

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys(acetyl)-AMC as substrate after 1 hr by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human HDAC10 (1 to 631 residues) expressed in baculovirus expression system in Sf9 cells RHKAcKAc after 2 hrs by ... | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of full length human HDAC8 expressed in an E. coli expression system using AMC-labeled RHKAcKAc as substrate after 2 hrs by fluorescence a... | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252391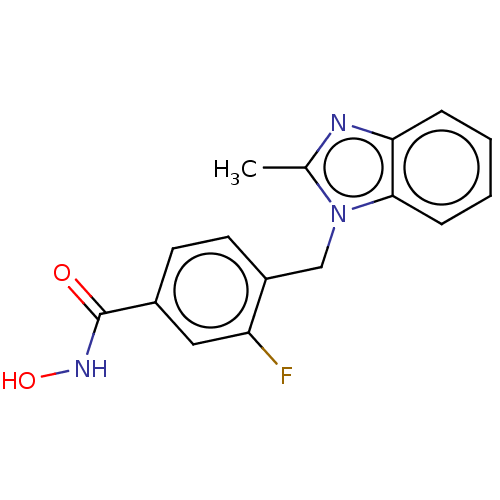 (CHEMBL4080014) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys(acetyl)-AMC as substrate after 1 hr by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163243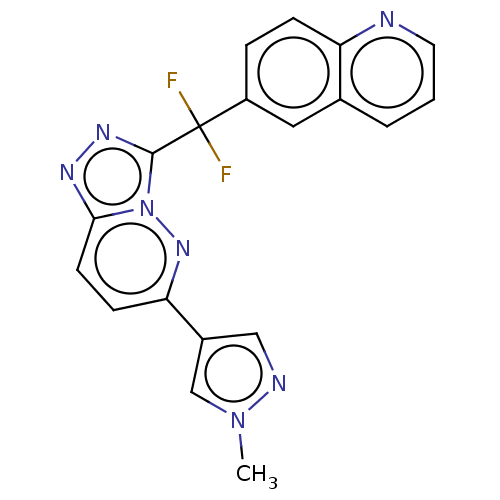 (US9062045, Comparator No. 1 (JNJ-38877605)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM119703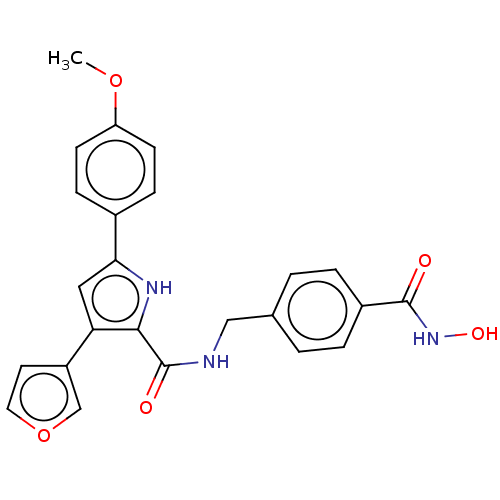 (US8685992, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged full length human HDAC6 expressed in baculovirus expression system in Sf9 cells using AMC-labeled RHKKAc as subst... | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50252394 (CHEMBL4090165) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3 using Boc-Lys (Ac)-AMC substrate by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163243 (US9062045, Comparator No. 1 (JNJ-38877605)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252395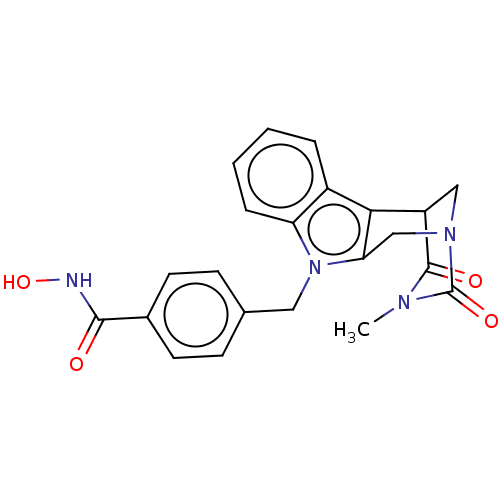 (CHEMBL4087616) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of human HDAC6 using RHKKAc as substrate by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252396 (CHEMBL4077645) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys(acetyl)-AMC as substrate after 1 hr by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Zea mays) | BDBM50105329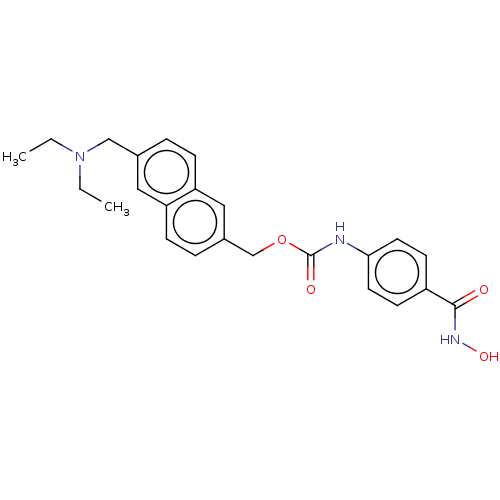 (CHEMBL1213492) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of maize HD1B | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252390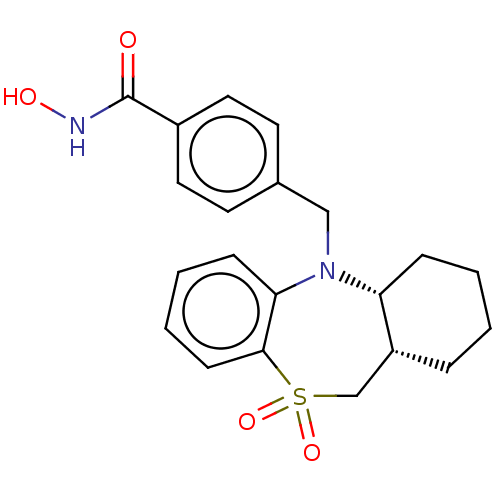 (CHEMBL4104247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys(acetyl)-AMC as substrate after 1 hr by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252392 (CHEMBL4069287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys(acetyl)-AMC as substrate after 1 hr by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50252393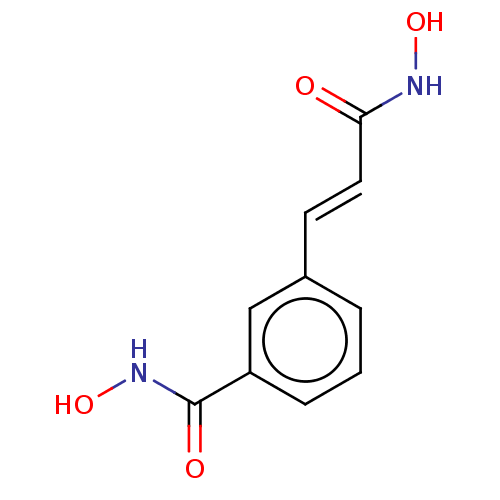 (CHEMBL4060412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50397360 (CHEMBL2170177 | US10188756, Compound CN110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of HDAC7 (unknown origin) using acetyl-Gly-Ala-(N-acetyl-Lys)-amino-4-methylcoumarin as substrate after 15 mins by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252394 (CHEMBL4090165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys (Ac)-AMC substrate by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50499377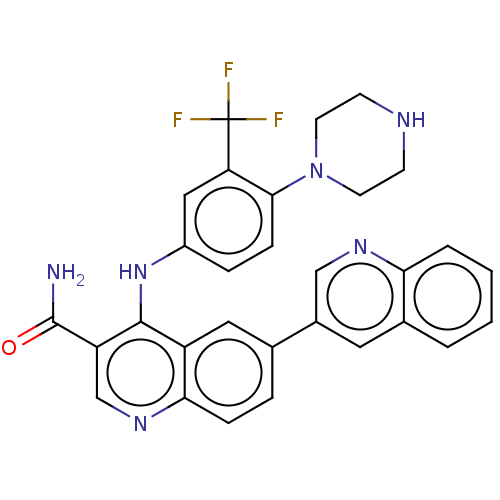 (CHEMBL3735194) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380399 (CHEMBL2018302 | Tubastatin A | US10227295, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys(acetyl)-AMC as substrate after 1 hr by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | CHEMBL5269658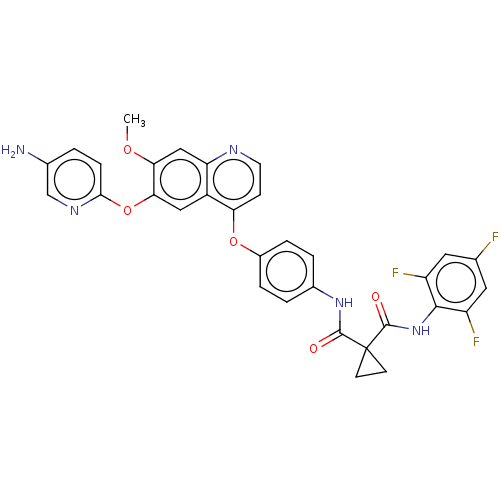 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | CHEMBL5288199 