

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106803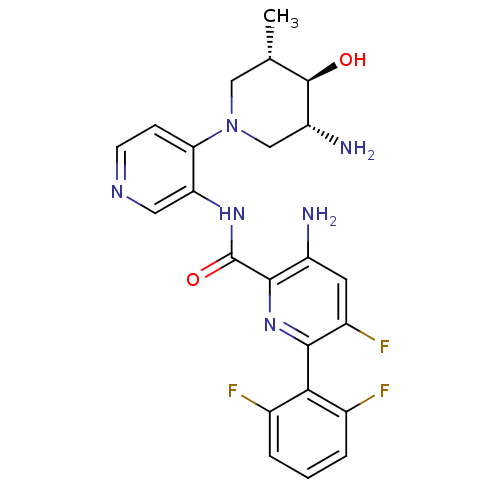 (US8592455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50445133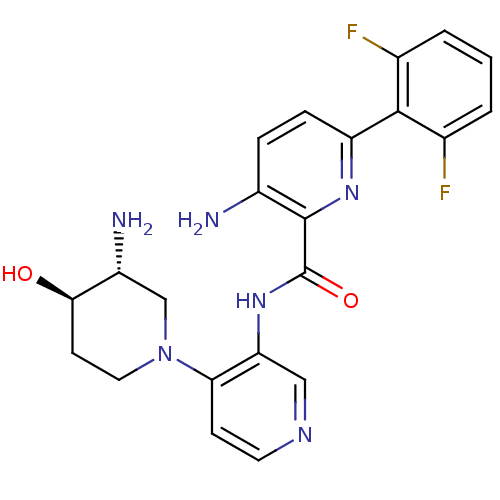 (CHEMBL3103869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106803 (US8592455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50445124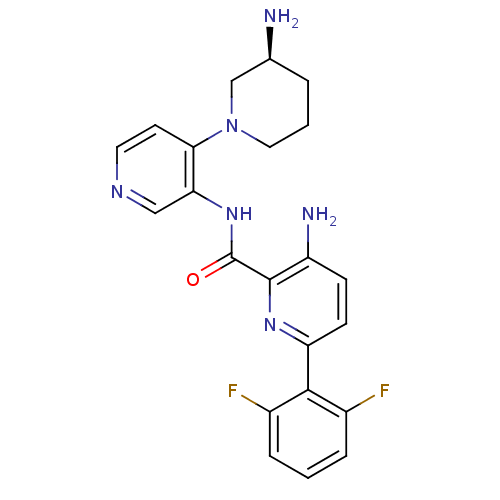 (CHEMBL3103868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106803 (US8592455, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50445124 (CHEMBL3103868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106870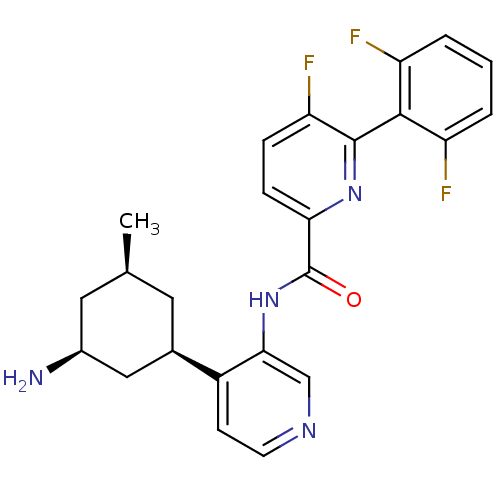 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50445124 (CHEMBL3103868) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50445133 (CHEMBL3103869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50445133 (CHEMBL3103869) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50387298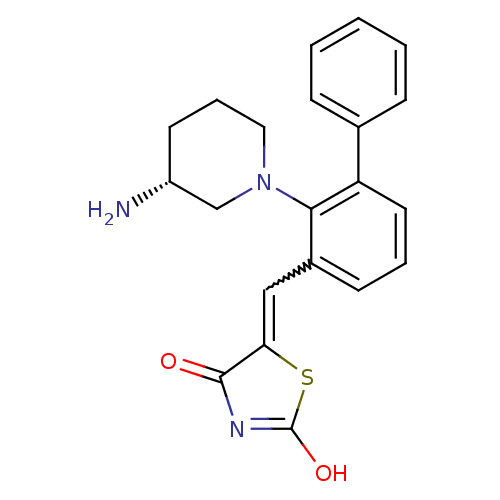 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50596723 (CHEMBL5205903 | US20230348421, Compound 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114246 BindingDB Entry DOI: 10.7270/Q20P142F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM23936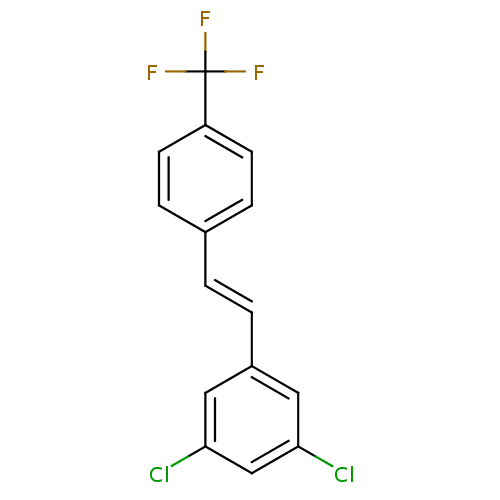 (1,3-dichloro-5-[(E)-2-[4-(trifluoromethyl)phenyl]e...