 Found 104 hits with Last Name = 'dal piaz' and Initial = 'f'
Found 104 hits with Last Name = 'dal piaz' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50428820
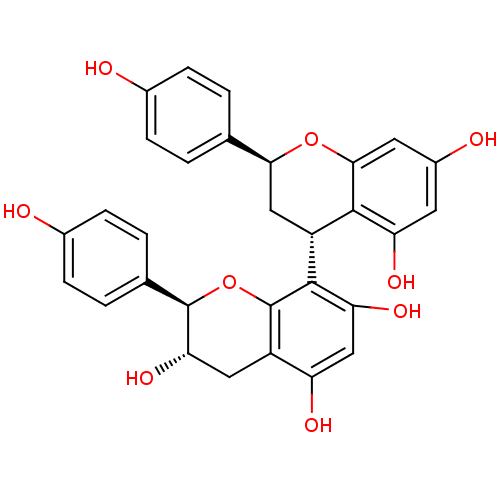
(CHEMBL2335722)Show SMILES O[C@H]1Cc2c(O)cc(O)c([C@@H]3C[C@H](Oc4cc(O)cc(O)c34)c3ccc(O)cc3)c2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H26O9/c31-16-5-1-14(2-6-16)25-12-20(27-22(35)9-18(33)10-26(27)38-25)28-23(36)13-21(34)19-11-24(37)29(39-30(19)28)15-3-7-17(32)8-4-15/h1-10,13,20,24-25,29,31-37H,11-12H2/t20-,24+,25+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PlGF-1/VEGFR-1 (unknown origin) interaction after 1 hr by ELISA |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50428820

(CHEMBL2335722)Show SMILES O[C@H]1Cc2c(O)cc(O)c([C@@H]3C[C@H](Oc4cc(O)cc(O)c34)c3ccc(O)cc3)c2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H26O9/c31-16-5-1-14(2-6-16)25-12-20(27-22(35)9-18(33)10-26(27)38-25)28-23(36)13-21(34)19-11-24(37)29(39-30(19)28)15-3-7-17(32)8-4-15/h1-10,13,20,24-25,29,31-37H,11-12H2/t20-,24+,25+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGF-A/VEGFR-1 (unknown origin) interaction after 1 hr by ELISA |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50428819
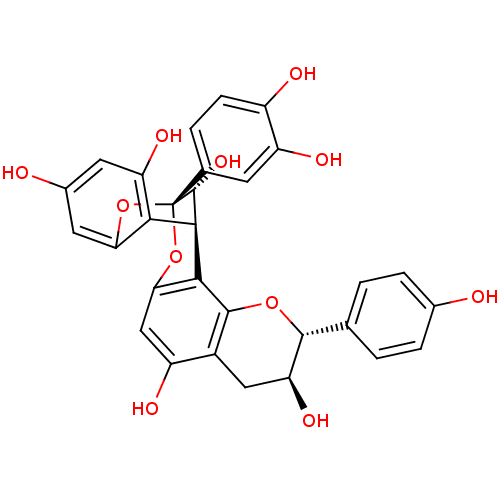
(GERANIN B)Show SMILES O[C@H]1Cc2c(O)cc3O[C@@]4(Oc5cc(O)cc(O)c5[C@@H]([C@H]4O)c3c2O[C@@H]1c1ccc(O)cc1)c1ccc(O)c(O)c1 |r,TLB:16:18:20:8.7.22,12:11:20:8.7.22| Show InChI InChI=1S/C30H24O11/c31-14-4-1-12(2-5-14)27-21(37)10-16-18(34)11-23-25(28(16)39-27)26-24-20(36)8-15(32)9-22(24)40-30(41-23,29(26)38)13-3-6-17(33)19(35)7-13/h1-9,11,21,26-27,29,31-38H,10H2/t21-,26+,27+,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGF-A/VEGFR-1 (unknown origin) interaction after 1 hr by ELISA |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50241990

(CHEMBL502489 | Camboginol | Garcinol | Garcinol, 1)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@@H](-[#6][C@@]12[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6]1=O)[#6]2=O)-[#6](-[#6])=[#6] |r,TLB:40:39:26.24.37:15.8.9| Show InChI InChI=1S/C38H50O6/c1-22(2)11-13-27(25(7)8)20-37-21-28(15-12-23(3)4)36(9,10)38(35(37)44,18-17-24(5)6)34(43)31(33(37)42)32(41)26-14-16-29(39)30(40)19-26/h11-12,14,16-17,19,27-28,31,39-40H,7,13,15,18,20-21H2,1-6,8-10H3/t27-,28+,31?,37+,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM84873
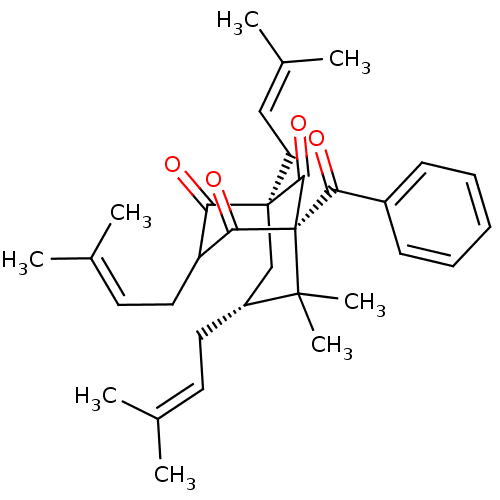
(Nemorosone, 2)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@@H]1-[#6][C@@]2([#6]\[#6]=[#6](\[#6])-[#6])[#6](=O)-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](=O)[C@@]([#6](=O)-c3ccccc3)([#6]2=O)C1([#6])[#6] |r,THB:33:32:15.21.13:34.6.5| Show InChI InChI=1S/C33H42O4/c1-21(2)14-16-25-20-32(19-18-23(5)6)28(35)26(17-15-22(3)4)29(36)33(30(32)37,31(25,7)8)27(34)24-12-10-9-11-13-24/h9-15,18,25-26H,16-17,19-20H2,1-8H3/t25-,26?,32+,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM84874