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | CHEMBL5290660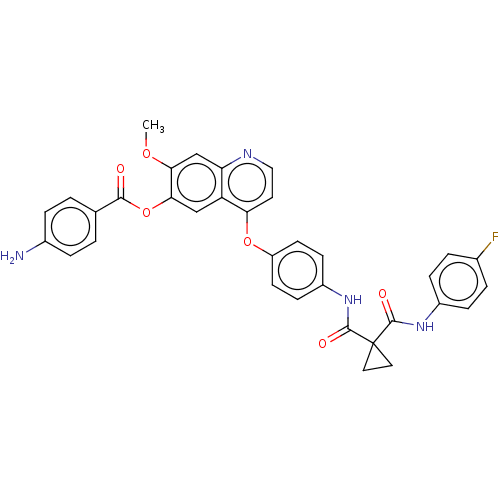 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | CHEMBL5283802 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | CHEMBL5267367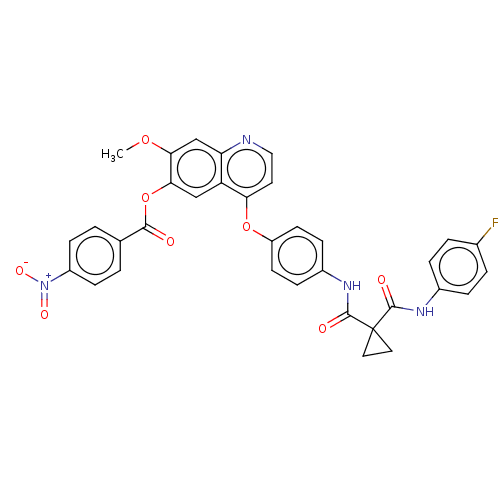 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50517959 (CHEMBL4536115) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50517960 (CHEMBL4447225) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50517974 (CHEMBL4457733) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50517966 (CHEMBL4587811) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50252394 (CHEMBL4090165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-Lys (Ac)-AMC substrate by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252388 (CHEMBL4079541) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of human HDAC6 after 15 mins by trypsin-coupled fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50517962 (CHEMBL4462318) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50517967 (CHEMBL4443828) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50517958 (CHEMBL4470236) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50252394 (CHEMBL4090165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC2 using Boc-Lys (Ac)-AMC substrate by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50252391 (CHEMBL4080014) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human HDAC7 (518 to end residues) expressed in baculovirus expression system using AMC-labeled RHKKAc as substrat... | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | CHEMBL5273741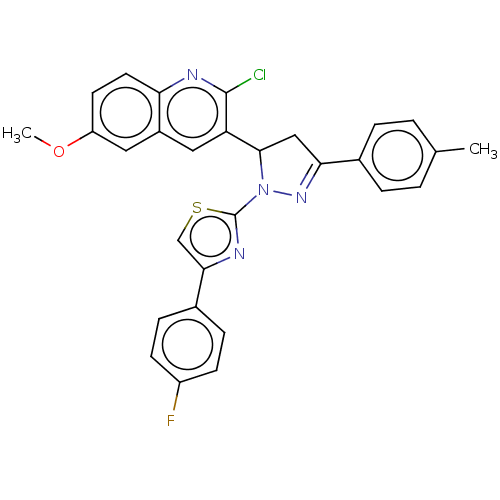 