 Found 1048 hits with Last Name = 'dalgarno' and Initial = 'd'
Found 1048 hits with Last Name = 'dalgarno' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23222
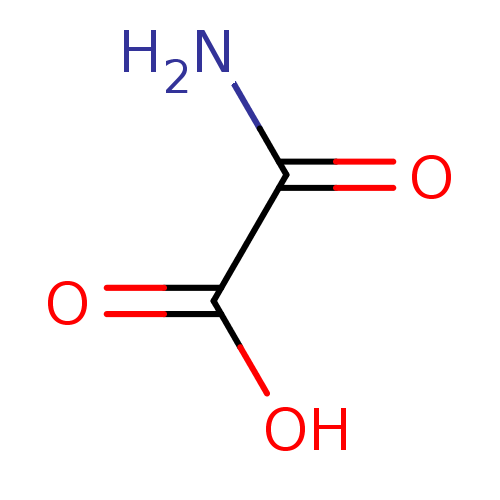
(Oxalamic acid | Oxamate | Oxamate, 3 | Oxamidic Ac...)Show InChI InChI=1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50062357

(AP26113 | CHEMBL3397300)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N(C)C Show InChI InChI=1S/C26H34ClN6O2P/c1-32(2)18-12-14-33(15-13-18)19-10-11-21(23(16-19)35-3)30-26-28-17-20(27)25(31-26)29-22-8-6-7-9-24(22)36(4,5)34/h6-11,16-18H,12-15H2,1-5H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185287
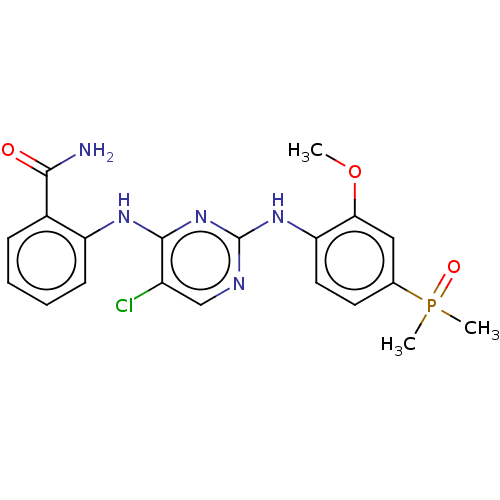
(CHEMBL3823268)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(N)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H21ClN5O3P/c1-29-17-10-12(30(2,3)28)8-9-16(17)25-20-23-11-14(21)19(26-20)24-15-7-5-4-6-13(15)18(22)27/h4-11H,1-3H3,(H2,22,27)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185237

(CHEMBL3824308)Show SMILES CCP(=O)(CC)c1ccccc1Nc1nc(Nc2ccc(cc2OC)N2CCC(CC2)N2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C31H43ClN7O2P/c1-5-42(40,6-2)29-10-8-7-9-27(29)34-30-25(32)22-33-31(36-30)35-26-12-11-24(21-28(26)41-4)38-15-13-23(14-16-38)39-19-17-37(3)18-20-39/h7-12,21-23H,5-6,13-20H2,1-4H3,(H2,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50345579
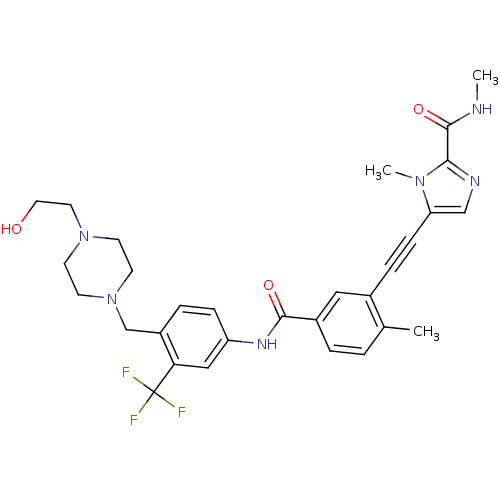
(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM482158
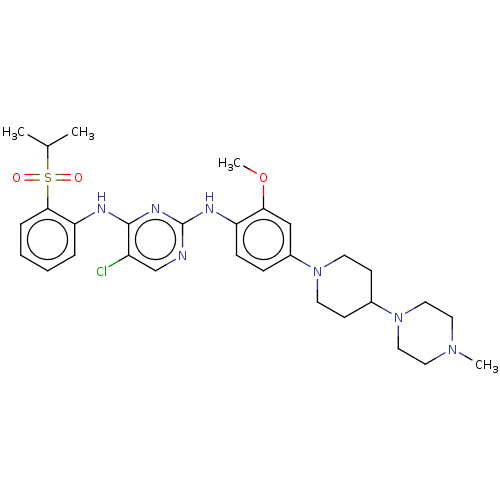
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185280

