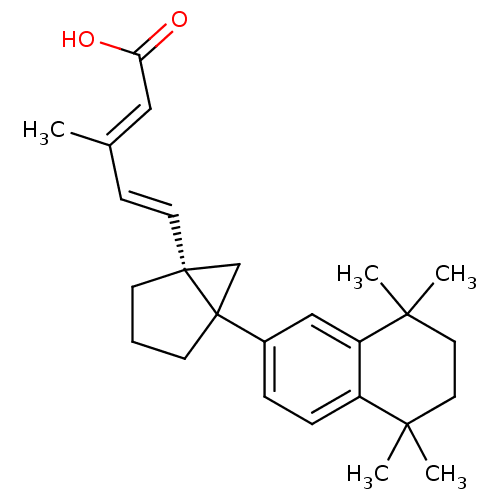
(3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...)Show SMILES C\C(\C=C\[C@]12CC1(CCC2)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H34O2/c1-18(15-22(27)28)9-12-25-10-6-11-26(25,17-25)19-7-8-20-21(16-19)24(4,5)14-13-23(20,2)3/h7-9,12,15-16H,6,10-11,13-14,17H2,1-5H3,(H,27,28)/b12-9+,18-15+/t25-,26?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409029
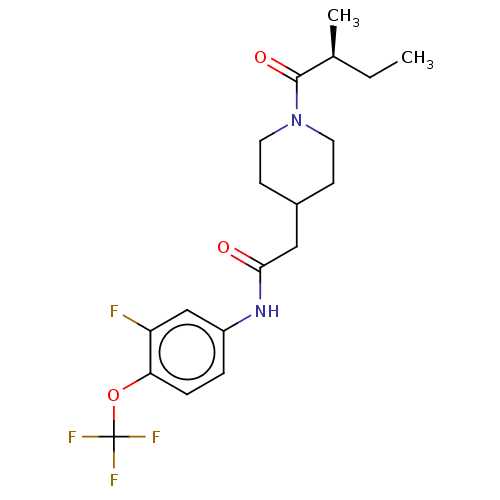
(US10377744, Compound No. 49 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC(=O)Nc2ccc(OC(F)(F)F)c(F)c2)CC1 |r| Show InChI InChI=1S/C19H24F4N2O3/c1-3-12(2)18(27)25-8-6-13(7-9-25)10-17(26)24-14-4-5-16(15(20)11-14)28-19(21,22)23/h4-5,11-13H,3,6-10H2,1-2H3,(H,24,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50052590
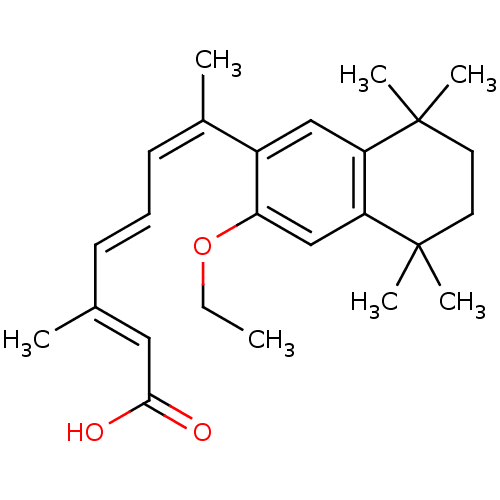
((2E,4E,6Z)-7-(3-Ethoxy-5,5,8,8-tetramethyl-5,6,7,8...)Show SMILES CCOc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H34O3/c1-8-28-22-16-21-20(24(4,5)12-13-25(21,6)7)15-19(22)18(3)11-9-10-17(2)14-23(26)27/h9-11,14-16H,8,12-13H2,1-7H3,(H,26,27)/b10-9+,17-14+,18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290188
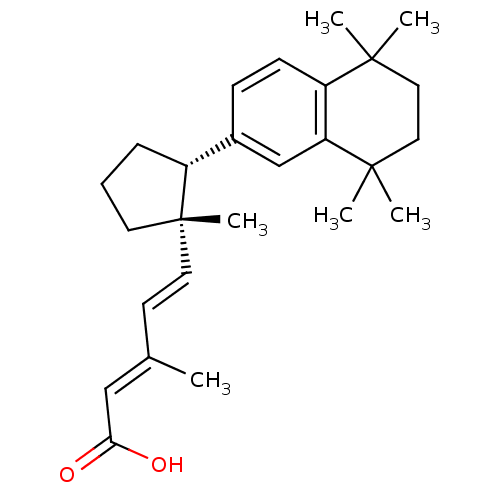
((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@]1(C)CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H36O2/c1-18(16-23(27)28)11-13-26(6)12-7-8-20(26)19-9-10-21-22(17-19)25(4,5)15-14-24(21,2)3/h9-11,13,16-17,20H,7-8,12,14-15H2,1-6H3,(H,27,28)/b13-11+,18-16+/t20-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290186
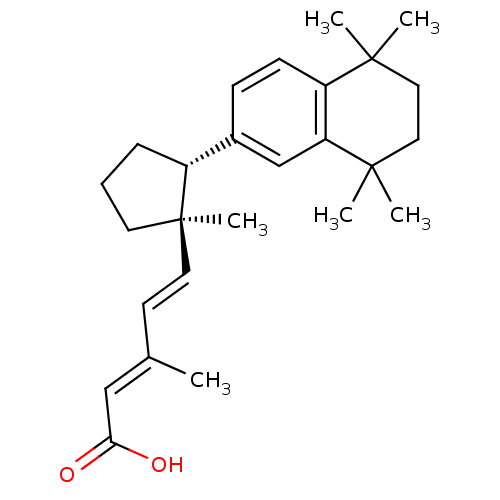
((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@]1(C)CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H36O2/c1-18(16-23(27)28)11-13-26(6)12-7-8-20(26)19-9-10-21-22(17-19)25(4,5)15-14-24(21,2)3/h9-11,13,16-17,20H,7-8,12,14-15H2,1-6H3,(H,27,28)/b13-11+,18-16+/t20-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50052588