(Guttiferone A, 3)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@H]1-[#6][C@@]2([#6]\[#6]=[#6](\[#6])-[#6])[#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6](=O)[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]2=O)[C@]1([#6])[#6]-[#6]=[#6](-[#6])-[#6] |r,THB:35:34:15.26.13:36.6.5| Show InChI InChI=1S/C37H48O6/c1-22(2)10-12-27-21-36(18-15-24(5)6)32(41)30(31(40)26-11-13-28(38)29(39)20-26)33(42)37(34(36)43,19-16-25(7)8)35(27,9)17-14-23(3)4/h10-11,13-16,20,27,30,38-39H,12,17-19,21H2,1-9H3/t27-,30?,35+,36-,37+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM84875
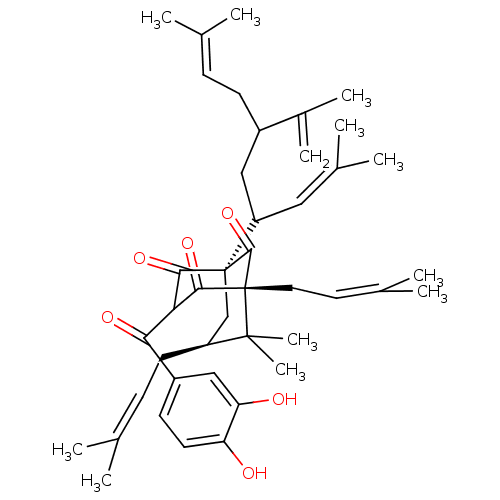
(Guttiferone E, 4)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6](-[#6]-[#6](-[#6]=[#6](-[#6])-[#6])[C@]12[#6]-[#6@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6]1=O)[#6]2=O)-[#6](-[#6])=[#6] |r,TLB:45:44:31.29.42:20.13.14| Show InChI InChI=1S/C43H58O6/c1-25(2)13-15-30(29(9)10)22-33(21-28(7)8)42-24-32(17-14-26(3)4)41(11,12)43(40(42)49,20-19-27(5)6)39(48)36(38(42)47)37(46)31-16-18-34(44)35(45)23-31/h13-14,16,18-19,21,23,30,32-33,36,44-45H,9,15,17,20,22,24H2,1-8,10-12H3/t30?,32-,33?,36?,42+,43+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM84877
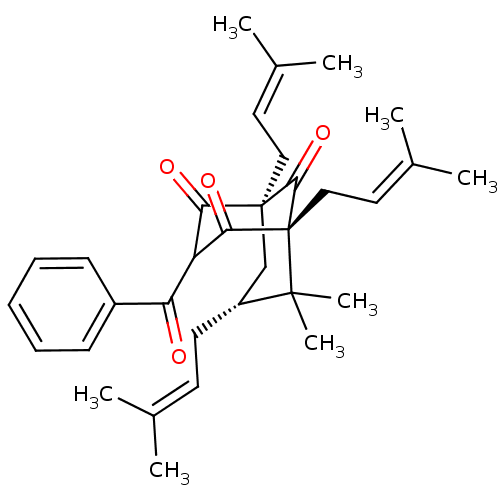
(Clusianone, 10)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@@H]1-[#6][C@@]2([#6]\[#6]=[#6](/[#6])-[#6])[#6](=O)-[#6](-[#6](=O)-c3ccccc3)-[#6](=O)[C@]([#6]\[#6]=[#6](/[#6])-[#6])([#6]2=O)C1([#6])[#6] |r,THB:33:32:15.24.13:34.6.5| Show InChI InChI=1S/C33H42O4/c1-21(2)14-15-25-20-32(18-16-22(3)4)28(35)26(27(34)24-12-10-9-11-13-24)29(36)33(30(32)37,31(25,7)8)19-17-23(5)6/h9-14,16-17,25-26H,15,18-20H2,1-8H3/t25-,26?,32+,33-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293285
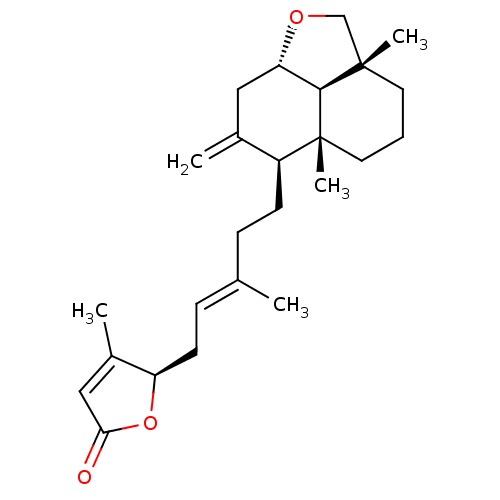
(23,6alpha-epoxy-labd-8,13(14),17-trien-16(R),19-ol...)Show SMILES C\C(CC[C@H]1C(=C)C[C@@H]2OC[C@]3(C)CCC[C@@]1(C)[C@@H]23)=C/C[C@H]1OC(=O)C=C1C |r,c:28| Show InChI InChI=1S/C25H36O3/c1-16(8-10-20-18(3)14-22(26)28-20)7-9-19-17(2)13-21-23-24(4,15-27-21)11-6-12-25(19,23)5/h8,14,19-21,23H,2,6-7,9-13,15H2,1,3-5H3/b16-8+/t19-,20+,21-,23-,24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293286

