

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174619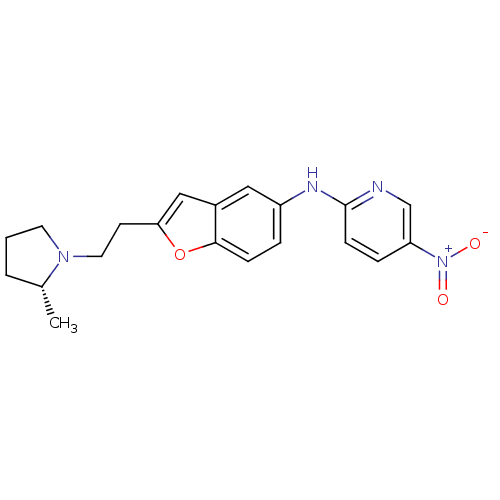 (CHEMBL197747 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in human K177 cell membrane incubated for 75 mins by liquid scintillation spectro... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115820 BindingDB Entry DOI: 10.7270/Q2WW7N96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in human K177 cell membrane incubated for 75 mins by liquid scintillation spectro... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115820 BindingDB Entry DOI: 10.7270/Q2WW7N96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074627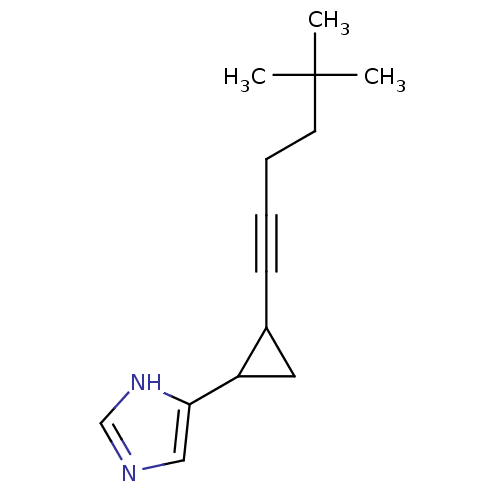 (4-[2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing rat histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158590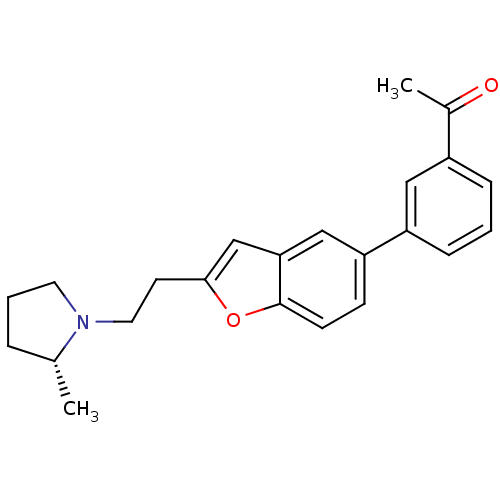 (1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174627 (CHEMBL199245 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158588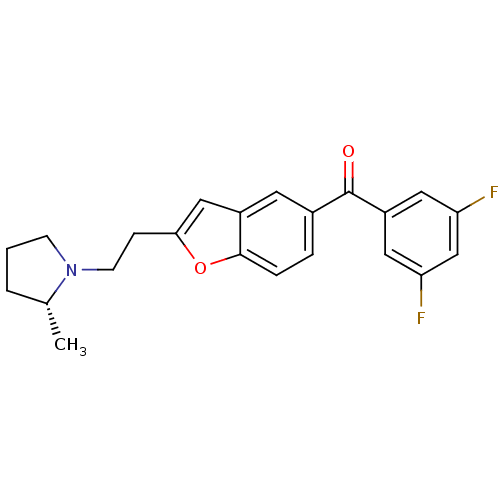 ((3,5-Difluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158596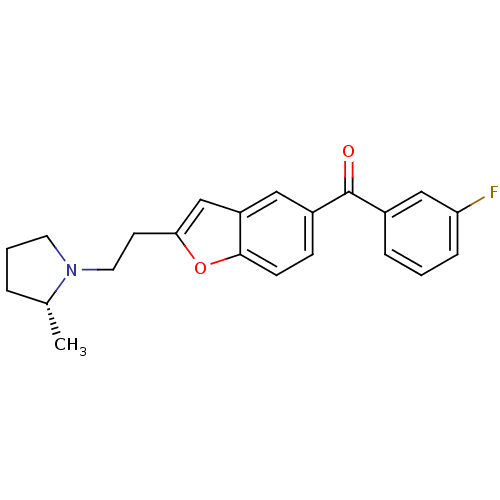 ((3-Fluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356873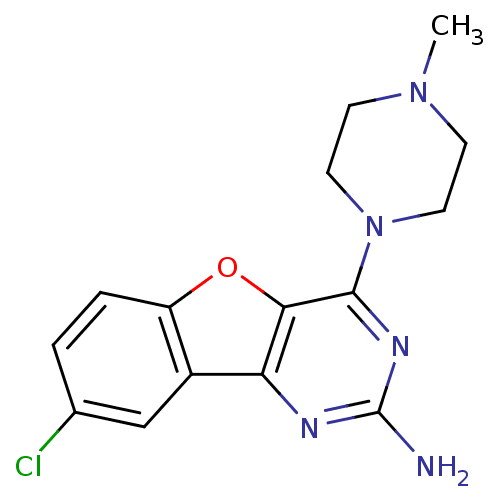 (CHEMBL1914541) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174621 (CHEMBL196467 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50174619 (CHEMBL197747 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for rat histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174613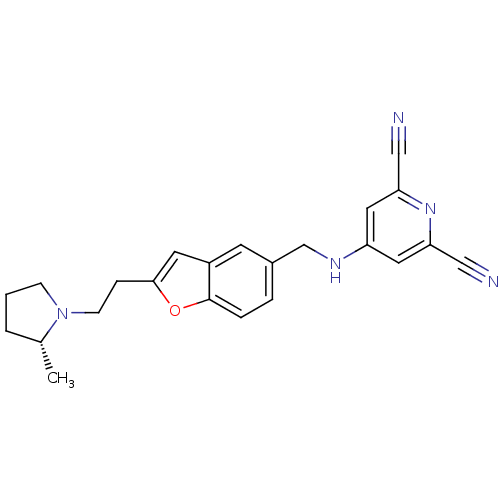 (4-({2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174637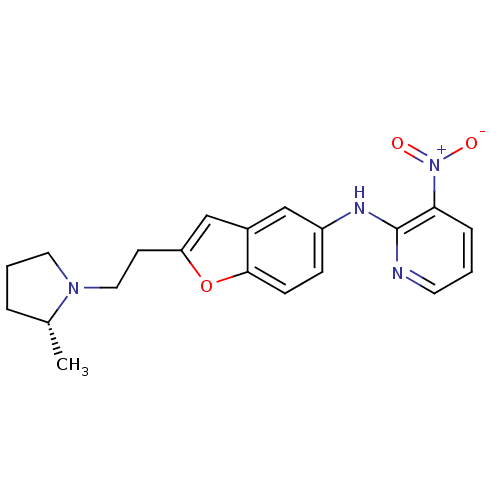 (CHEMBL196294 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174615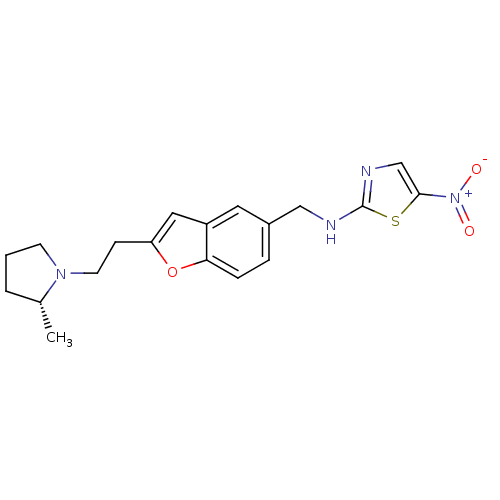 (CHEMBL424842 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158609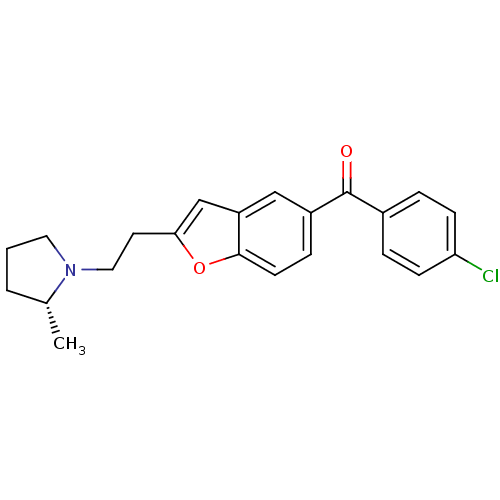 ((4-Chloro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158607 ((3-Chloro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158599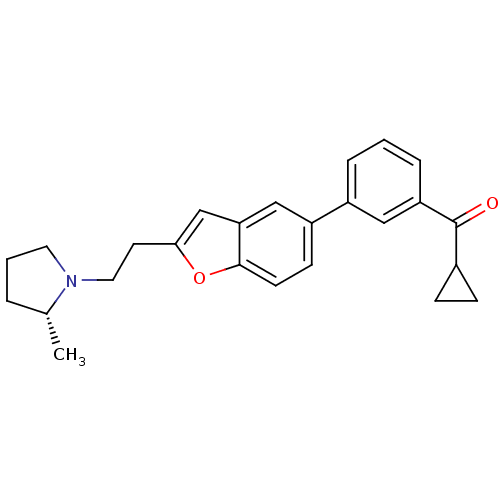 (CHEMBL369502 | Cyclopropyl-(3-{2-[2-((R)-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158603 ((4-Fluoro-3-methyl-phenyl)-{2-[2-((R)-2-methyl-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158592 (CHEMBL368699 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174620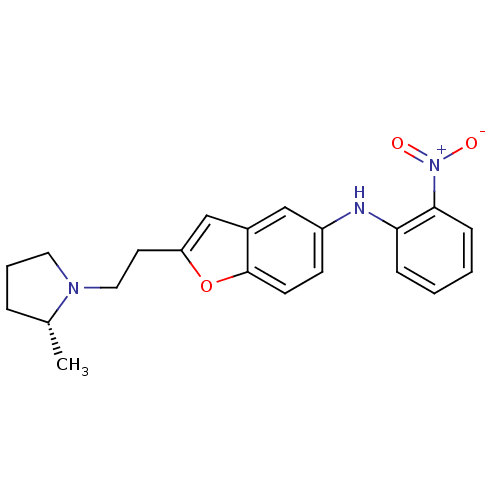 (CHEMBL371258 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158605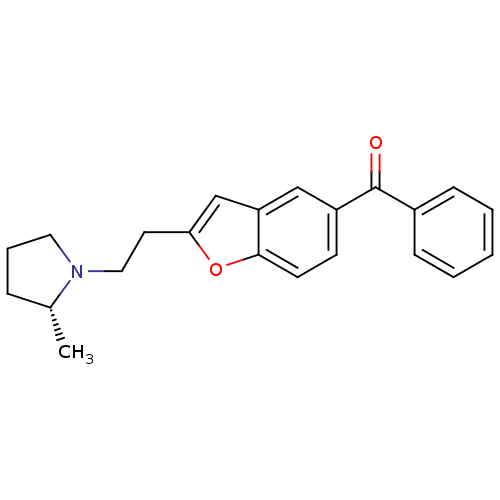 (CHEMBL362662 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human alpha3beta4 nAChR | Citation and Details Article DOI: 10.1016/j.bmc.2020.115820 BindingDB Entry DOI: 10.7270/Q2WW7N96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158593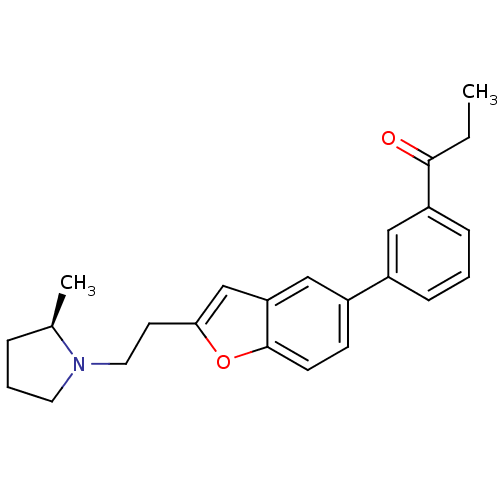 (1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158604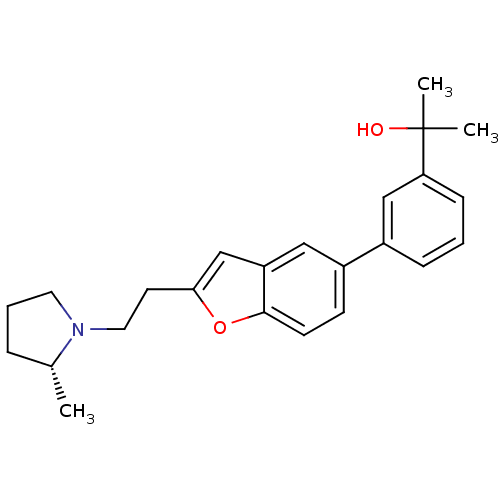 (1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158598 (CHEMBL178950 | Cyclopropyl-(4-{2-[2-((R)-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158602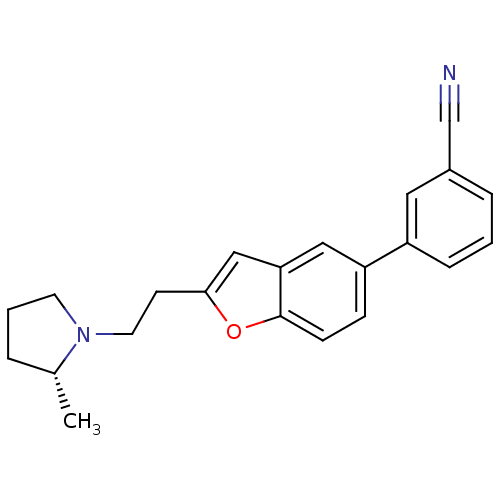 (3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50174627 (CHEMBL199245 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for rat histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174614 (4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158610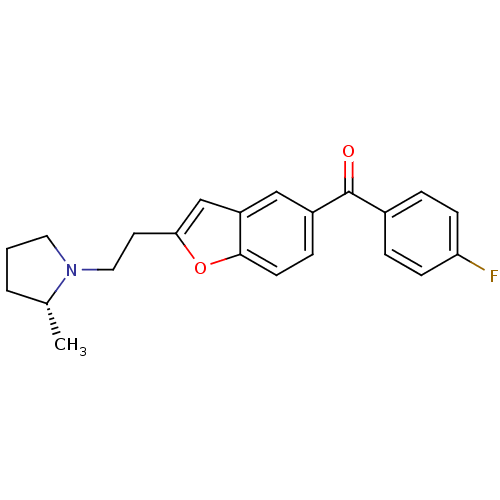 ((4-Fluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50143282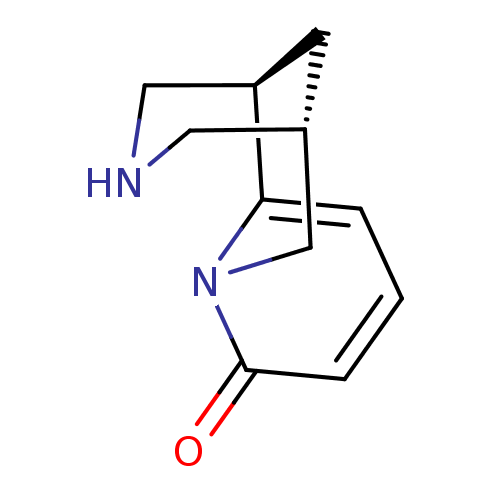 ((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR by Cheng-Prusoff equation analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115820 BindingDB Entry DOI: 10.7270/Q2WW7N96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174624 (CHEMBL371210 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174634 (3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356794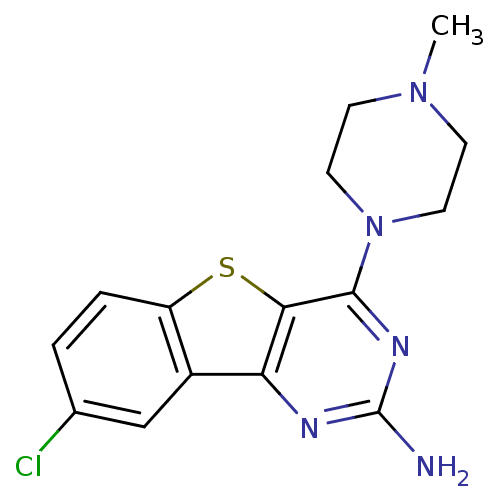 (CHEMBL1914462) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174631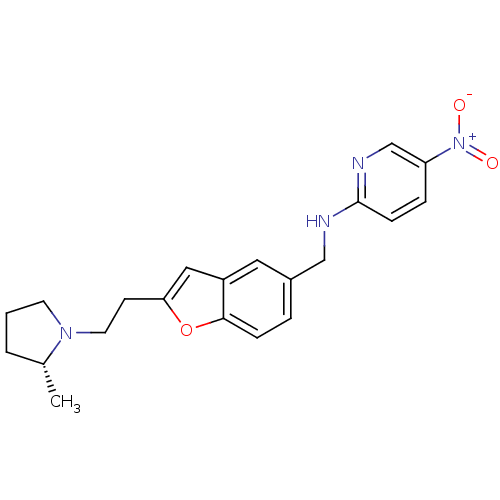 (CHEMBL196629 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174636 (CHEMBL198703 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174629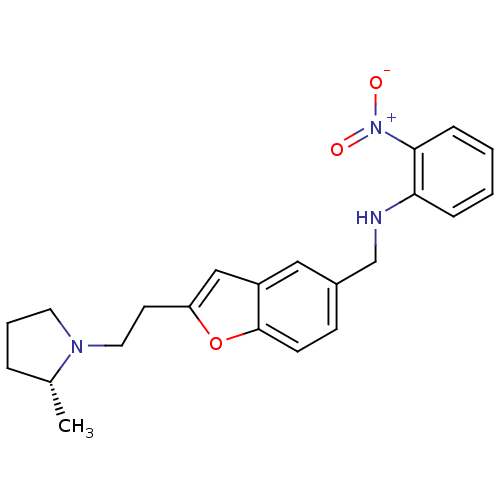 (CHEMBL194620 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174623 ((6-Chloro-pyridazin-3-yl)-{2-[2-((R)-2-methyl-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50548705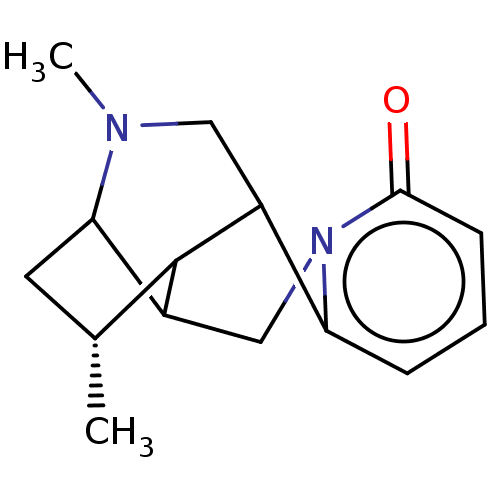 (CHEMBL4740159) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR by Cheng-Prusoff equation analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115820 BindingDB Entry DOI: 10.7270/Q2WW7N96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174625 ((5-Ethyl-pyrimidin-2-yl)-{2-[2-((R)-2-methyl-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174639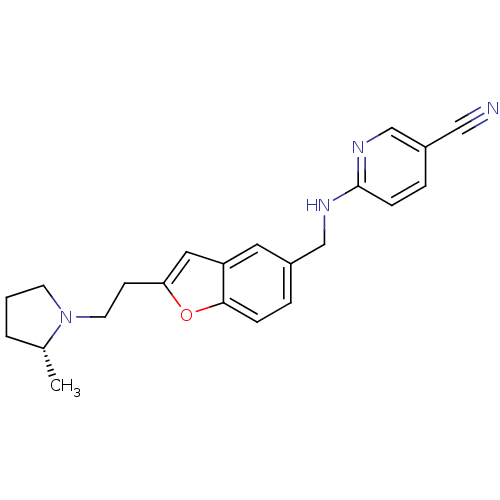 (6-({2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50174637 (CHEMBL196294 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for rat histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158608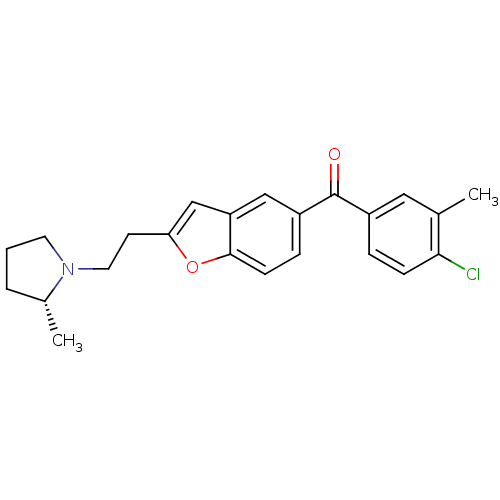 ((4-Chloro-3-methyl-phenyl)-{2-[2-((R)-2-methyl-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50174621 (CHEMBL196467 