

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29100 (dibenzothiazepine, 12h) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29098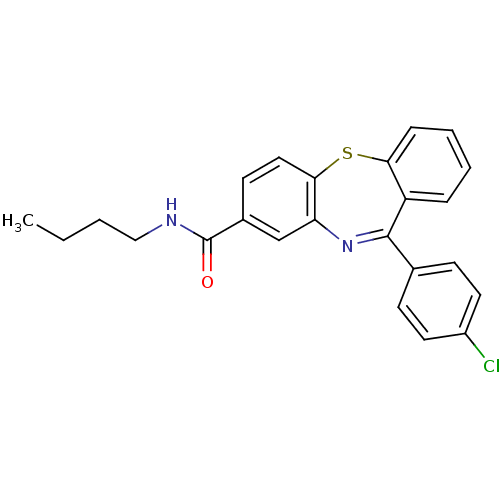 (dibenzothiazepine, 12e) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29096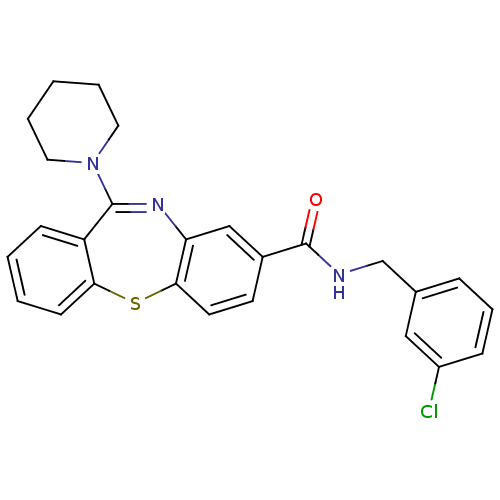 (dibenzothiazepine, 12b) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29097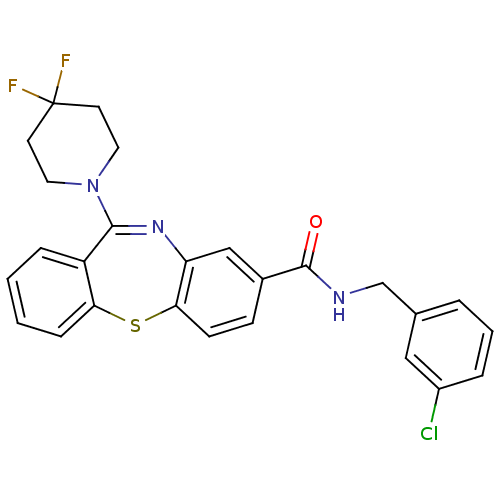 (dibenzothiazepine, 12c) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.790 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29101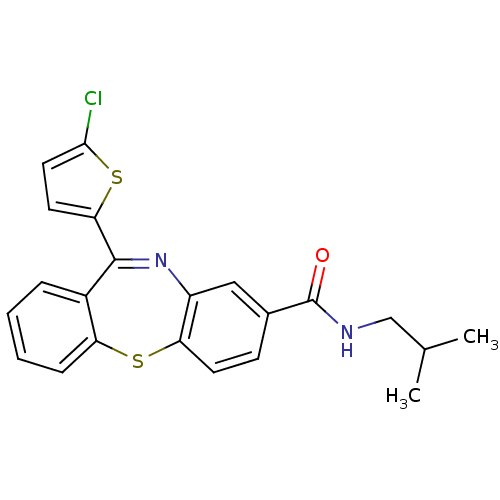 (dibenzothiazepine, 12j) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.20 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29102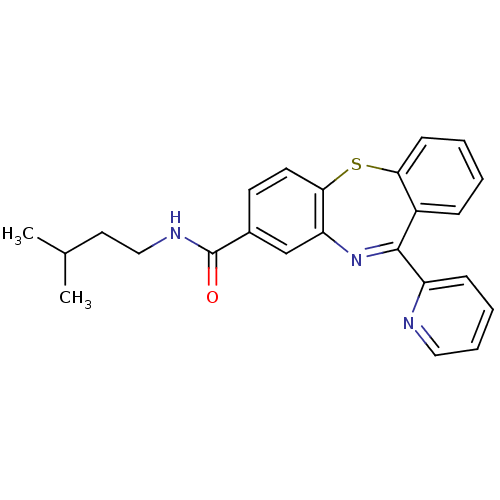 (dibenzothiazepine, 12k) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29095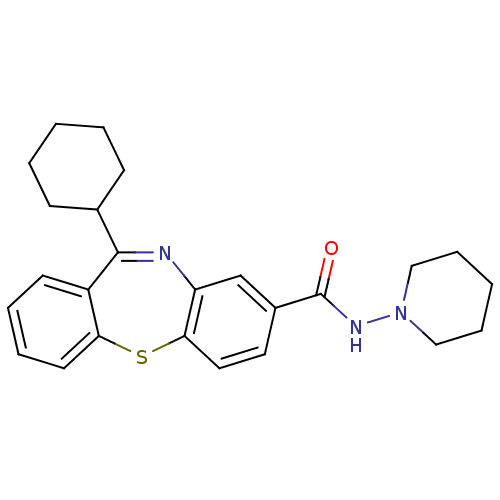 (dibenzothiazepine, 12a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29094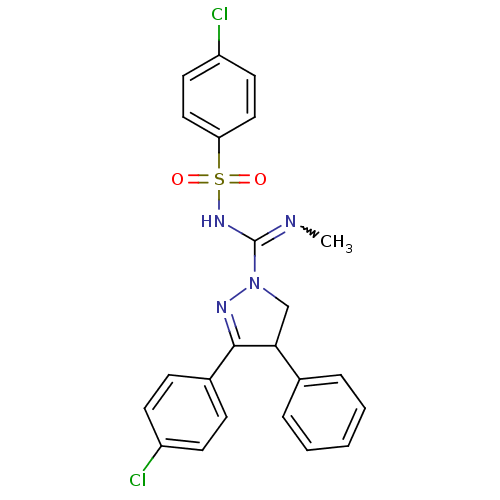 ((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29099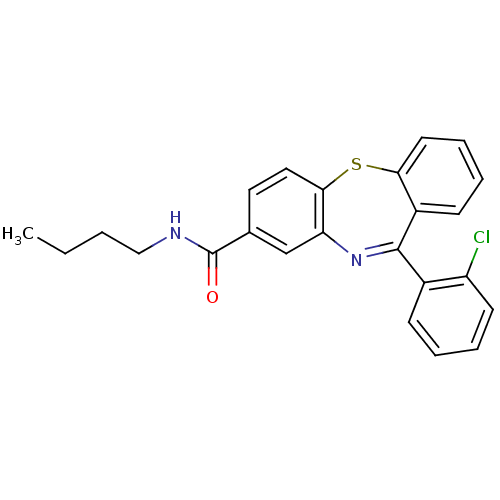 (dibenzothiazepine, 12g) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29103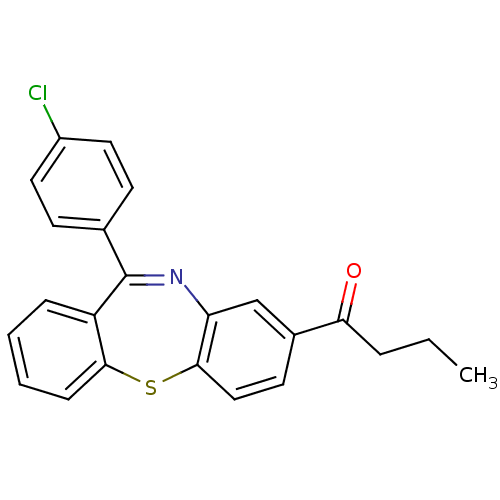 (dibenzothiazepine, 13) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | -41.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 398 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29100 (dibenzothiazepine, 12h) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29097 (dibenzothiazepine, 12c) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29094 ((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29099 (dibenzothiazepine, 12g) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.01E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G-protein coupled receptor member X2 (Homo sapiens (Human)) | BDBM50413340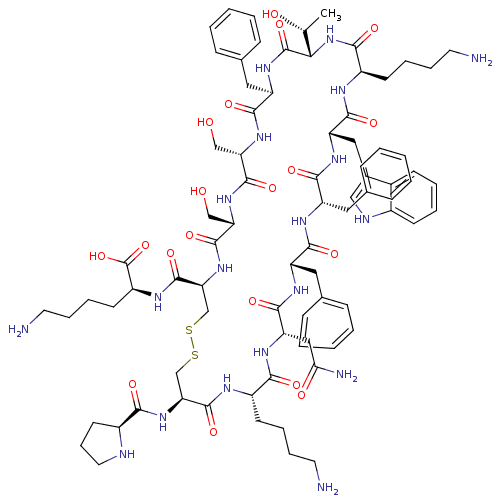 (CHEMBL505715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human MrgX2 receptor | Bioorg Med Chem Lett 19: 1729-32 (2009) Article DOI: 10.1016/j.bmcl.2009.01.085 BindingDB Entry DOI: 10.7270/Q29S1S8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29101 (dibenzothiazepine, 12j) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29102 (dibenzothiazepine, 12k) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29095 (dibenzothiazepine, 12a) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29096 (dibenzothiazepine, 12b) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29098 (dibenzothiazepine, 12e) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412708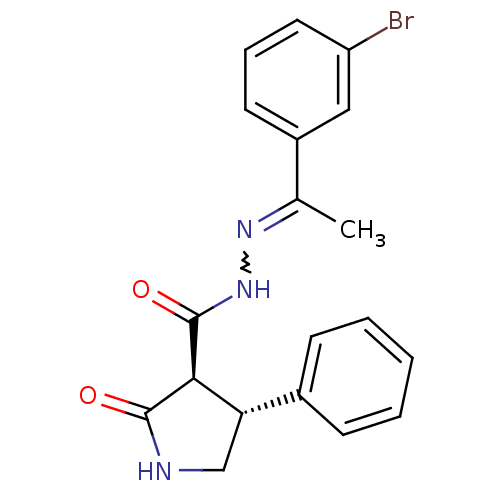 (AC-264613 | CHEMBL494502) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412708 (AC-264613 | CHEMBL494502) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414757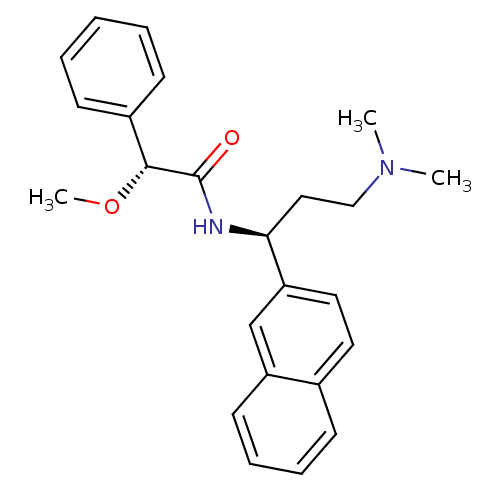 (CHEMBL572454) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 741 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414758 (CHEMBL574305) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414759 (CHEMBL574304) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 871 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414760 (CHEMBL573837) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414761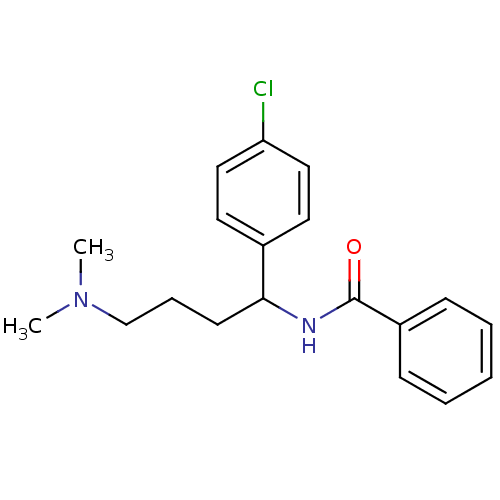 (CHEMBL573836) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414762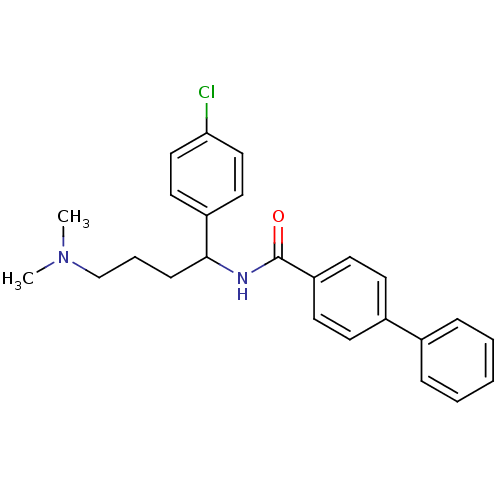 (CHEMBL576746) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414763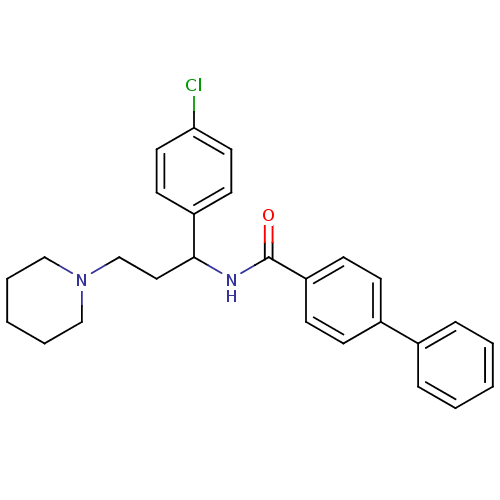 (CHEMBL572467) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 661 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414764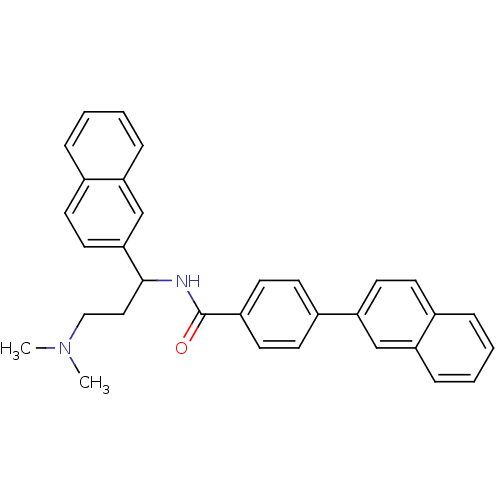 (CHEMBL578853) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 525 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414765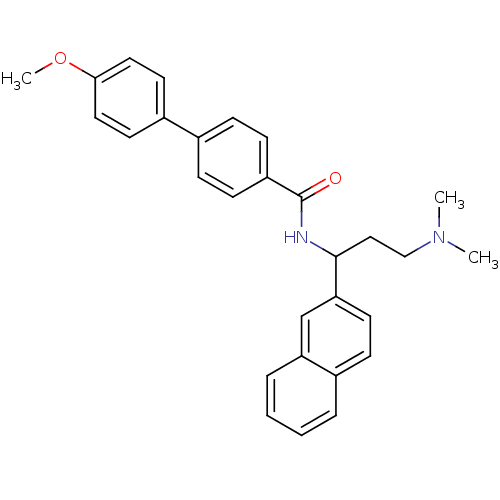 (CHEMBL578654) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 105 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414766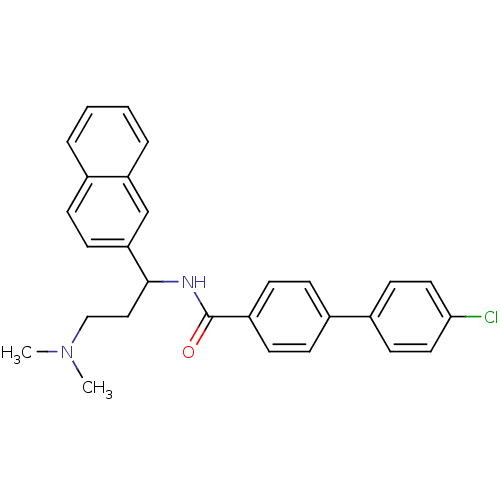 (CHEMBL572439) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 43.6 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM85517 (CAS_171436-38-7 | SL-NH2 | SLIGRL-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412709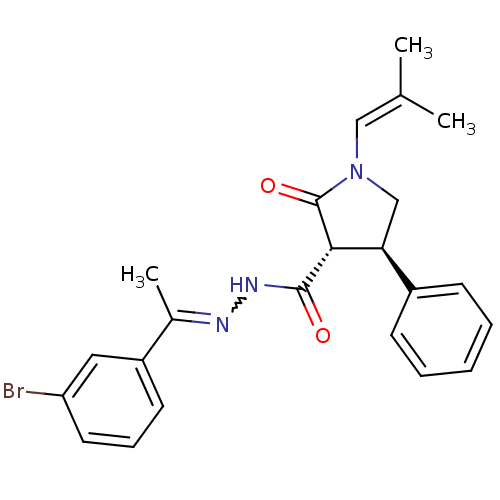 (CHEMBL461639) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412710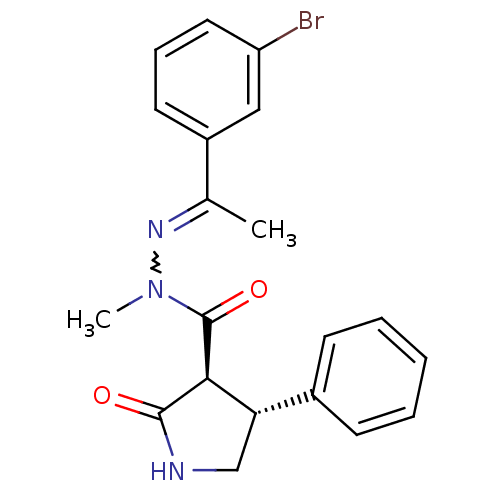 (CHEMBL459724) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412711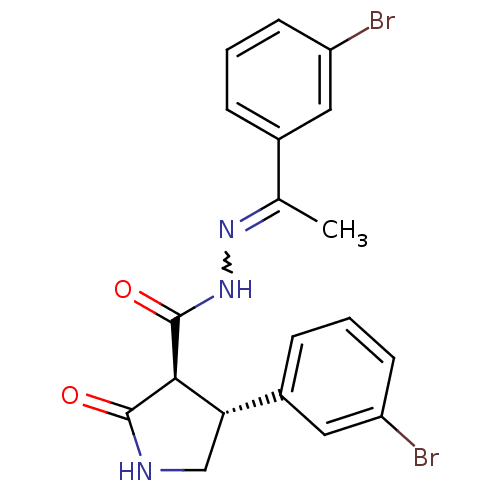 (CHEMBL459723) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412712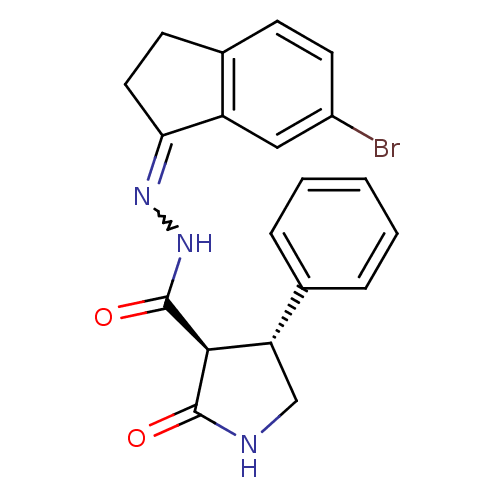 (CHEMBL459509) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412713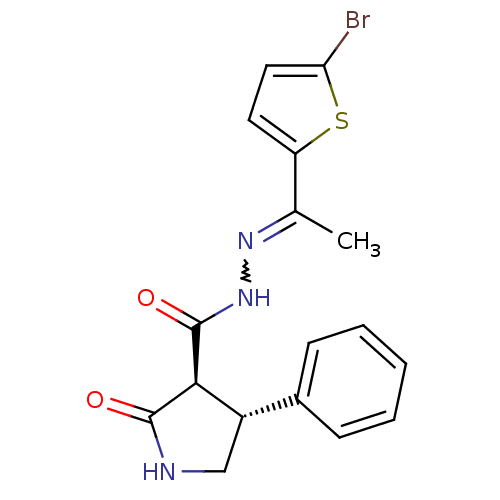 (CHEMBL493704) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412714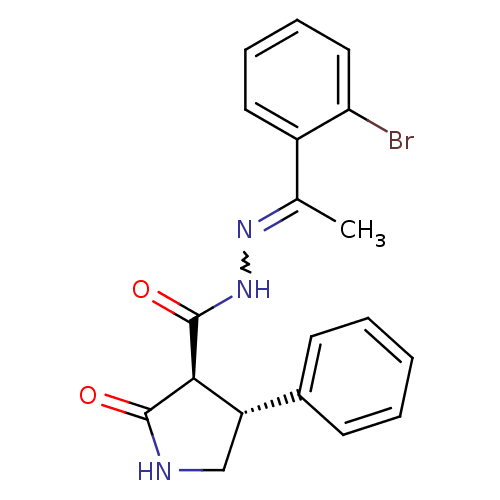 (CHEMBL493494) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412715 (CHEMBL494503) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412708 (AC-264613 | CHEMBL494502) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412716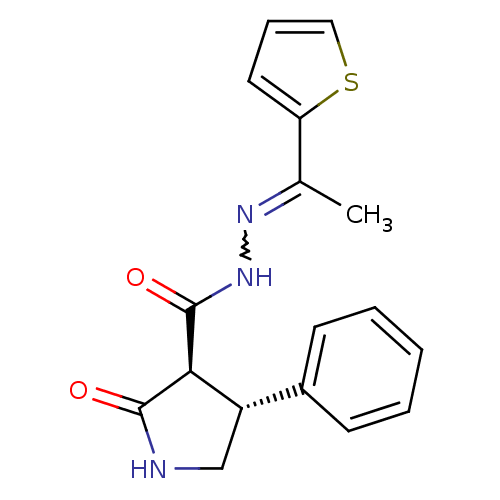 (AC-98170 | CHEMBL494303) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412717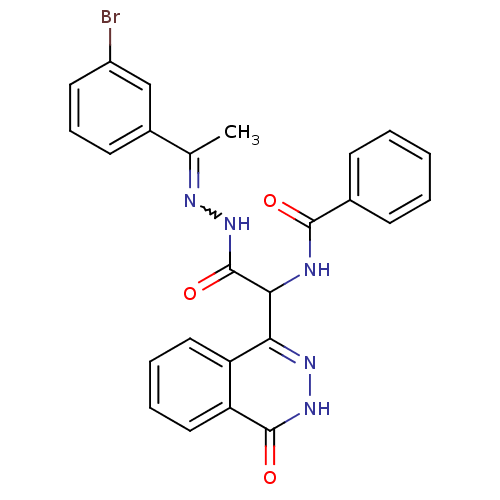 (AC-55541 | CHEMBL493076) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50412718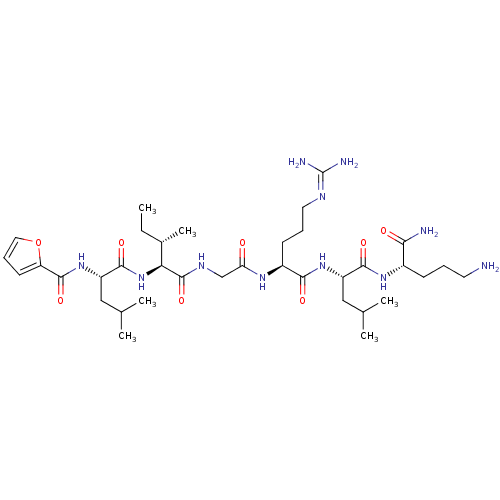 (CHEMBL509819) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in HEK293T cells assessed as effect on intracellular calcium mobilization by R-SAT assay | J Med Chem 51: 5490-3 (2008) Article DOI: 10.1021/jm800754r BindingDB Entry DOI: 10.7270/Q290251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414767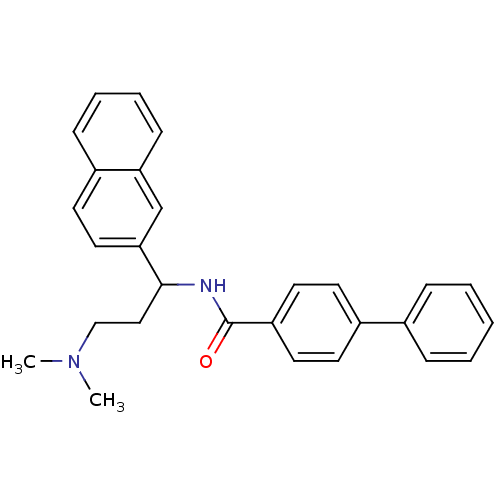 (CHEMBL574527) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414768 (CHEMBL572423) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414769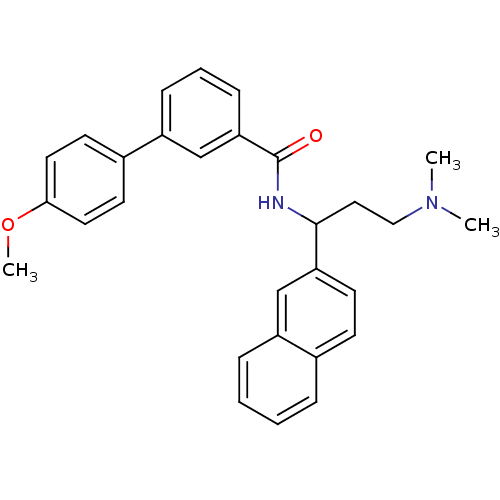 (CHEMBL573574) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 646 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 (Homo sapiens (Human)) | BDBM50414770 (CHEMBL574726) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Agonist activity against human urotensin 2 receptor expressed in human NIH373 cells assessed as beta-galactosidase activity after 5 days by R-SAT ass... | Bioorg Med Chem 17: 4657-65 (2009) Article DOI: 10.1016/j.bmc.2009.04.062 BindingDB Entry DOI: 10.7270/Q20866J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 217 total ) | Next | Last >> |