(8alpha-23-dihydroxy-23,6alpha-epoxy-labd-13(14),15...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@@H]2OC(O)[C@]3(C)CCC[C@@]1(C)[C@@H]23)=C/C=C1\OC(=O)C=C1C |r,c:30| Show InChI InChI=1S/C25H36O5/c1-15(7-9-17-16(2)13-20(26)29-17)8-10-19-23(3)11-6-12-24(4)21(23)18(30-22(24)27)14-25(19,5)28/h7,9,13,18-19,21-22,27-28H,6,8,10-12,14H2,1-5H3/b15-7+,17-9-/t18-,19+,21+,22?,23+,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293287

(8alpha-hydroxylabd-13(14),15,17-trien-6alpha,23-16...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@@H]2OC(=O)[C@]3(C)CCC[C@@]1(C)[C@@H]23)=C/C=C1\OC(=O)C=C1C |r,c:30| Show InChI InChI=1S/C25H34O5/c1-15(7-9-17-16(2)13-20(26)29-17)8-10-19-23(3)11-6-12-24(4)21(23)18(30-22(24)27)14-25(19,5)28/h7,9,13,18-19,21,28H,6,8,10-12,14H2,1-5H3/b15-7+,17-9-/t18-,19+,21+,23+,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293288

(6alpha,8alpha-dihydroxy-23-oxo-13(14),15,17-trien-...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@H]2[C@@](C)(CCC[C@]12C)C=O)=C/C=C1\OC(=O)C=C1C |r,c:29| Show InChI InChI=1S/C25H36O5/c1-16(7-9-19-17(2)13-21(28)30-19)8-10-20-24(4)12-6-11-23(3,15-26)22(24)18(27)14-25(20,5)29/h7,9,13,15,18,20,22,27,29H,6,8,10-12,14H2,1-5H3/b16-7+,19-9-/t18-,20+,22-,23-,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293289

(6alpha,8alpha-dihydroxy-23-carbossi-labd-13(14),15...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@@H]2[C@]1(C)CCC[C@@]2(C)C(O)=O)=C/C=C1\OC(=O)C=C1C |r,c:30| Show InChI InChI=1S/C25H36O6/c1-15(7-9-18-16(2)13-20(27)31-18)8-10-19-23(3)11-6-12-24(4,22(28)29)21(23)17(26)14-25(19,5)30/h7,9,13,17,19,21,26,30H,6,8,10-12,14H2,1-5H3,(H,28,29)/b15-7+,18-9-/t17-,19+,21+,23+,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293290

(6alpha,8alpha,23-trihydroxy-labd-13(14),15,17-trie...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@H]2[C@](C)(CO)CCC[C@]12C)=C/C=C1\OC(=O)C=C1C |r,c:29| Show InChI InChI=1S/C25H38O5/c1-16(7-9-19-17(2)13-21(28)30-19)8-10-20-24(4)12-6-11-23(3,15-26)22(24)18(27)14-25(20,5)29/h7,9,13,18,20,22,26-27,29H,6,8,10-12,14-15H2,1-5H3/b16-7+,19-9-/t18-,20+,22-,23-,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293291

(6alpha,15(S)-dihydroxy-23-oxo-labd-8(22),13(14),17...)Show SMILES C\C(CC[C@H]1C(=C)C[C@H](O)[C@H]2[C@@](C)(CCC[C@]12C)C=O)=C/[C@H](O)[C@H]1OC(=O)C=C1C |r,c:29| Show InChI InChI=1S/C25H36O5/c1-15(11-19(27)22-17(3)13-21(29)30-22)7-8-18-16(2)12-20(28)23-24(4,14-26)9-6-10-25(18,23)5/h11,13-14,18-20,22-23,27-28H,2,6-10,12H2,1,3-5H3/b15-11+/t18-,19-,20-,22-,23-,24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293292

(6alpha,15(S),23-trihydroxy-labd-8(22),13(14),17-tr...)Show SMILES C\C(CC[C@H]1C(=C)C[C@H](O)[C@H]2[C@](C)(CO)CCC[C@]12C)=C/[C@H](O)[C@H]1OC(=O)C=C1C |r,c:29| Show InChI InChI=1S/C25H38O5/c1-15(11-19(27)22-17(3)13-21(29)30-22)7-8-18-16(2)12-20(28)23-24(4,14-26)9-6-10-25(18,23)5/h11,13,18-20,22-23,26-28H,2,6-10,12,14H2,1,3-5H3/b15-11+/t18-,19-,20-,22-,23-,24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293293
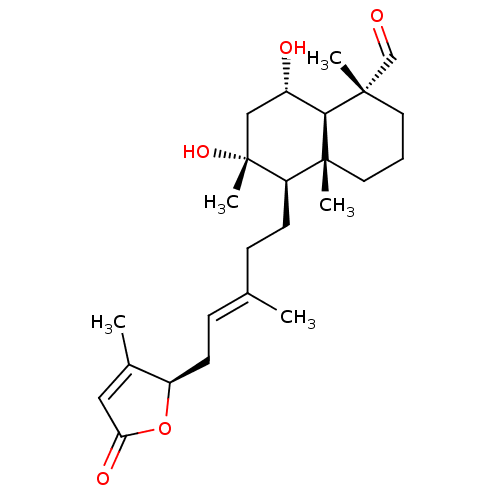
(6alpha,8alpha-dihydroxy-23-oxo-labd-13(14),17-dien...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@H]2[C@@](C)(CCC[C@]12C)C=O)=C/C[C@H]1OC(=O)C=C1C |r,c:29| Show InChI InChI=1S/C25H38O5/c1-16(7-9-19-17(2)13-21(28)30-19)8-10-20-24(4)12-6-11-23(3,15-26)22(24)18(27)14-25(20,5)29/h7,13,15,18-20,22,27,29H,6,8-12,14H2,1-5H3/b16-7+/t18-,19+,20+,22-,23-,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293294

