

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473230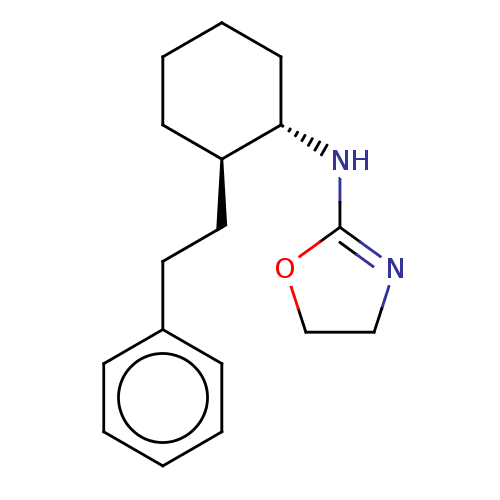 (CHEMBL47313) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473228 (AGN-190837 | CHEMBL297752) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473228 (AGN-190837 | CHEMBL297752) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473230 (CHEMBL47313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50054539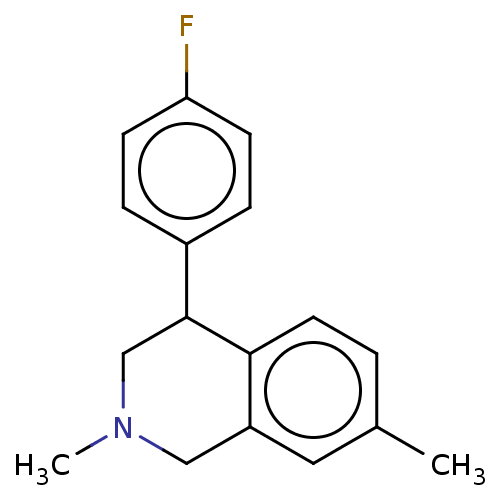 (CHEMBL3323088) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting | ACS Med Chem Lett 5: 760-5 (2014) Article DOI: 10.1021/ml500053b BindingDB Entry DOI: 10.7270/Q2154JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473230 (CHEMBL47313) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant, using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473229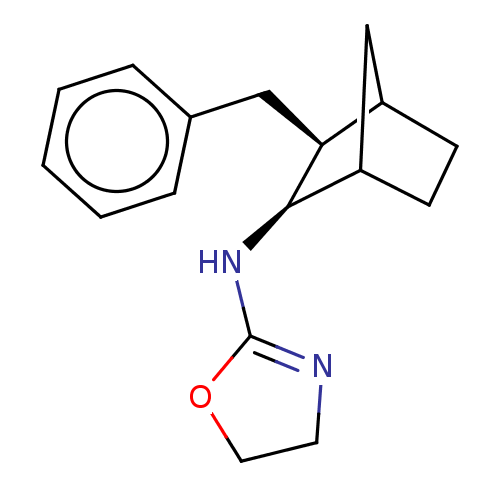 (CHEMBL295186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473227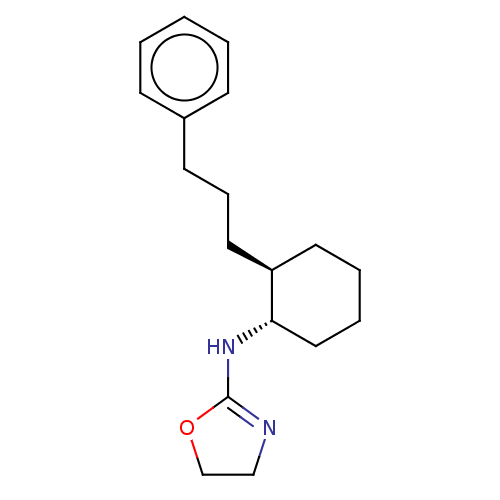 (CHEMBL50720) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473226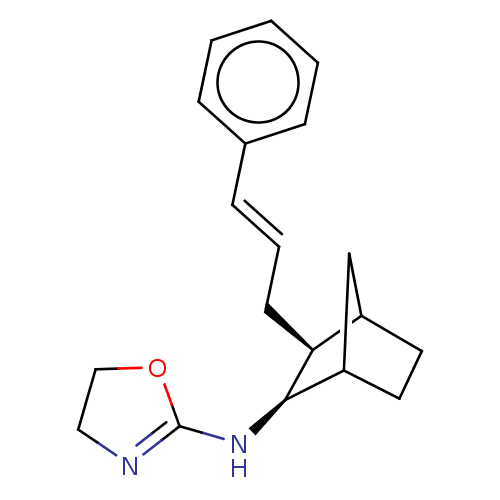 (CHEMBL297827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473225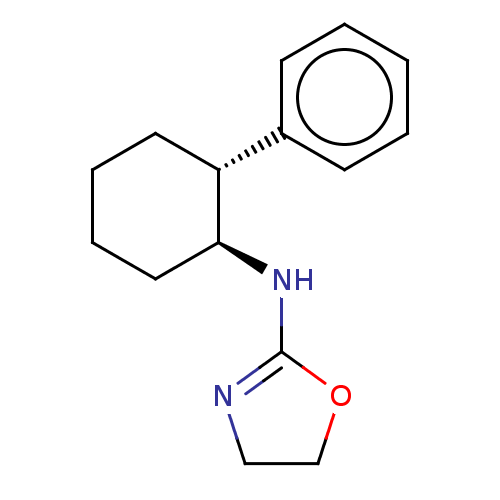 (CHEMBL297228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473232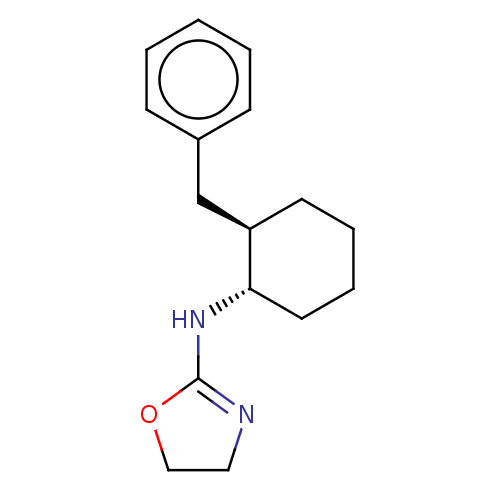 (CHEMBL50719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473225 (CHEMBL297228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473228 (AGN-190837 | CHEMBL297752) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant determined using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473224 (CHEMBL296660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50473231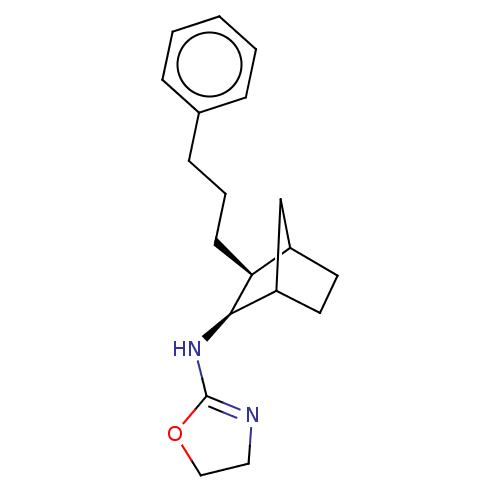 (CHEMBL48341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2A adrenergic receptor expressed in LM(tk-) cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473227 (CHEMBL50720) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473227 (CHEMBL50720) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant, using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50062912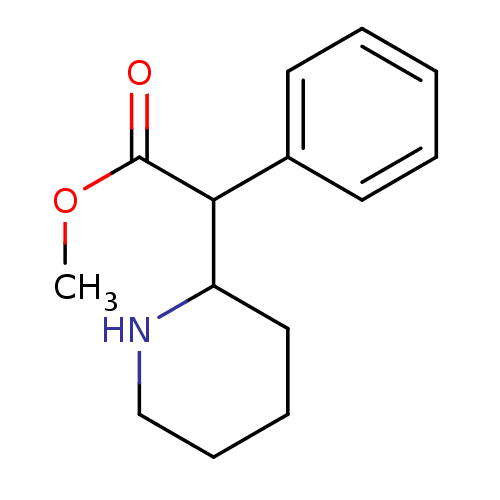 (CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting | ACS Med Chem Lett 5: 760-5 (2014) Article DOI: 10.1021/ml500053b BindingDB Entry DOI: 10.7270/Q2154JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50054539 (CHEMBL3323088) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting | ACS Med Chem Lett 5: 760-5 (2014) Article DOI: 10.1021/ml500053b BindingDB Entry DOI: 10.7270/Q2154JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473232 (CHEMBL50719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473229 (CHEMBL295186) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473225 (CHEMBL297228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant, using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473232 (CHEMBL50719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant, using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473229 (CHEMBL295186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant, using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473226 (CHEMBL297827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant, using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473224 (CHEMBL296660) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473226 (CHEMBL297827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473231 (CHEMBL48341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant, using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50473224 (CHEMBL296660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant, using [3H]rauwolscine in LM(tk-) cells stably transfected with cloned human Alpha-2C adrenergic receptor | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50473231 (CHEMBL48341) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against human Alpha-2B adrenergic receptor expressed in Y-1 cells using [3H]rauwolscine | J Med Chem 43: 1699-704 (2000) Article DOI: 10.1021/jm9905256 BindingDB Entry DOI: 10.7270/Q2D221C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50054539 (CHEMBL3323088) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting | ACS Med Chem Lett 5: 760-5 (2014) Article DOI: 10.1021/ml500053b BindingDB Entry DOI: 10.7270/Q2154JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50062912 (CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting | ACS Med Chem Lett 5: 760-5 (2014) Article DOI: 10.1021/ml500053b BindingDB Entry DOI: 10.7270/Q2154JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50062912 (CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting | ACS Med Chem Lett 5: 760-5 (2014) Article DOI: 10.1021/ml500053b BindingDB Entry DOI: 10.7270/Q2154JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM148993 (US8962648, 72 | US8962648, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149054 (US8962648, 319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149042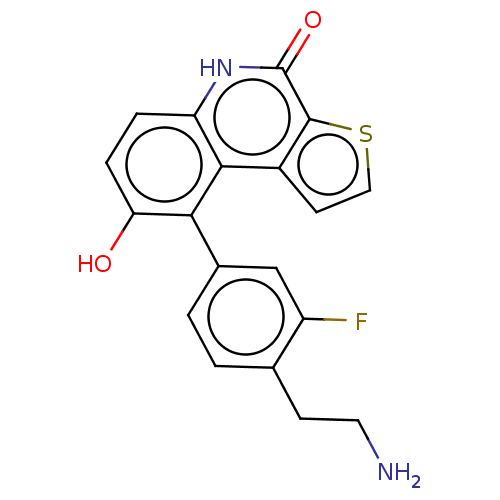 (US8962648, 276) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149041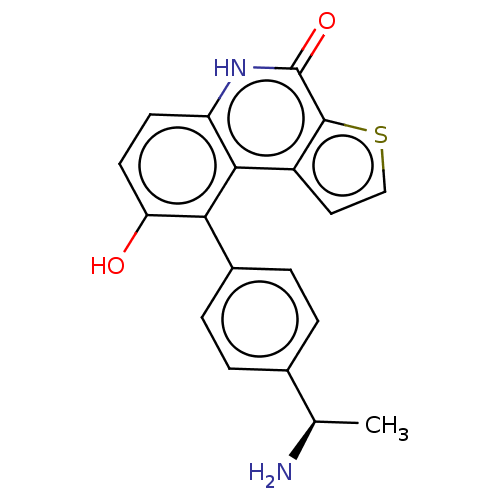 (US8962648, 275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149027 (US8962648, 235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50034641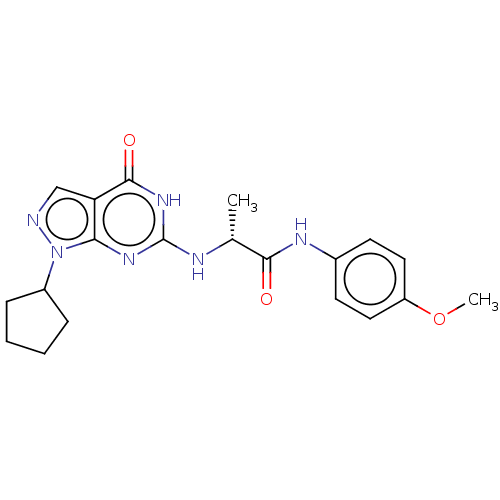 (CHEMBL3360415) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysis | J Med Chem 57: 10304-13 (2014) Article DOI: 10.1021/jm500836h BindingDB Entry DOI: 10.7270/Q28P624W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM148993 (US8962648, 72 | US8962648, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149118 (US8962648, 1160) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149043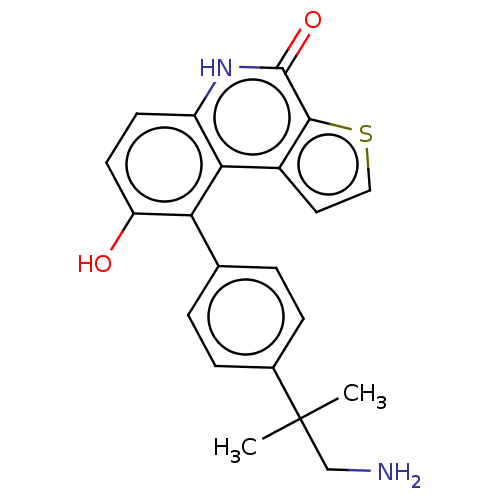 (US8962648, 277) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149016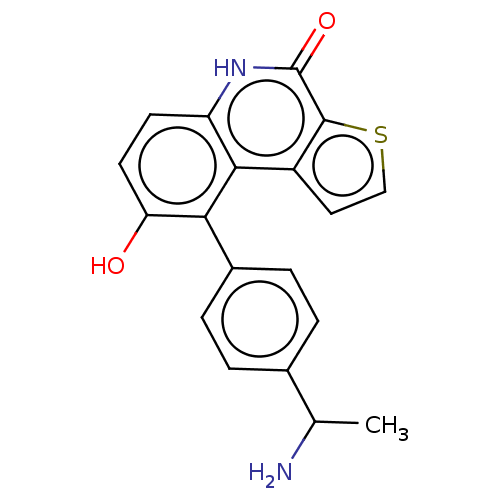 (US8962648, 195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149039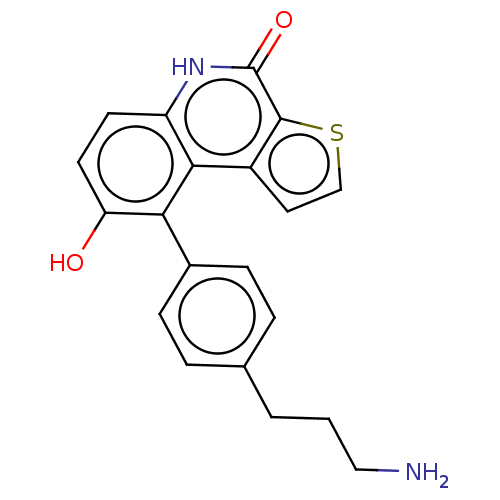 (US8962648, 273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149192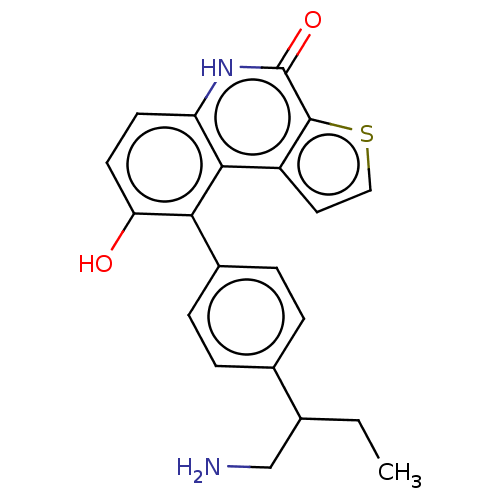 (US8962648, 1309) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149034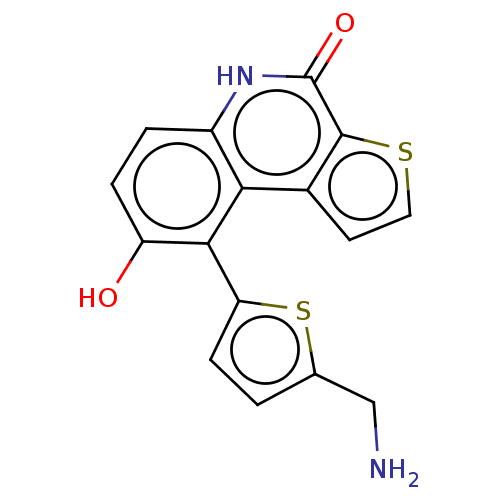 (US8962648, 266) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM148997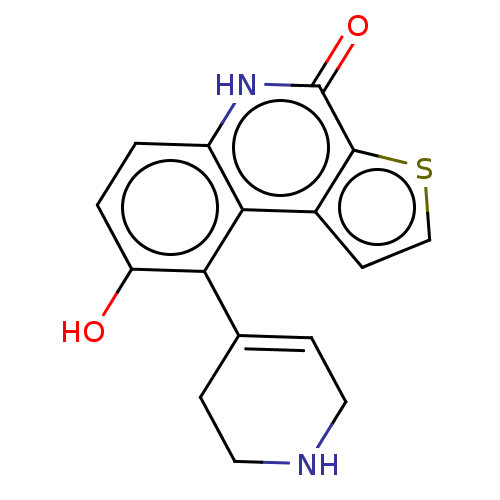 (US8962648, 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149026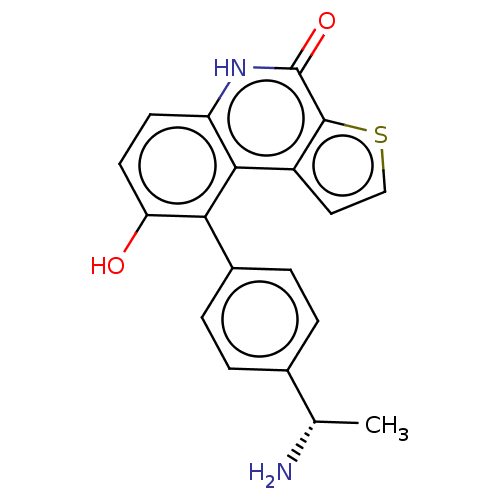 (US8962648, 233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149062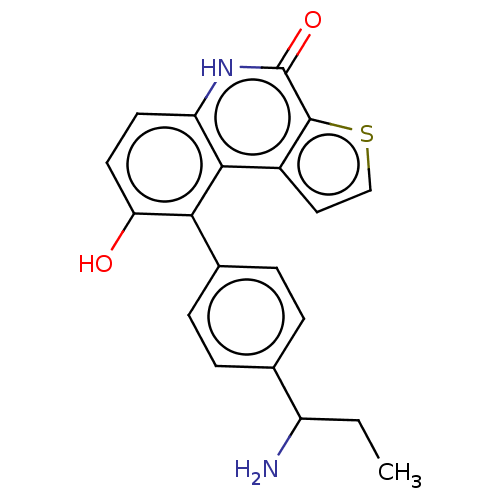 (US8962648, 336) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149097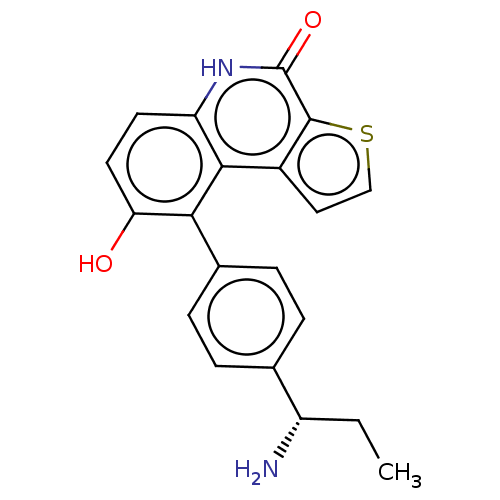 (US8962648, 1120) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 489 total ) | Next | Last >> |