((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...)Show SMILES COc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H32O3/c1-16(13-22(25)26)9-8-10-17(2)18-14-19-20(15-21(18)27-7)24(5,6)12-11-23(19,3)4/h8-10,13-15H,11-12H2,1-7H3,(H,25,26)/b9-8+,16-13+,17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR gamma |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50290660
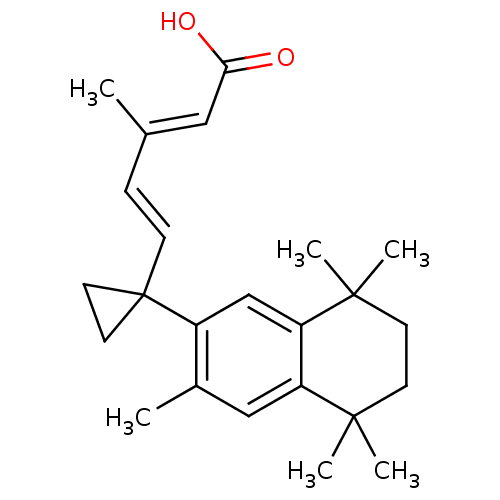
((2E,4E)-3-Methyl-5-[1-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES C\C(\C=C\C1(CC1)c1cc2c(cc1C)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-24(11-12-24)18-15-20-19(14-17(18)2)22(3,4)9-10-23(20,5)6/h7-8,13-15H,9-12H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to RXR beta receptor |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290660

((2E,4E)-3-Methyl-5-[1-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES C\C(\C=C\C1(CC1)c1cc2c(cc1C)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-24(11-12-24)18-15-20-19(14-17(18)2)22(3,4)9-10-23(20,5)6/h7-8,13-15H,9-12H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to RXR alpha receptor |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50052033
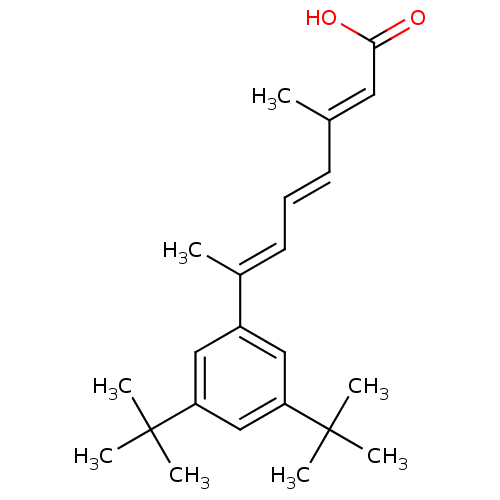
((2E,4E,6E)-7-(3,5-Di-tert-butyl-phenyl)-3-methyl-o...)Show SMILES C\C(\C=C\C=C(/C)c1cc(cc(c1)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C23H32O2/c1-16(12-21(24)25)10-9-11-17(2)18-13-19(22(3,4)5)15-20(14-18)23(6,7)8/h9-15H,1-8H3,(H,24,25)/b10-9+,16-12+,17-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Mus musculus) | BDBM50290187