(6alpha,8alpha,15(S)-trihydroxy-23-oxo-labd-13(14),...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@H]2[C@@](C)(CCC[C@]12C)C=O)=C/[C@H](O)[C@H]1OC(=O)C=C1C |r,c:30| Show InChI InChI=1S/C25H38O6/c1-15(11-17(27)21-16(2)12-20(29)31-21)7-8-19-24(4)10-6-9-23(3,14-26)22(24)18(28)13-25(19,5)30/h11-12,14,17-19,21-22,27-28,30H,6-10,13H2,1-5H3/b15-11+/t17-,18-,19+,21-,22-,23-,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293295

(6alpha,8alpha,15(S)-trihydroxy-23-carbossimethylla...)Show SMILES COC(=O)[C@]1(C)CCC[C@]2(C)[C@@H](CC\C(C)=C\[C@H](O)[C@H]3OC(=O)C=C3C)[C@](C)(O)C[C@H](O)[C@@H]12 |r,c:23| Show InChI InChI=1S/C26H40O7/c1-15(12-17(27)21-16(2)13-20(29)33-21)8-9-19-24(3)10-7-11-25(4,23(30)32-6)22(24)18(28)14-26(19,5)31/h12-13,17-19,21-22,27-28,31H,7-11,14H2,1-6H3/b15-12+/t17-,18-,19+,21-,22+,24+,25+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293296

(6alpha,8alpha-dihydroxy-23-carbossi-labd-13(14),17...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@@H]2[C@]1(C)CCC[C@@]2(C)C(O)=O)=C/CC1OC(=O)C=C1C |r,c:30| Show InChI InChI=1S/C25H38O6/c1-15(7-9-18-16(2)13-20(27)31-18)8-10-19-23(3)11-6-12-24(4,22(28)29)21(23)17(26)14-25(19,5)30/h7,13,17-19,21,26,30H,6,8-12,14H2,1-5H3,(H,28,29)/b15-7+/t17-,18?,19+,21+,23+,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293297
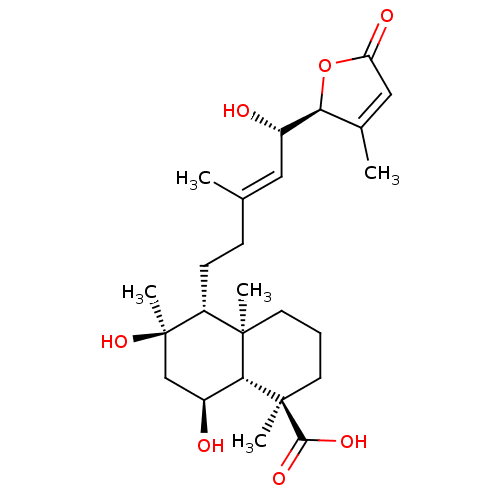
(6alpha,8alpha,15(S)-trihydroxy-23-carbossi-labd-13...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@@H]2[C@]1(C)CCC[C@@]2(C)C(O)=O)=C/[C@H](O)[C@H]1OC(=O)C=C1C |r,c:31| Show InChI InChI=1S/C25H38O7/c1-14(11-16(26)20-15(2)12-19(28)32-20)7-8-18-23(3)9-6-10-24(4,22(29)30)21(23)17(27)13-25(18,5)31/h11-12,16-18,20-21,26-27,31H,6-10,13H2,1-5H3,(H,29,30)/b14-11+/t16-,17-,18+,20-,21+,23+,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293298

(6alpha,8alpha,23-trihydroxy-labd-13(14),17-dien-16...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@H]2[C@](C)(CO)CCC[C@]12C)=C/C[C@H]1OC(=O)C=C1C |r,c:29| Show InChI InChI=1S/C25H40O5/c1-16(7-9-19-17(2)13-21(28)30-19)8-10-20-24(4)12-6-11-23(3,15-26)22(24)18(27)14-25(20,5)29/h7,13,18-20,22,26-27,29H,6,8-12,14-15H2,1-5H3/b16-7+/t18-,19+,20+,22-,23-,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293299

(6alpha,8alpha,15(S),23-tetrahydroxy-labd-13(14),17...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@H](O)[C@H]2[C@](C)(CO)CCC[C@]12C)=C/[C@H](O)[C@H]1OC(=O)C=C1C |r,c:30| Show InChI InChI=1S/C25H40O6/c1-15(11-17(27)21-16(2)12-20(29)31-21)7-8-19-24(4)10-6-9-23(3,14-26)22(24)18(28)13-25(19,5)30/h11-12,17-19,21-22,26-28,30H,6-10,13-14H2,1-5H3/b15-11+/t17-,18-,19+,21-,22-,23-,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293300

