

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50169441 (CHEMBL3806205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting | ACS Med Chem Lett 7: 283-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00448 BindingDB Entry DOI: 10.7270/Q2D79D95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50169441 (CHEMBL3806205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting | ACS Med Chem Lett 7: 283-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00448 BindingDB Entry DOI: 10.7270/Q2D79D95 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM258470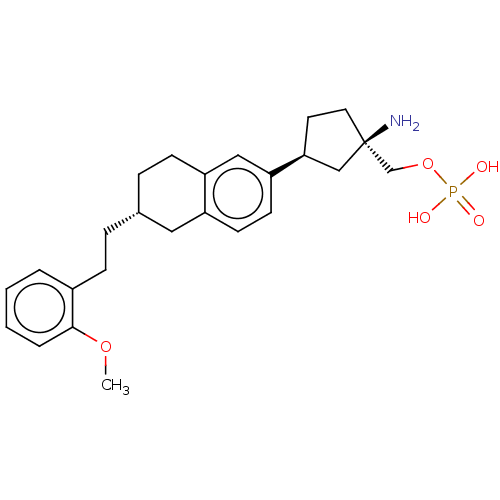 (US9522888, 697) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230106 (US10106559, Example 31 | US10435415, Example 31 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194718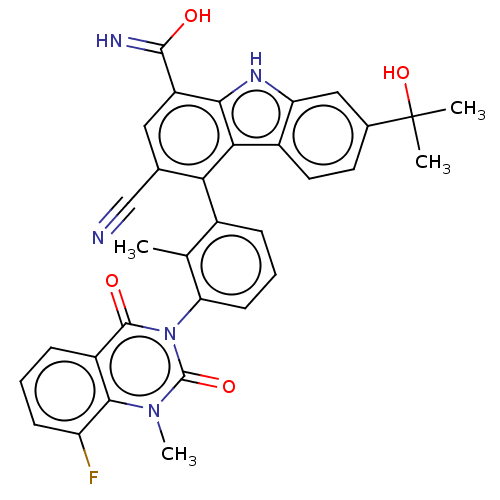 (CHEMBL3899411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM197654 (US9216972, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human S1P1 | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5CD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194722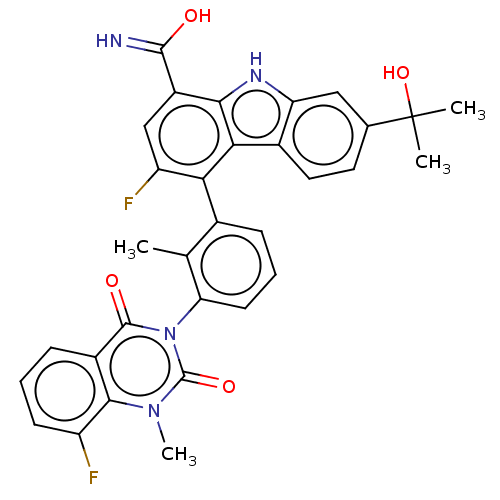 (CHEMBL3896019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50547850 (CHEMBL4741099) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194720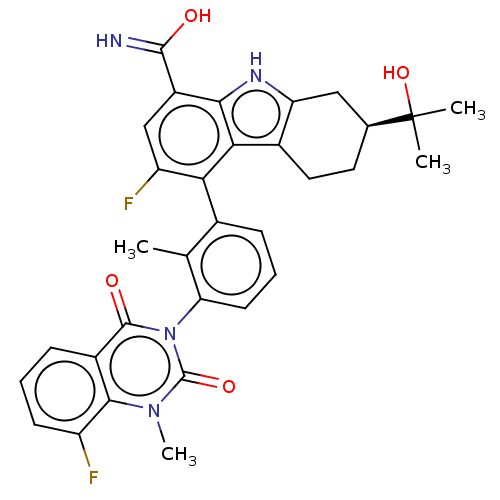 (CHEMBL3900554) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194720 (CHEMBL3900554) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264137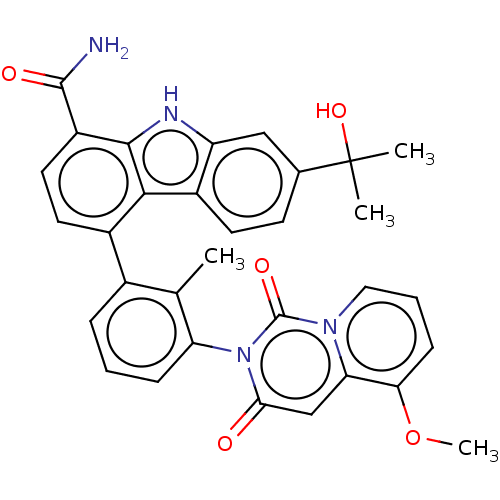 (7-(2-Hydroxypropan-2-yl)-4-(3-(5-methoxy-1,3-dioxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230107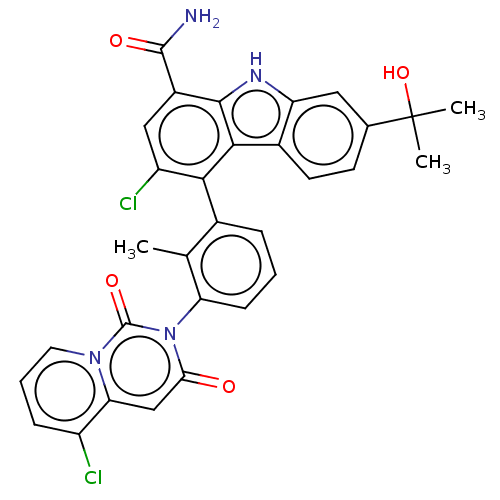 (US10106559, Example 33 | US10435415, Example 33 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50547849 (CHEMBL4789404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194717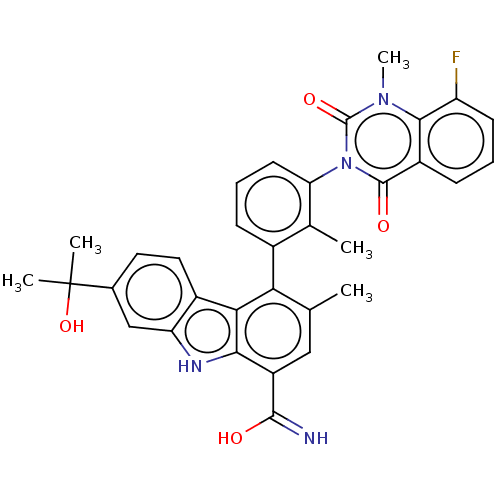 (CHEMBL3931086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264146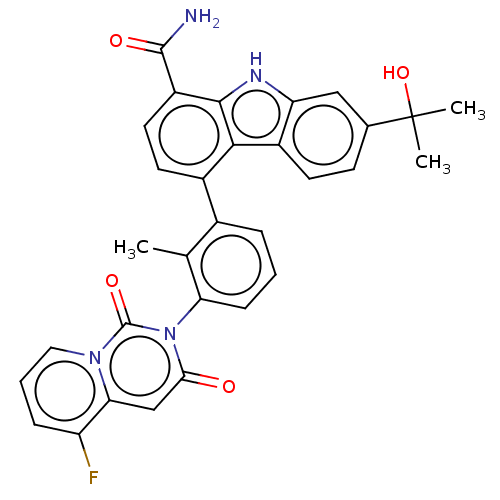 (4-(3-(5-Fluoro-1,3-dioxo-1H-pyrido[1,2-c]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264146 (4-(3-(5-Fluoro-1,3-dioxo-1H-pyrido[1,2-c]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264139 (4-(3-(R)-(5-Chloro-1,3-dioxo-1H-pyrido[1,2-c]pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230081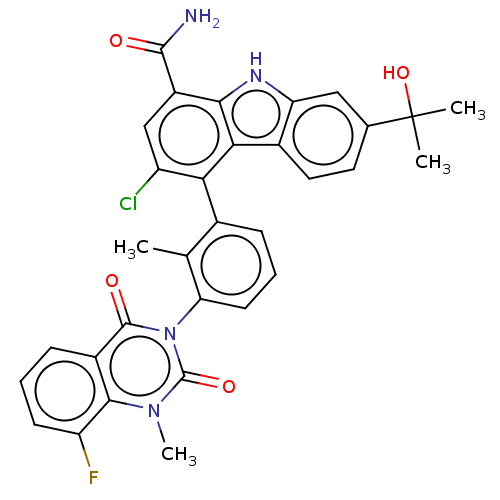 (US9334290, 1 | US9334290, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264139 (4-(3-(R)-(5-Chloro-1,3-dioxo-1H-pyrido[1,2-c]pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264139 (4-(3-(R)-(5-Chloro-1,3-dioxo-1H-pyrido[1,2-c]pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230081 (US9334290, 1 | US9334290, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230107 (US10106559, Example 33 | US10435415, Example 