((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...)Show SMILES C\C(\C=C\[C@H]1CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C25H34O2/c1-17(15-23(26)27)9-10-18-7-6-8-20(18)19-11-12-21-22(16-19)25(4,5)14-13-24(21,2)3/h9-12,15-16,18,20H,6-8,13-14H2,1-5H3,(H,26,27)/b10-9+,17-15+/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Effective potency in transcriptional activation assay in CV-1 cells expressing retinoic acid receptor RAR gamma |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290193
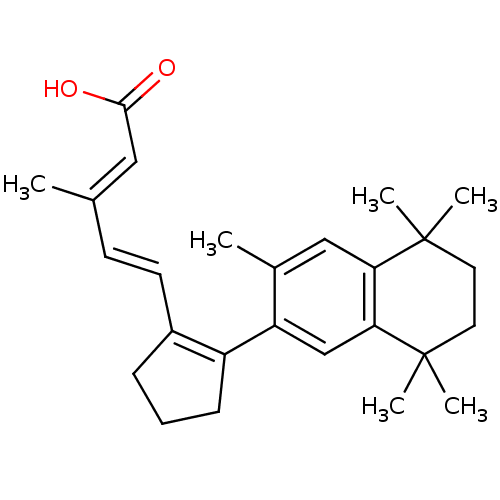
(3-Methyl-5-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetra...)Show SMILES C\C(\C=C\C1=C(CCC1)c1cc2c(cc1C)C(C)(C)CCC2(C)C)=C/C(O)=O |t:4| Show InChI InChI=1S/C26H34O2/c1-17(14-24(27)28)10-11-19-8-7-9-20(19)21-16-23-22(15-18(21)2)25(3,4)12-13-26(23,5)6/h10-11,14-16H,7-9,12-13H2,1-6H3,(H,27,28)/b11-10+,17-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50290660

((2E,4E)-3-Methyl-5-[1-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES C\C(\C=C\C1(CC1)c1cc2c(cc1C)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-24(11-12-24)18-15-20-19(14-17(18)2)22(3,4)9-10-23(20,5)6/h7-8,13-15H,9-12H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR gamma |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074300
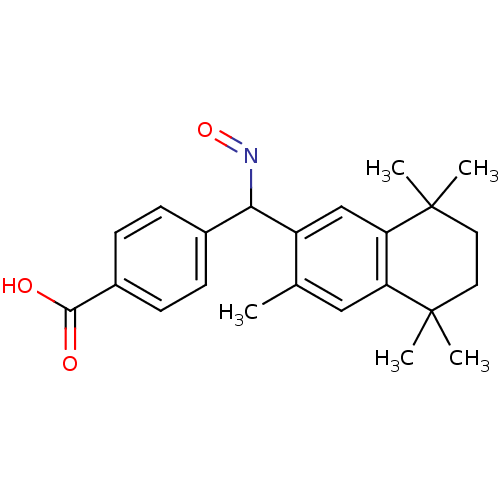
(4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES Cc1cc2c(cc1C(N=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H27NO3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24-27)15-6-8-16(9-7-15)21(25)26/h6-9,12-13,20H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Mus musculus) | BDBM50290192

(3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...)Show SMILES C\C(\C=C\[C@]12CC1(CCC2)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H34O2/c1-18(15-22(27)28)9-12-25-10-6-11-26(25,17-25)19-7-8-20-21(16-19)24(4,5)14-13-23(20,2)3/h7-9,12,15-16H,6,10-11,13-14,17H2,1-5H3,(H,27,28)/b12-9+,18-15+/t25-,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50074300