(8alpha-hydroxy,23alpha-O-ethyl-23,6alpha-epoxy-lab...)Show SMILES CCO[C@H]1O[C@H]2C[C@@](C)(O)[C@H](CC\C(C)=C\C[C@H]3OC(=O)C=C3C)[C@@]3(C)CCC[C@]1(C)[C@H]23 |r,c:21| Show InChI InChI=1S/C27H42O5/c1-7-30-24-26(5)14-8-13-25(4)21(27(6,29)16-20(32-24)23(25)26)12-10-17(2)9-11-19-18(3)15-22(28)31-19/h9,15,19-21,23-24,29H,7-8,10-14,16H2,1-6H3/b17-9+/t19-,20+,21-,23-,24+,25-,26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293301
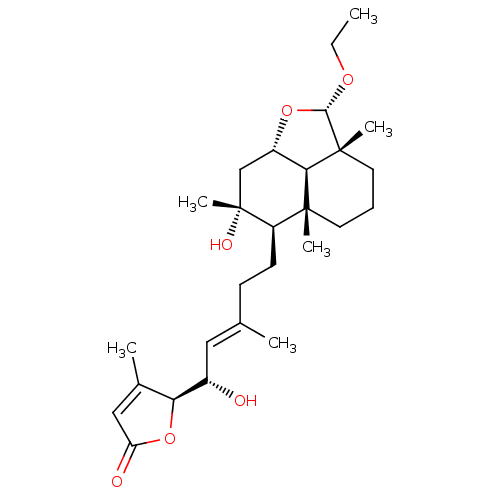
(8alpha,15(S)-dihydroxy,23alpha-O-ethyl-23,6alpha-e...)Show SMILES CCO[C@H]1O[C@H]2C[C@@](C)(O)[C@H](CC\C(C)=C\[C@H](O)[C@H]3OC(=O)C=C3C)[C@@]3(C)CCC[C@]1(C)[C@H]23 |r,c:22| Show InChI InChI=1S/C27H42O6/c1-7-31-24-26(5)12-8-11-25(4)20(27(6,30)15-19(32-24)23(25)26)10-9-16(2)13-18(28)22-17(3)14-21(29)33-22/h13-14,18-20,22-24,28,30H,7-12,15H2,1-6H3/b16-13+/t18-,19-,20+,22-,23+,24-,25+,26+,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Tubulin--tyrosine ligase
(Homo sapiens (Human)) | BDBM50293302

(8alpha,15(S),23alpha-trihydroxy-23,6alpha-epoxy-la...)Show SMILES C\C(CC[C@H]1[C@](C)(O)C[C@@H]2O[C@H](O)[C@]3(C)CCC[C@@]1(C)[C@@H]23)=C/[C@H](O)[C@H]1OC(=O)C=C1C |r,c:31| Show InChI InChI=1S/C25H38O6/c1-14(11-16(26)20-15(2)12-19(27)31-20)7-8-18-23(3)9-6-10-24(4)21(23)17(30-22(24)28)13-25(18,5)29/h11-12,16-18,20-22,26,28-29H,6-10,13H2,1-5H3/b14-11+/t16-,17-,18+,20-,21+,22-,23+,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to TTL by surface plasmon resonance |
J Med Chem 52: 3814-28 (2009)
Article DOI: 10.1021/jm801637f
BindingDB Entry DOI: 10.7270/Q2JD4WQD |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50206427
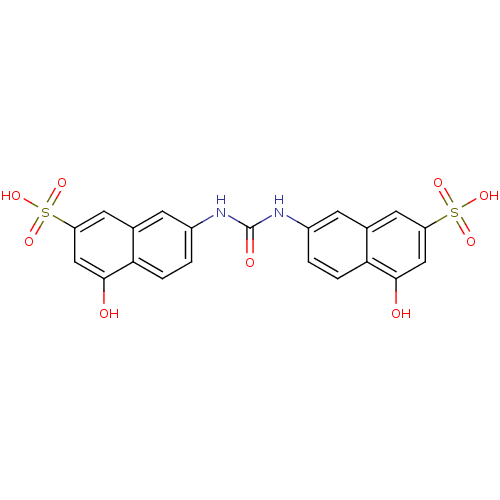
(7,7'-carbonylbis(azanediyl)bis(4-hydroxynaphthalen...)Show SMILES Oc1cc(cc2cc(NC(=O)Nc3ccc4c(O)cc(cc4c3)S(O)(=O)=O)ccc12)S(O)(=O)=O Show InChI InChI=1S/C21H16N2O9S2/c24-19-9-15(33(27,28)29)7-11-5-13(1-3-17(11)19)22-21(26)23-14-2-4-18-12(6-14)8-16(10-20(18)25)34(30,31)32/h1-10,24-25H,(H2,22,23,26)(H,27,28,29)(H,30,31,32) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant GST-PRMT1 expressed Escherichia coli BL21 by SPR assay |
J Med Chem 55: 9875-90 (2012)
Article DOI: 10.1021/jm301097p
BindingDB Entry DOI: 10.7270/Q2445NM2 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429448

(CHEMBL2332347 | Catalpin)Show SMILES O[C@@H]1C[C@H]2[C@@H](C[C@@]3(O)CO[C@@H](O1)[C@@H]23)OC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C16H18O7/c17-9-3-1-8(2-4-9)14(19)22-11-6-16(20)7-21-15-13(16)10(11)5-12(18)23-15/h1-4,10-13,15,17-18,20H,5-7H2/t10-,11+,12-,13+,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.93E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429449

(CHEMBL2332356 | Rehmaglutin A)Show SMILES O[C@@H]1[C@@H](O)[C@@]2(O)CO[C@H]3OCC[C@@H]1[C@@H]23 |r| Show InChI InChI=1S/C9H14O5/c10-6-4-1-2-13-8-5(4)9(12,3-14-8)7(6)11/h4-8,10-12H,1-3H2/t4-,5-,6+,7-,8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429450