33 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human peripheral B cells assessed as reduction in anti-IgM/IgG-induced CD86 surface expression | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230101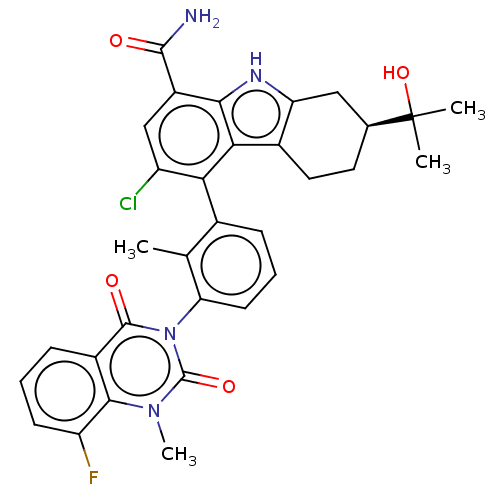 (US10106559, Example 58 | US10435415, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230091 (US10106559, Example 14 | US10435415, Example 14 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230091 (US10106559, Example 14 | US10435415, Example 14 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50547848 (CHEMBL4741884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264146 (4-(3-(5-Fluoro-1,3-dioxo-1H-pyrido[1,2-c]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50547848 (CHEMBL4741884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50550026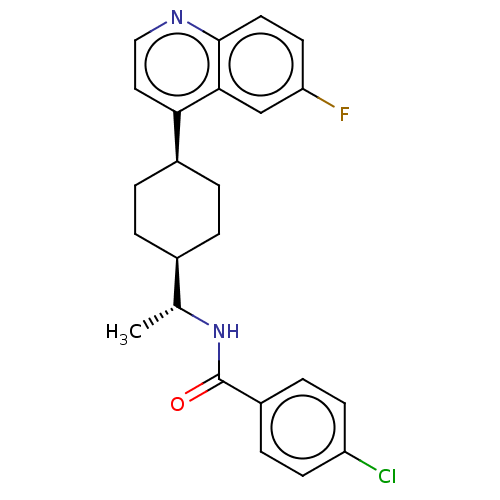 (CHEMBL4786690) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated mouse M109 cells preincubated for 2 hrs followed by recombinant murine IFNgamma stimulation and measured af... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50550026 (CHEMBL4786690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230107 (US10106559, Example 33 | US10435415, Example 33 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC cells assessed as inhibition of FCepsilonR1/immune complex-induced TNFalpha production | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50571879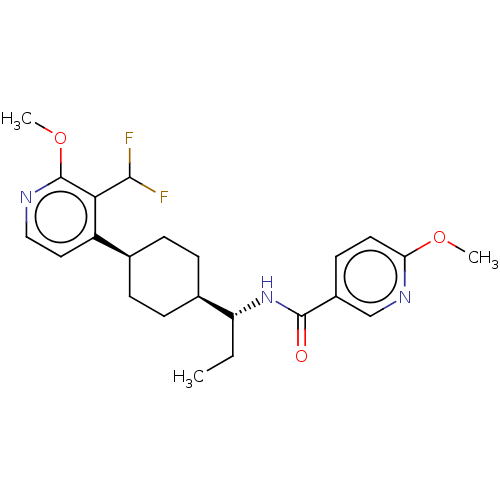 (CHEMBL4848094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50571880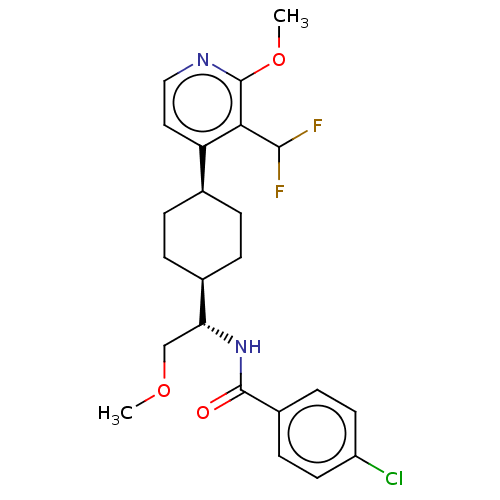 (CHEMBL4851220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50571881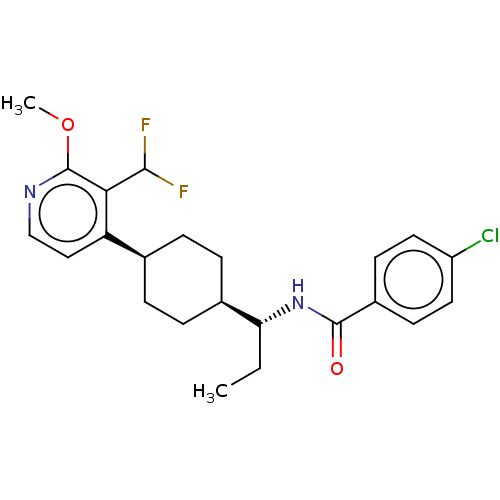 (CHEMBL4845988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50571883 (CHEMBL4868709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230103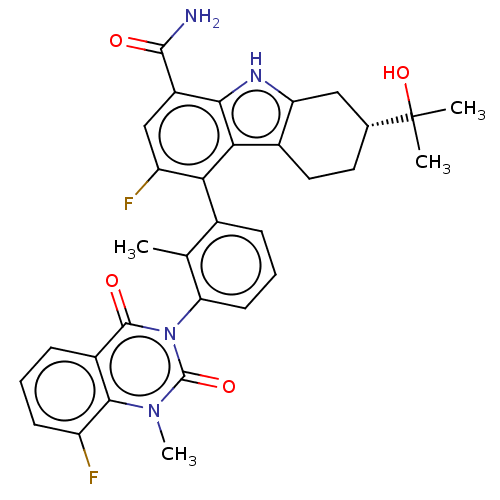 (US10106559, Example 27 | US10435415, Example 27 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50571879 (CHEMBL4848094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma/LPS-stimulated human whole blood preincubated for 4 hrs followed by IFNgamma/LPS stimulation and incubated for 18 hrs ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194715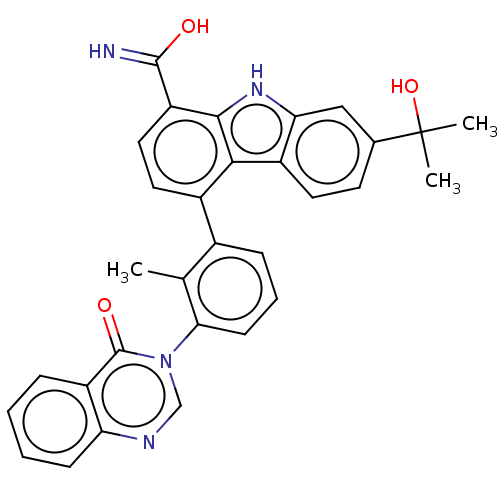 (CHEMBL3918580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194723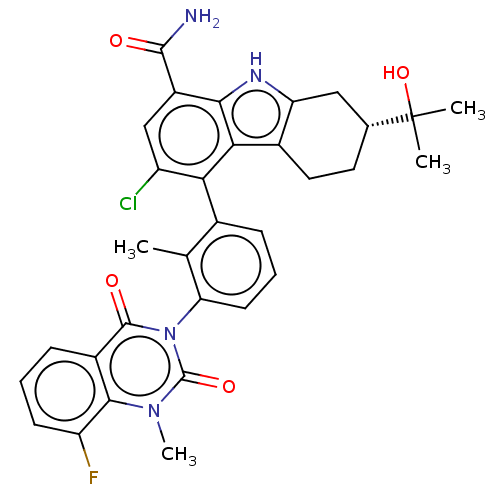 (CHEMBL3944049) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194724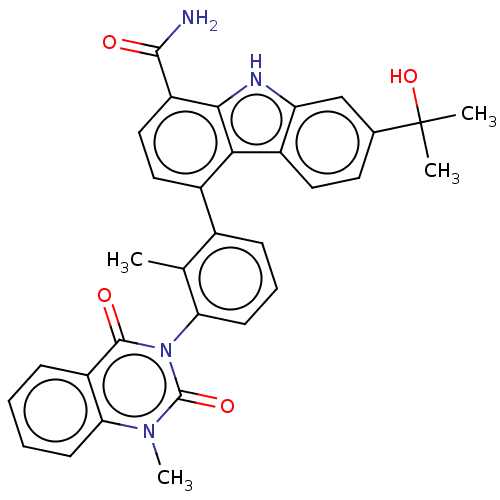 (CHEMBL3941224) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194719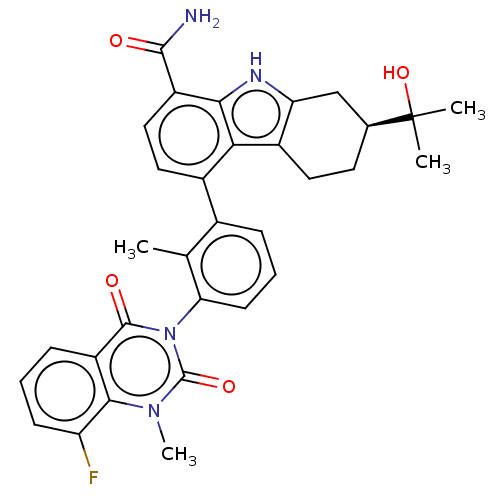 (CHEMBL3976719) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194721 (CHEMBL3908310 | US9714234, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... | J