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220432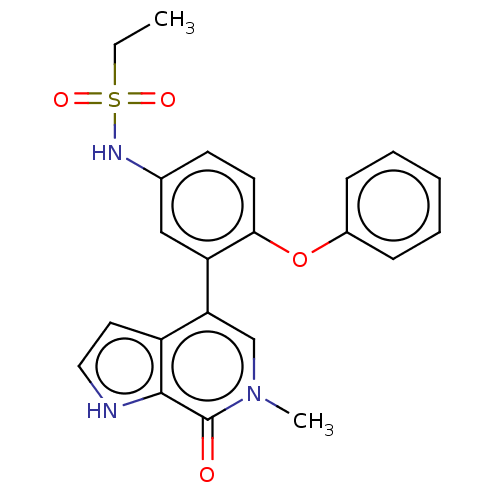 (US9296741, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220432 (US9296741, 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.390 | -53.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220626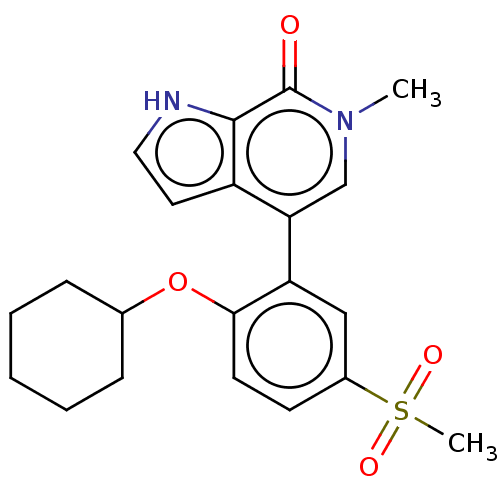 (US9296741, 215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.460 | -53.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50457489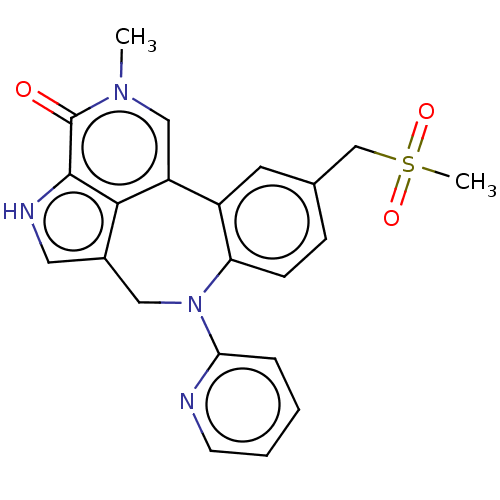 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD2 bromodomain 1 to 2 (G73 to A560 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by b... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220518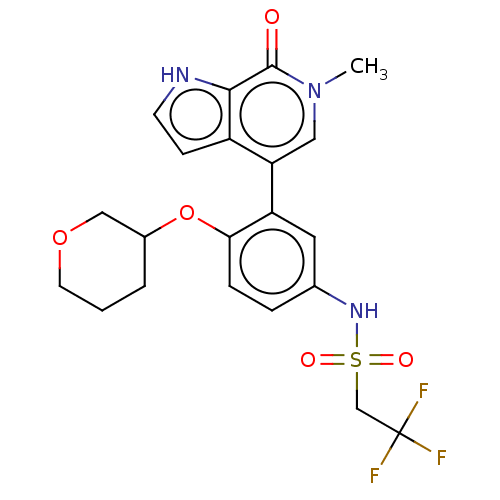 (US9296741, 107) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220484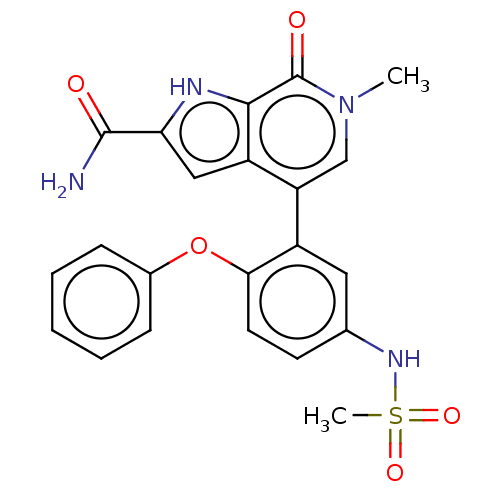 (US9296741, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220452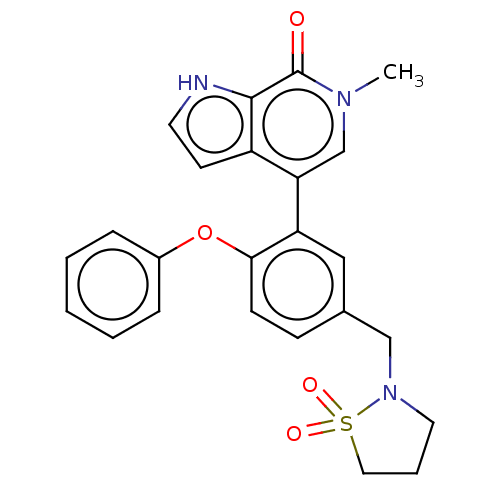 (US9296741, 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.510 | -53.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220516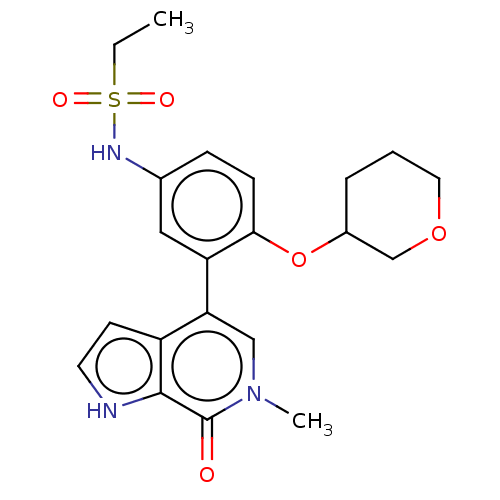 (US9296741, 105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | -53.