(VERMINOSIDE)Show SMILES OC[C@H]1O[C@@H](O[C@H]2OC=CC3[C@@H](OC(=O)\C=C\c4ccc(O)c(O)c4)[C@H]4O[C@@]4(CO)C23)[C@H](O)[C@@H](O)[C@@H]1O |r,c:8| Show InChI InChI=1S/C24H28O13/c25-8-14-17(30)18(31)19(32)23(34-14)36-22-16-11(5-6-33-22)20(21-24(16,9-26)37-21)35-15(29)4-2-10-1-3-12(27)13(28)7-10/h1-7,11,14,16-23,25-28,30-32H,8-9H2/b4-2+/t11?,14-,16?,17-,18+,19-,20-,21-,22-,23+,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM15359
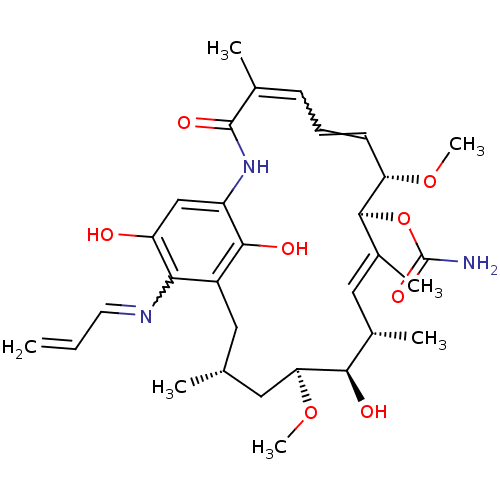
((4E,6Z,8S,9S,10E,12S,13R,14S,16R)-13-hydroxy-8,14-...)Show SMILES CO[C@H]1C[C@H](C)Cc2c(O)c(NC(=O)C(C)=CC=C[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)cc(O)c2N=CC=C |r,w:16.16,38.39,t:28| Show InChI InChI=1S/C31H43N3O8/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35/h8-12,15-17,19,24-25,27,29,35-37H,1,13-14H2,2-7H3,(H2,32,39)(H,34,38)/b11-9?,18-10?,20-15+,33-12?/t17-,19+,24+,25+,27-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429451

(CHEMBL2332366)Show SMILES OC[C@]1(O)C[C@@H](OC(=O)c2ccc(O)cc2)[C@@H]2C=CO[C@@H](O[C@@H]3O[C@H](COC(=O)c4ccc(O)cc4)[C@@H](O)[C@H](O)[C@H]3O)[C@H]12 |r,c:18| Show InChI InChI=1S/C29H32O14/c30-13-29(38)11-19(41-26(37)15-3-7-17(32)8-4-15)18-9-10-39-27(21(18)29)43-28-24(35)23(34)22(33)20(42-28)12-40-25(36)14-1-5-16(31)6-2-14/h1-10,18-24,27-28,30-35,38H,11-13H2/t18-,19+,20+,21+,22+,23-,24+,27-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429452

(CHEMBL2332361)Show SMILES OC[C@H]1O[C@@H](O[C@@H]2OC=C[C@H]3[C@@H](C[C@@](O)(CO)[C@@H]23)OC(=O)\C=C/c2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r,c:8| Show InChI InChI=1S/C24H30O12/c25-10-16-19(29)20(30)21(31)23(35-16)36-22-18-14(7-8-33-22)15(9-24(18,32)11-26)34-17(28)6-3-12-1-4-13(27)5-2-12/h1-8,14-16,18-23,25-27,29-32H,9-11H2/b6-3-/t14-,15+,16+,18+,19+,20-,21+,22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429453
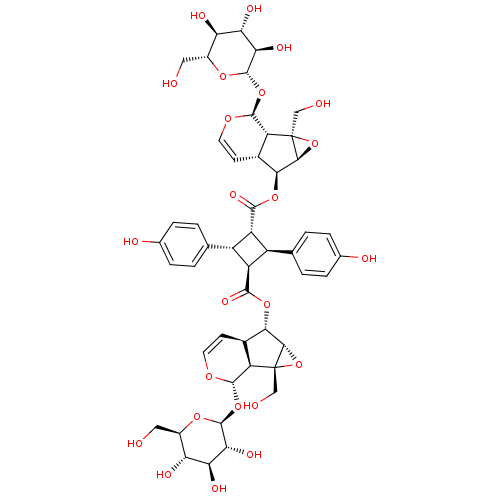
(Argenteoside B | CHEMBL2332353)Show SMILES OC[C@H]1O[C@@H](O[C@@H]2OC=C[C@H]3[C@H](OC(=O)[C@H]4[C@@H]([C@@H]([C@H]4c4ccc(O)cc4)C(=O)O[C@@H]4[C@@H]5O[C@]5(CO)[C@@H]5[C@H]4C=CO[C@H]5O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)c4ccc(O)cc4)[C@@H]4O[C@]4(CO)[C@@H]23)[C@H](O)[C@@H](O)[C@@H]1O |r,c:8,41| Show InChI InChI=1S/C48H56O24/c49-13-23-31(55)33(57)35(59)45(65-23)69-43-29-21(9-11-63-43)37(39-47(29,15-51)71-39)67-41(61)27-25(17-1-5-19(53)6-2-17)28(26(27)18-3-7-20(54)8-4-18)42(62)68-38-22-10-12-64-44(30(22)48(16-52)40(38)72-48)70-46-36(60)34(58)32(56)24(14-50)66-46/h1-12,21-40,43-46,49-60H,13-16H2/t21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33+,34+,35-,36-,37+,38+,39+,40+,43+,44+,45+,46+,47-,48-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429454