(4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES Cc1cc2c(cc1C(N=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H27NO3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24-27)15-6-8-16(9-7-15)21(25)26/h6-9,12-13,20H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074307
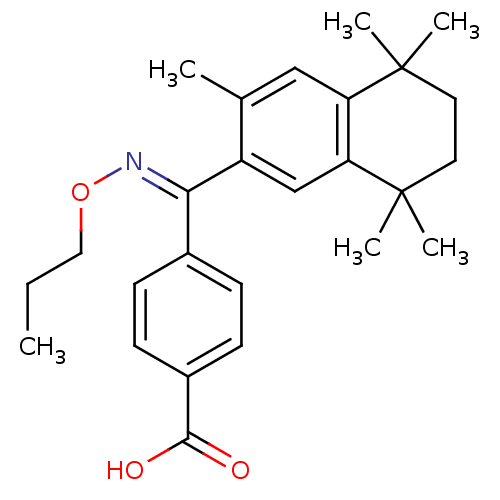
(4-{(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CCCO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H33NO3/c1-7-14-30-27-23(18-8-10-19(11-9-18)24(28)29)20-16-22-21(15-17(20)2)25(3,4)12-13-26(22,5)6/h8-11,15-16H,7,12-14H2,1-6H3,(H,28,29)/b27-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074300

(4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES Cc1cc2c(cc1C(N=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H27NO3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24-27)15-6-8-16(9-7-15)21(25)26/h6-9,12-13,20H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Mus musculus) | BDBM50290187

((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...)Show SMILES C\C(\C=C\[C@H]1CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C25H34O2/c1-17(15-23(26)27)9-10-18-7-6-8-20(18)19-11-12-21-22(16-19)25(4,5)14-13-24(21,2)3/h9-12,15-16,18,20H,6-8,13-14H2,1-5H3,(H,26,27)/b10-9+,17-15+/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR beta |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074304
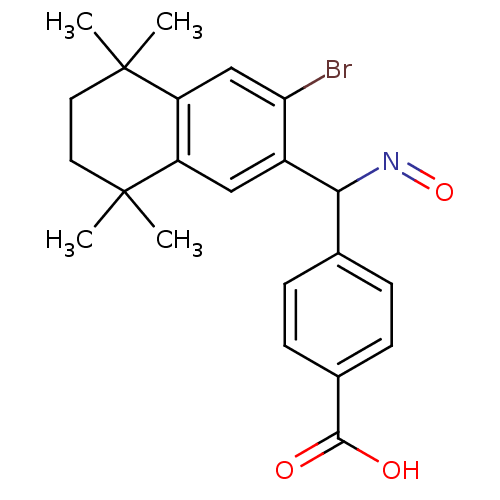
(4-{(3-Bromo-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...)Show SMILES CC1(C)CCC(C)(C)c2cc(C(N=O)c3ccc(cc3)C(O)=O)c(Br)cc12 Show InChI InChI=1S/C22H24BrNO3/c1-21(2)9-10-22(3,4)17-12-18(23)15(11-16(17)21)19(24-27)13-5-7-14(8-6-13)20(25)26/h5-8,11-12,19H,9-10H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50290659

((2E,4E)-3-Methyl-5-[1-(5,5,8,8-tetramethyl-5,6,7,8...)Show SMILES C\C(\C=C\C1(CC1)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C23H30O2/c1-16(14-20(24)25)8-9-23(12-13-23)17-6-7-18-19(15-17)22(4,5)11-10-21(18,2)3/h6-9,14-15H,10-13H2,1-5H3,(H,24,25)/b9-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to RXR gamma receptor |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Mus musculus) | BDBM50290188

((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@]1(C)CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H36O2/c1-18(16-23(27)28)11-13-26(6)12-7-8-20(26)19-9-10-21-22(17-19)25(4,5)15-14-24(21,2)3/h9-11,13,16-17,20H,7-8,12,14-15H2,1-6H3,(H,27,28)/b13-11+,18-16+/t20-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR gamma |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50290659