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220679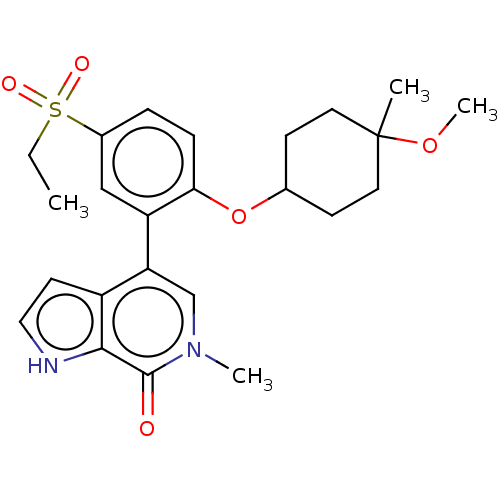 (US9296741, 268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | -52.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260067 (CHEMBL4078267 | US9957263, Example 78) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Compound dilution series were prepared in DMSO via a 3-fold serial dilution from 2.5 mM to 42 nM. Compounds were then diluted 6:100 in assay buffer (... | Bioorg Med Chem Lett 19: 360-4 (2009) BindingDB Entry DOI: 10.7270/Q2Q81GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM390899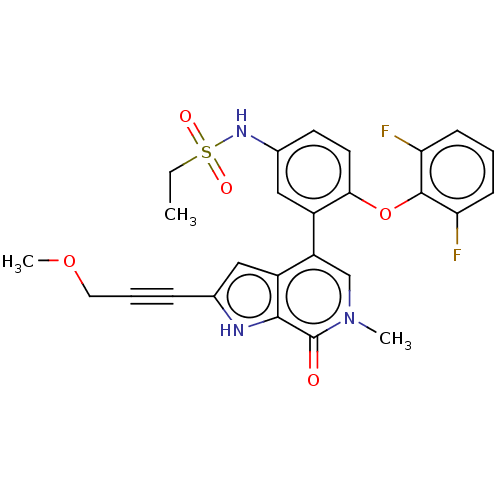 (N-{4-(2,4-difluorophenoxy)-3-[2-(3-methoxyprop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.605 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Compound dilution series were prepared in DMSO via a 3-fold serial dilution from 2.5 mM to 42 nM. Compounds were then diluted 6:100 in assay buffer (... | Bioorg Med Chem Lett 19: 360-4 (2009) BindingDB Entry DOI: 10.7270/Q2Q81GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457489 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220507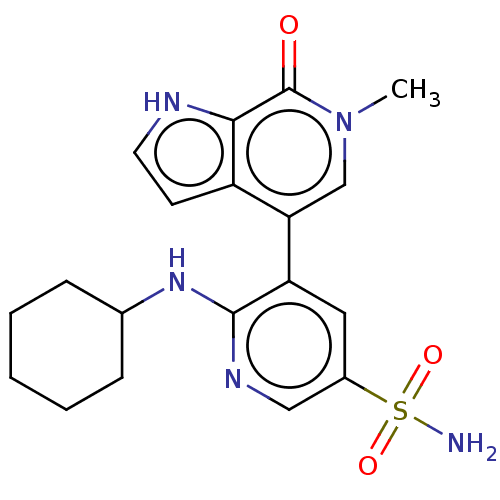 (US9296741, 96) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | -52.