(CHEMBL2332346)Show SMILES OC[C@H]1O[C@@H](O[C@@H]2OC=C[C@H]3[C@@H](C[C@@](O)(CO)[C@@H]23)OC(=O)c2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r,c:8| Show InChI InChI=1S/C22H28O12/c23-8-14-16(26)17(27)18(28)21(33-14)34-20-15-12(5-6-31-20)13(7-22(15,30)9-24)32-19(29)10-1-3-11(25)4-2-10/h1-6,12-18,20-21,23-28,30H,7-9H2/t12-,13+,14+,15+,16+,17-,18+,20-,21-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429455

(CHEMBL2332355 | Specioside)Show SMILES OC[C@H]1O[C@@H](O[C@@H]2OC=C[C@H]3[C@H](OC(=O)\C=C\c4ccc(O)cc4)[C@@H]4O[C@]4(CO)[C@@H]23)[C@H](O)[C@@H](O)[C@@H]1O |r,c:8| Show InChI InChI=1S/C24H28O12/c25-9-14-17(29)18(30)19(31)23(33-14)35-22-16-13(7-8-32-22)20(21-24(16,10-26)36-21)34-15(28)6-3-11-1-4-12(27)5-2-11/h1-8,13-14,16-23,25-27,29-31H,9-10H2/b6-3+/t13-,14-,16-,17-,18+,19-,20+,21+,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429456
(3,4-Dihydrocatalposide | CHEMBL2332365)Show SMILES OC[C@H]1O[C@@H](O[C@@H]2OCC[C@H]3[C@H](OC(=O)c4ccc(O)cc4)[C@@H]4O[C@]4(CO)[C@@H]23)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C22H28O12/c23-7-12-14(26)15(27)16(28)21(31-12)33-20-13-11(5-6-30-20)17(18-22(13,8-24)34-18)32-19(29)9-1-3-10(25)4-2-9/h1-4,11-18,20-21,23-28H,5-8H2/t11-,12-,13-,14-,15+,16-,17+,18+,20+,21+,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429457
(Argenteoside A | CHEMBL2332352)Show SMILES OC[C@H]1O[C@@H](O[C@@H]2OC=C[C@H]3[C@H](OC(=O)[C@H]4[C@@H]([C@H]([C@@H]4c4ccc(O)cc4)c4ccc(O)cc4)C(=O)O[C@@H]4[C@@H]5O[C@]5(CO)[C@@H]5[C@H]4C=CO[C@H]5O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)[C@@H]4O[C@]4(CO)[C@@H]23)[C@H](O)[C@@H](O)[C@@H]1O |r,c:8,49| Show InChI InChI=1S/C48H56O24/c49-13-23-31(55)33(57)35(59)45(65-23)69-43-29-21(9-11-63-43)37(39-47(29,15-51)71-39)67-41(61)27-25(17-1-5-19(53)6-2-17)26(18-3-7-20(54)8-4-18)28(27)42(62)68-38-22-10-12-64-44(30(22)48(16-52)40(38)72-48)70-46-36(60)34(58)32(56)24(14-50)66-46/h1-12,21-40,43-46,49-60H,13-16H2/t21-,22-,23-,24-,25+,26+,27-,28-,29-,30-,31-,32-,33+,34+,35-,36-,37+,38+,39+,40+,43+,44+,45+,46+,47-,48-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50429458
(CHEMBL2332354 | Catalposide)Show SMILES OC[C@H]1O[C@@H](O[C@@H]2OC=C[C@H]3[C@H](OC(=O)c4ccc(O)cc4)[C@@H]4O[C@]4(CO)[C@@H]23)[C@H](O)[C@@H](O)[C@@H]1O |r,c:8| Show InChI InChI=1S/C22H26O12/c23-7-12-14(26)15(27)16(28)21(31-12)33-20-13-11(5-6-30-20)17(18-22(13,8-24)34-18)32-19(29)9-1-3-10(25)4-2-9/h1-6,11-18,20-21,23-28H,7-8H2/t11-,12-,13-,14-,15+,16-,17+,18+,20+,21+,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50347503
(RADICICOL)Show SMILES C[C@@H]1C[C@@H]2O[C@@H]2C=CC=CC(=O)Cc2c(Cl)c(O)cc(O)c2C(=O)O1 |r,w:9.10,7.8| Show InChI InChI=1S/C18H17ClO6/c1-9-6-15-14(25-15)5-3-2-4-10(20)7-11-16(18(23)24-9)12(21)8-13(22)17(11)19/h2-5,8-9,14-15,21-22H,6-7H2,1H3/t9-,14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant Hsp90 alpha (unknown origin) by surface plasmon resonance |
J Med Chem 56: 1583-95 (2013)
Article DOI: 10.1021/jm301398y
BindingDB Entry DOI: 10.7270/Q2K938W3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant VEGFA (unknown origin) measured for 60 seconds by surface plasmon resonance assay |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50428821
(PROANTHOCYANIDIN A1)Show SMILES O[C@H]1Cc2c(O)cc3O[C@@]4(Oc5cc(O)cc(O)c5[C@@H]([C@H]4O)c3c2O[C@@H]1c1ccc(O)c(O)c1)c1ccc(O)c(O)c1 |r,THB:16:18:7.22.8:20,12:11:7.22.8:20| Show InChI InChI=1S/C30H24O12/c31-13-7-20(37)24-22(8-13)41-30(12-2-4-16(33)19(36)6-12)29(39)26(24)25-23(42-30)10-17(34)14-9-21(38)27(40-28(14)25)11-1-3-15(32)18(35)5-11/h1-8,10,21,26-27,29,31-39H,9H2/t21-,26+,27+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 476 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant VEGFA (unknown origin) measured for 60 seconds by surface plasmon resonance assay |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50428819