((2E,4E)-3-Methyl-5-[1-(5,5,8,8-tetramethyl-5,6,7,8...)Show SMILES C\C(\C=C\C1(CC1)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C23H30O2/c1-16(14-20(24)25)8-9-23(12-13-23)17-6-7-18-19(15-17)22(4,5)11-10-21(18,2)3/h6-9,14-15H,10-13H2,1-5H3,(H,24,25)/b9-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to RXR alpha receptor |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074306
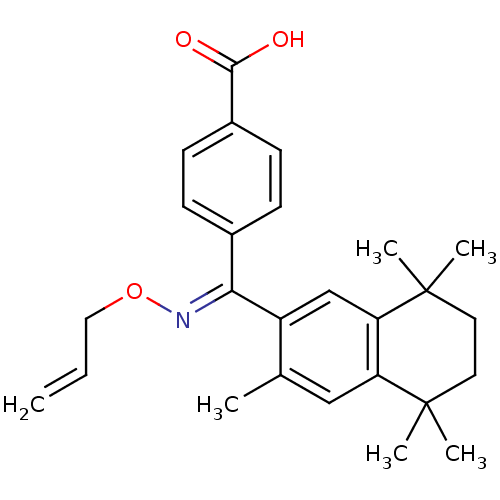
(4-[[(E)-Allyloxyimino]-(3,5,5,8,8-pentamethyl-5,6,...)Show SMILES Cc1cc2c(cc1\C(=N\OCC=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H31NO3/c1-7-14-30-27-23(18-8-10-19(11-9-18)24(28)29)20-16-22-21(15-17(20)2)25(3,4)12-13-26(22,5)6/h7-11,15-16H,1,12-14H2,2-6H3,(H,28,29)/b27-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Mus musculus) | BDBM50290186

((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@]1(C)CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H36O2/c1-18(16-23(27)28)11-13-26(6)12-7-8-20(26)19-9-10-21-22(17-19)25(4,5)15-14-24(21,2)3/h9-11,13,16-17,20H,7-8,12,14-15H2,1-6H3,(H,27,28)/b13-11+,18-16+/t20-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR beta |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074295
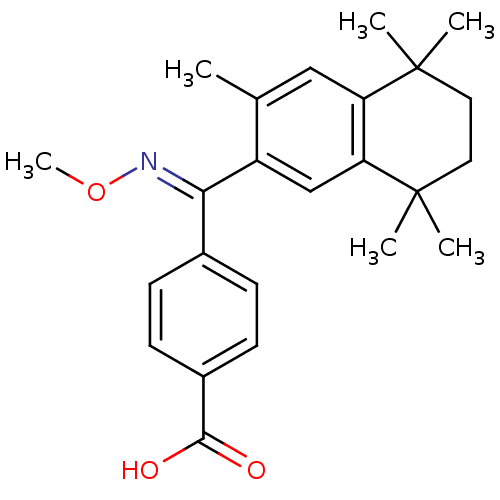
(4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES CO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO3/c1-15-13-19-20(24(4,5)12-11-23(19,2)3)14-18(15)21(25-28-6)16-7-9-17(10-8-16)22(26)27/h7-10,13-14H,11-12H2,1-6H3,(H,26,27)/b25-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50074295

(4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES CO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO3/c1-15-13-19-20(24(4,5)12-11-23(19,2)3)14-18(15)21(25-28-6)16-7-9-17(10-8-16)22(26)27/h7-10,13-14H,11-12H2,1-6H3,(H,26,27)/b25-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50052589
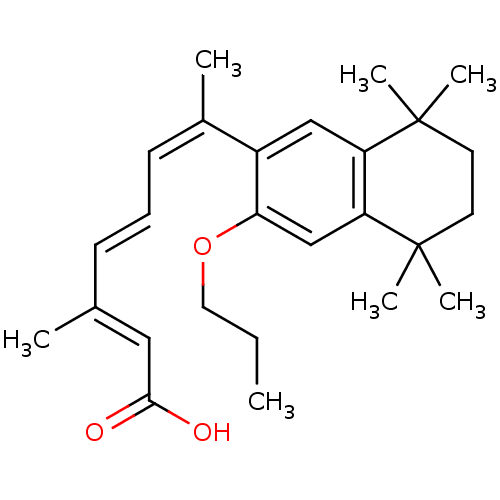
((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...)Show SMILES CCCOc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H36O3/c1-8-14-29-23-17-22-21(25(4,5)12-13-26(22,6)7)16-20(23)19(3)11-9-10-18(2)15-24(27)28/h9-11,15-17H,8,12-14H2,1-7H3,(H,27,28)/b10-9+,18-15+,19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50052589