Med Chem 59: 9173-9200 (2016) Article DOI: 10.1021/acs.jmedchem.6b01088 BindingDB Entry DOI: 10.7270/Q23T9K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50571883 (CHEMBL4868709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma/LPS-stimulated human whole blood preincubated for 4 hrs followed by IFNgamma/LPS stimulation and incubated for 18 hrs ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50571884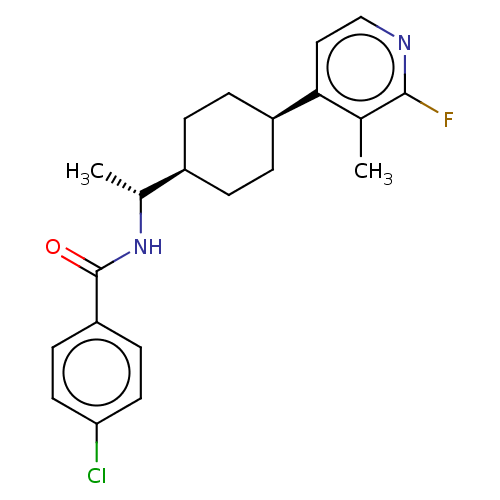 (CHEMBL4859400) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated mouse M109 cells preincubated for 2 hrs followed by recombinant murine IFNgamma stimulation and measured af... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM230107 (US10106559, Example 33 | US10435415, Example 33 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TEC (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50571882 (CHEMBL4860353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50194715 (CHEMBL3918580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50571886 (CHEMBL4861763) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated mouse M109 cells preincubated for 2 hrs followed by recombinant murine IFNgamma stimulation and measured af... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00236 BindingDB Entry DOI: 10.7270/Q2KS6W9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 437 total ) | Next | Last >> |