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252389 (CHEMBL4103801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys(acetyl)-AMC as substrate after 1 hr by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | CHEMBL5274305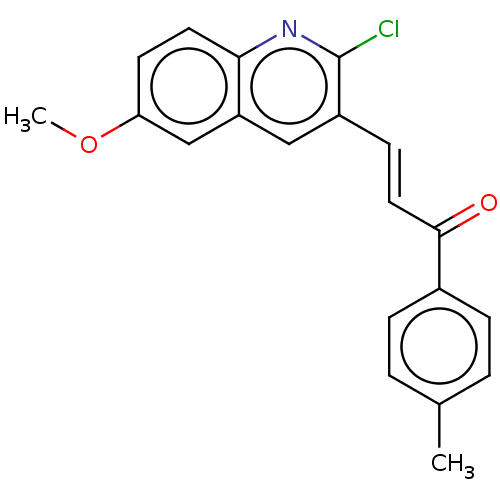 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50021574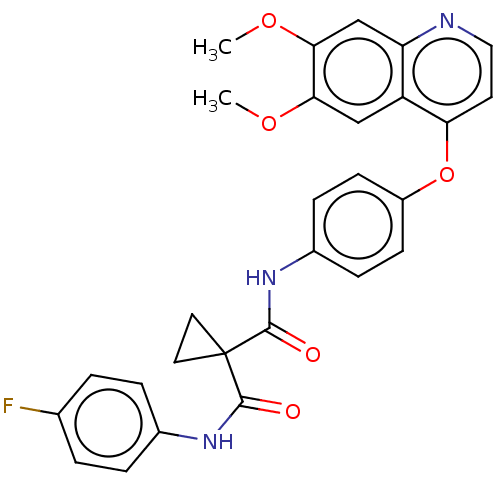 (BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | CHEMBL5269307 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | CHEMBL5287460 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM198121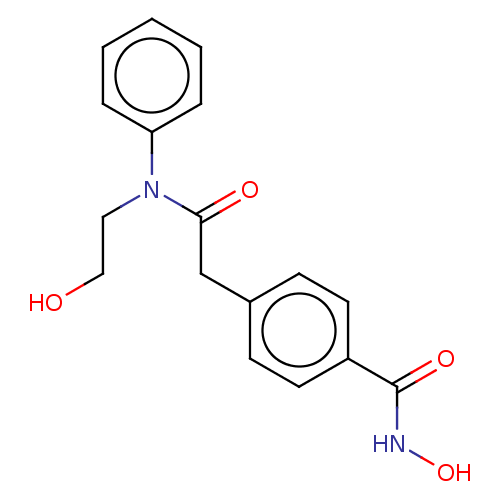 (HPOB) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of human HDAC6 after 15 mins by trypsin-coupled fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50252391 (CHEMBL4080014) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human HDAC9 (604 to 1066 residues) expressed in baculovirus expression system after 2 hrs by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50252393 (CHEMBL4060412) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM119703 (US8685992, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of C-terminal GST-tagged full length human HDAC1 expressed in baculovirus expression system in Sf9 cells using AMC-labeled RHKKAc as subst... | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | CHEMBL5290778 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | <75 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | CHEMBL5286891 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | <75 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 165 total ) | Next | Last >> |