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for rat histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50174613 (4-({2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for rat histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143282 ((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]nicotine from rat alpha4beta2 nAChR by liquid scintillation counting | Citation and Details Article DOI: 10.1016/j.bmc.2020.115820 BindingDB Entry DOI: 10.7270/Q2WW7N96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315348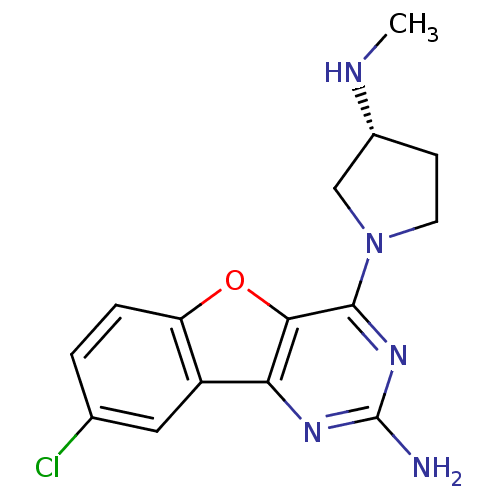 ((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174612 (3-({2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50158590 (1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing rat histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174640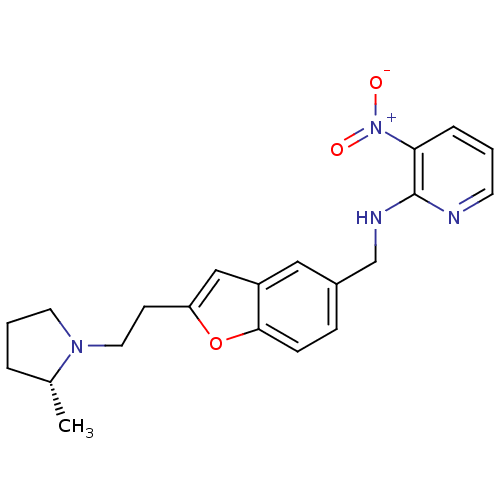 (CHEMBL199187 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158606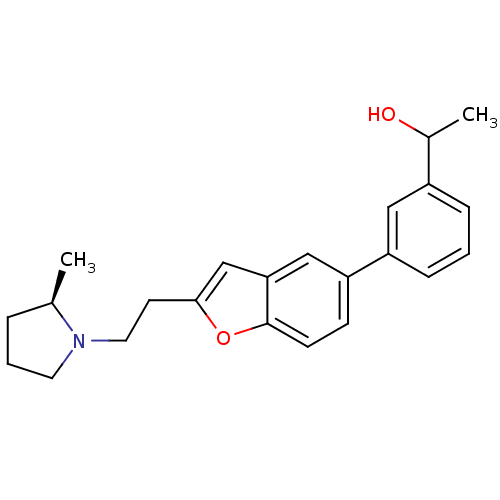 (1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 730 total ) | Next | Last >> |