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220495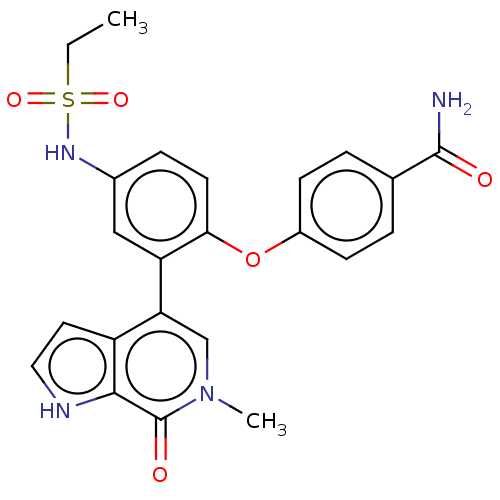 (US9296741, 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | -52.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50156345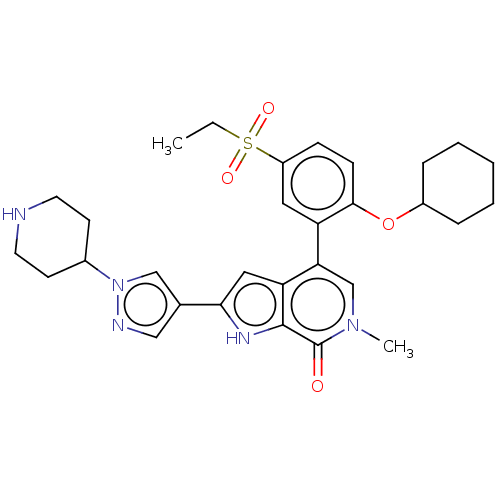 (CHEMBL3787680 | US9957263, Example 149) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.669 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Compound dilution series were prepared in DMSO via a 3-fold serial dilution from 2.5 mM to 42 nM. Compounds were then diluted 6:100 in assay buffer (... | Bioorg Med Chem Lett 19: 360-4 (2009) BindingDB Entry DOI: 10.7270/Q2Q81GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220431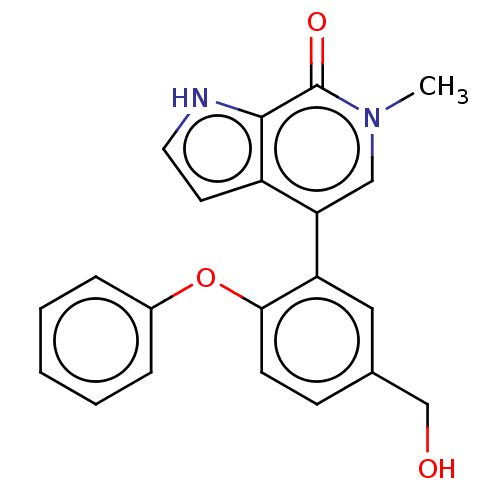 (US10633379, Compound Z | US9296741, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.680 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220614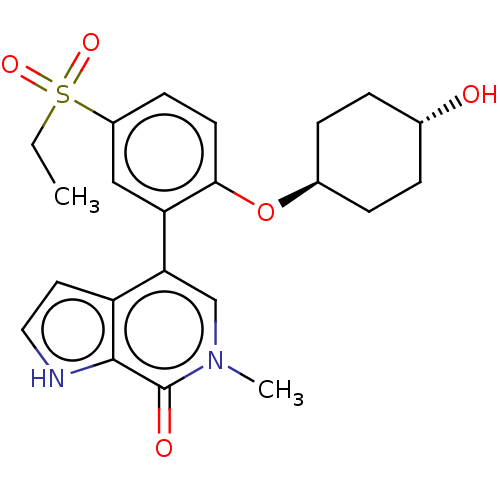 (US9296741, 203) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.690 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50457489 (CHEMBL4208129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRDT bromodomain 1 to 2 (N21to P380 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by br... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220520 (US9296741, 109) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457496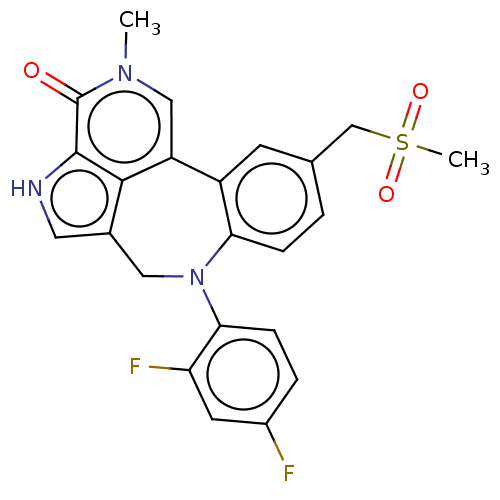 (CHEMBL4217457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD4 bromodomain 2 (E352 to M457 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromo... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220477 (US9296741, 66) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.740 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220614 (US9296741, 203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220471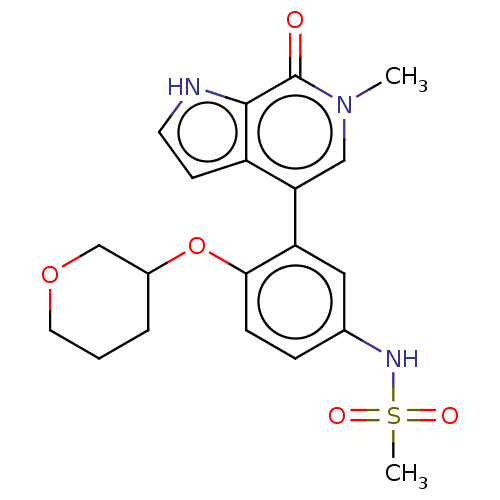 (US9296741, 60) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220434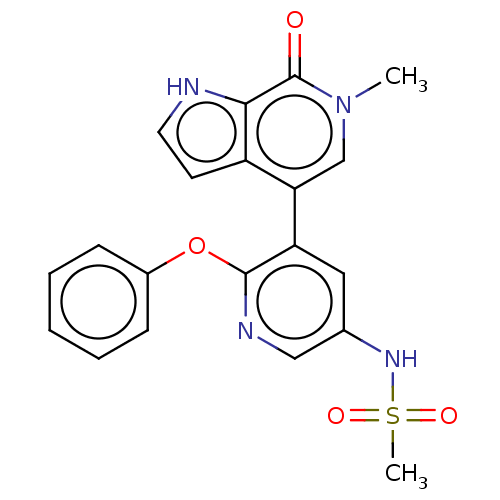 (US9296741, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220679 (US9296741, 268) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.760 | -52.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220649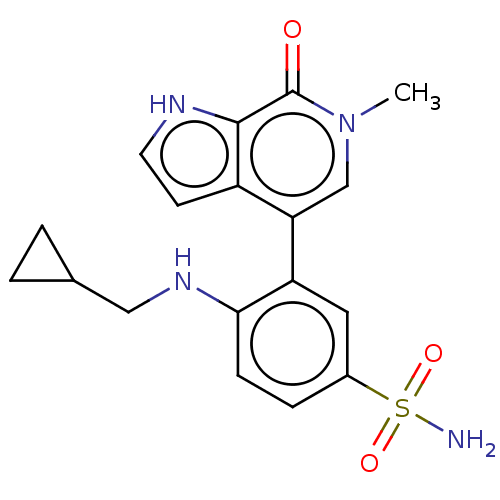 (US9296741, 238) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.780 | -52.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220608 (US9296741, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.790 | -52.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3564 total ) | Next | Last >> |