((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...)Show SMILES CCCOc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H36O3/c1-8-14-29-23-17-22-21(25(4,5)12-13-26(22,6)7)16-20(23)19(3)11-9-10-18(2)15-24(27)28/h9-11,15-17H,8,12-14H2,1-7H3,(H,27,28)/b10-9+,18-15+,19-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074295

(4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES CO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO3/c1-15-13-19-20(24(4,5)12-11-23(19,2)3)14-18(15)21(25-28-6)16-7-9-17(10-8-16)22(26)27/h7-10,13-14H,11-12H2,1-6H3,(H,26,27)/b25-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50052033

((2E,4E,6E)-7-(3,5-Di-tert-butyl-phenyl)-3-methyl-o...)Show SMILES C\C(\C=C\C=C(/C)c1cc(cc(c1)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C23H32O2/c1-16(12-21(24)25)10-9-11-17(2)18-13-19(22(3,4)5)15-20(14-18)23(6,7)8/h9-15H,1-8H3,(H,24,25)/b10-9+,16-12+,17-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR gamma |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50052033

((2E,4E,6E)-7-(3,5-Di-tert-butyl-phenyl)-3-methyl-o...)Show SMILES C\C(\C=C\C=C(/C)c1cc(cc(c1)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C23H32O2/c1-16(12-21(24)25)10-9-11-17(2)18-13-19(22(3,4)5)15-20(14-18)23(6,7)8/h9-15H,1-8H3,(H,24,25)/b10-9+,16-12+,17-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR gamma |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Mus musculus) | BDBM50290192

(3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...)Show SMILES C\C(\C=C\[C@]12CC1(CCC2)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H34O2/c1-18(15-22(27)28)9-12-25-10-6-11-26(25,17-25)19-7-8-20-21(16-19)24(4,5)14-13-23(20,2)3/h7-9,12,15-16H,6,10-11,13-14,17H2,1-5H3,(H,27,28)/b12-9+,18-15+/t25-,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Mus musculus) | BDBM50290186

((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@]1(C)CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H36O2/c1-18(16-23(27)28)11-13-26(6)12-7-8-20(26)19-9-10-21-22(17-19)25(4,5)15-14-24(21,2)3/h9-11,13,16-17,20H,7-8,12,14-15H2,1-6H3,(H,27,28)/b13-11+,18-16+/t20-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR beta |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Mus musculus) | BDBM50290188

((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@]1(C)CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H36O2/c1-18(16-23(27)28)11-13-26(6)12-7-8-20(26)19-9-10-21-22(17-19)25(4,5)15-14-24(21,2)3/h9-11,13,16-17,20H,7-8,12,14-15H2,1-6H3,(H,27,28)/b13-11+,18-16+/t20-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Effective potency in transcriptional activation assay in CV-1 cells expressing retinoic acid receptor RAR beta |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50052033

((2E,4E,6E)-7-(3,5-Di-tert-butyl-phenyl)-3-methyl-o...)Show SMILES C\C(\C=C\C=C(/C)c1cc(cc(c1)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C23H32O2/c1-16(12-21(24)25)10-9-11-17(2)18-13-19(22(3,4)5)15-20(14-18)23(6,7)8/h9-15H,1-8H3,(H,24,25)/b10-9+,16-12+,17-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR beta |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50052033

((2E,4E,6E)-7-(3,5-Di-tert-butyl-phenyl)-3-methyl-o...)Show SMILES C\C(\C=C\C=C(/C)c1cc(cc(c1)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C23H32O2/c1-16(12-21(24)25)10-9-11-17(2)18-13-19(22(3,4)5)15-20(14-18)23(6,7)8/h9-15H,1-8H3,(H,24,25)/b10-9+,16-12+,17-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR beta |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data