(GERANIN B)Show SMILES O[C@H]1Cc2c(O)cc3O[C@@]4(Oc5cc(O)cc(O)c5[C@@H]([C@H]4O)c3c2O[C@@H]1c1ccc(O)cc1)c1ccc(O)c(O)c1 |r,TLB:16:18:20:8.7.22,12:11:20:8.7.22| Show InChI InChI=1S/C30H24O11/c31-14-4-1-12(2-5-14)27-21(37)10-16-18(34)11-23-25(28(16)39-27)26-24-20(36)8-15(32)9-22(24)40-30(41-23,29(26)38)13-3-6-17(33)19(35)7-13/h1-9,11,21,26-27,29,31-38H,10H2/t21-,26+,27+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant VEGFA (unknown origin) measured for 60 seconds by surface plasmon resonance assay |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50428820

(CHEMBL2335722)Show SMILES O[C@H]1Cc2c(O)cc(O)c([C@@H]3C[C@H](Oc4cc(O)cc(O)c34)c3ccc(O)cc3)c2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H26O9/c31-16-5-1-14(2-6-16)25-12-20(27-22(35)9-18(33)10-26(27)38-25)28-23(36)13-21(34)19-11-24(37)29(39-30(19)28)15-3-7-17(32)8-4-15/h1-10,13,20,24-25,29,31-37H,11-12H2/t20-,24+,25+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant VEGFA (unknown origin) measured for 60 seconds by surface plasmon resonance assay |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant PIGF1 (unknown origin) measured for 60 seconds by surface plasmon resonance assay |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50428821

(PROANTHOCYANIDIN A1)Show SMILES O[C@H]1Cc2c(O)cc3O[C@@]4(Oc5cc(O)cc(O)c5[C@@H]([C@H]4O)c3c2O[C@@H]1c1ccc(O)c(O)c1)c1ccc(O)c(O)c1 |r,THB:16:18:7.22.8:20,12:11:7.22.8:20| Show InChI InChI=1S/C30H24O12/c31-13-7-20(37)24-22(8-13)41-30(12-2-4-16(33)19(36)6-12)29(39)26(24)25-23(42-30)10-17(34)14-9-21(38)27(40-28(14)25)11-1-3-15(32)18(35)5-11/h1-8,10,21,26-27,29,31-39H,9H2/t21-,26+,27+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant PIGF1 (unknown origin) measured for 60 seconds by surface plasmon resonance assay |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50428819

(GERANIN B)Show SMILES O[C@H]1Cc2c(O)cc3O[C@@]4(Oc5cc(O)cc(O)c5[C@@H]([C@H]4O)c3c2O[C@@H]1c1ccc(O)cc1)c1ccc(O)c(O)c1 |r,TLB:16:18:20:8.7.22,12:11:20:8.7.22| Show InChI InChI=1S/C30H24O11/c31-14-4-1-12(2-5-14)27-21(37)10-16-18(34)11-23-25(28(16)39-27)26-24-20(36)8-15(32)9-22(24)40-30(41-23,29(26)38)13-3-6-17(33)19(35)7-13/h1-9,11,21,26-27,29,31-38H,10H2/t21-,26+,27+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant PIGF1 (unknown origin) measured for 60 seconds by surface plasmon resonance assay |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50428820

(CHEMBL2335722)Show SMILES O[C@H]1Cc2c(O)cc(O)c([C@@H]3C[C@H](Oc4cc(O)cc(O)c34)c3ccc(O)cc3)c2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H26O9/c31-16-5-1-14(2-6-16)25-12-20(27-22(35)9-18(33)10-26(27)38-25)28-23(36)13-21(34)19-11-24(37)29(39-30(19)28)15-3-7-17(32)8-4-15/h1-10,13,20,24-25,29,31-37H,11-12H2/t20-,24+,25+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant PIGF1 (unknown origin) measured for 60 seconds by surface plasmon resonance assay |
J Nat Prod 76: 29-35 (2013)
Article DOI: 10.1021/np300614u
BindingDB Entry DOI: 10.7270/Q24F1S3K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50085041
(2-(4-(3-(4-acetyl-3-hydroxy-2-propylphenoxy)propox...)Show SMILES CCCc1c(O)c(ccc1OCCCOc1ccc(OCC(O)=O)cc1)C(C)=O Show InChI InChI=1S/C22H26O7/c1-3-5-19-20(11-10-18(15(2)23)22(19)26)28-13-4-12-27-16-6-8-17(9-7-16)29-14-21(24)25/h6-11,26H,3-5,12-14H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro"
Curated by ChEMBL
| Assay Description
Binding affinity to PPARdelta (unknown origin) assessed as kinetic dissociation constant by SPR assay |
Eur J Med Chem 127: 379-397 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.047
BindingDB Entry DOI: 10.7270/Q2474D4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50235984

(CHEMBL4064900)Show SMILES CC(Oc1ccc(Cc2ccc(OCc3coc(n3)-c3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C26H23NO5/c1-18(26(28)29)32-24-13-9-20(10-14-24)15-19-7-11-23(12-8-19)30-16-22-17-31-25(27-22)21-5-3-2-4-6-21/h2-14,17-18H,15-16H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of FTase-catalyzed incorporation of [3H]- FPP radioligand into recombinant Ha-Ras by 50% at an enzyme concentration of 1 nM. |
Eur J Med Chem 127: 379-397 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.047
BindingDB Entry DOI: 10.7270/Q2474D4J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data