(CHEMBL3822611)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(C)CC1 Show InChI InChI=1S/C24H30ClN6O2P/c1-30-11-13-31(14-12-30)17-9-10-19(21(15-17)33-2)28-24-26-16-18(25)23(29-24)27-20-7-5-6-8-22(20)34(3,4)32/h5-10,15-16H,11-14H2,1-4H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185275
(CHEMBL3823235)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C25H32ClN6O3P/c1-35-22-16-18(32-12-10-31(11-13-32)14-15-33)8-9-20(22)29-25-27-17-19(26)24(30-25)28-21-6-4-5-7-23(21)36(2,3)34/h4-9,16-17,33H,10-15H2,1-3H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RET using KKKSPGEYVNIEFG by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ARIAD Pharmaceuticals Inc
| Assay Description
Inhibition of wild-type Abl and Abl T315I kinase activity was measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FRET... |
Chem Biol Drug Des 70: 171-81 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00556.x
BindingDB Entry DOI: 10.7270/Q228063T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185284
(CHEMBL3823549)Show SMILES COc1cc(OCCCN2CCCC2)ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C26H33ClN5O3P/c1-34-23-17-19(35-16-8-15-32-13-6-7-14-32)11-12-21(23)30-26-28-18-20(27)25(31-26)29-22-9-4-5-10-24(22)36(2,3)33/h4-5,9-12,17-18H,6-8,13-16H2,1-3H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244622
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES CN1CCN(CC1)c1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C28H34N7OP/c1-20-7-6-8-21(2)24(20)13-14-35-19-29-25-26(30-22-9-11-23(12-10-22)37(4,5)36)31-28(32-27(25)35)34-17-15-33(3)16-18-34/h6-14,19H,15-18H2,1-5H3,(H,30,31,32)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244621
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(nc12)N1CCOCC1 Show InChI InChI=1S/C27H31N6O2P/c1-19-6-5-7-20(2)23(19)12-13-33-18-28-24-25(29-21-8-10-22(11-9-21)36(3,4)34)30-27(31-26(24)33)32-14-16-35-17-15-32/h5-13,18H,14-17H2,1-4H3,(H,29,30,31)/b13-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185281
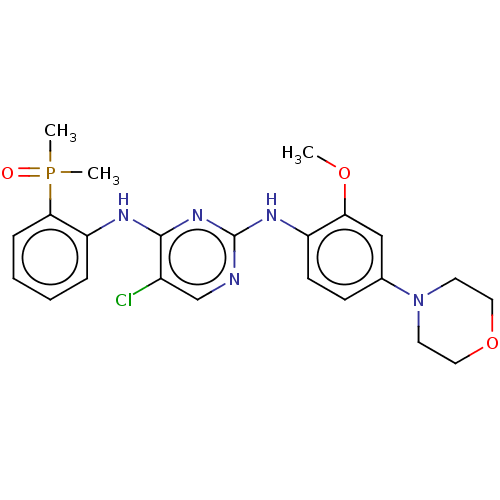
(CHEMBL3823416)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCOCC1 Show InChI InChI=1S/C23H27ClN5O3P/c1-31-20-14-16(29-10-12-32-13-11-29)8-9-18(20)27-23-25-15-17(24)22(28-23)26-19-6-4-5-7-21(19)33(2,3)30/h4-9,14-15H,10-13H2,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185268

(CHEMBL3824327)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(CC1)C1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-13-11-21(12-14-35)36-15-17-37(18-16-36)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132351
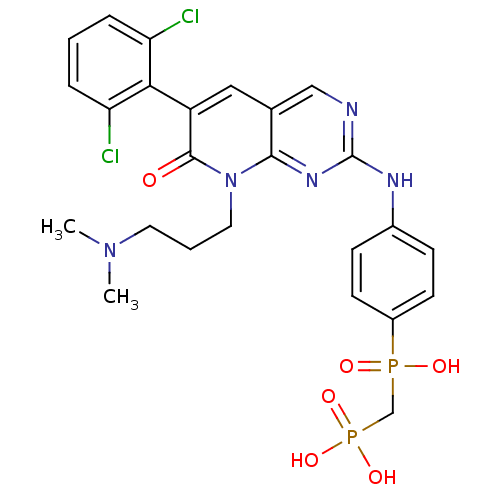
(({4-[6-(2,6-Dichloro-phenyl)-8-(3-dimethylamino-pr...)Show SMILES CN(C)CCCn1c2nc(Nc3ccc(cc3)P(O)(=O)CP(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(3.9,-7.22,;3.9,-5.68,;2.57,-4.91,;5.23,-4.91,;5.23,-3.37,;6.56,-2.59,;6.56,-1.05,;5.21,-.28,;3.88,-1.05,;2.55,-.29,;1.22,-1.06,;-.11,-.29,;-.11,1.25,;-1.44,2.01,;-2.78,1.23,;-2.77,-.31,;-1.44,-1.07,;-4.12,2,;-4.89,.67,;-3.35,3.34,;-5.45,2.77,;-5.45,4.31,;-6.99,4.32,;-5.86,5.8,;-4.12,5.09,;2.55,1.26,;3.88,2.03,;5.21,1.26,;6.54,2.03,;7.89,1.25,;9.22,2.02,;9.22,3.57,;7.89,4.33,;10.55,4.36,;11.88,3.57,;11.88,2.02,;10.55,1.25,;10.55,-.29,;7.89,-.28,;9.22,-1.05,)| Show InChI InChI=1S/C25H27Cl2N5O6P2/c1-31(2)11-4-12-32-23-16(13-19(24(32)33)22-20(26)5-3-6-21(22)27)14-28-25(30-23)29-17-7-9-18(10-8-17)39(34,35)15-40(36,37)38/h3,5-10,13-14H,4,11-12,15H2,1-2H3,(H,34,35)(H,28,29,30)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185290
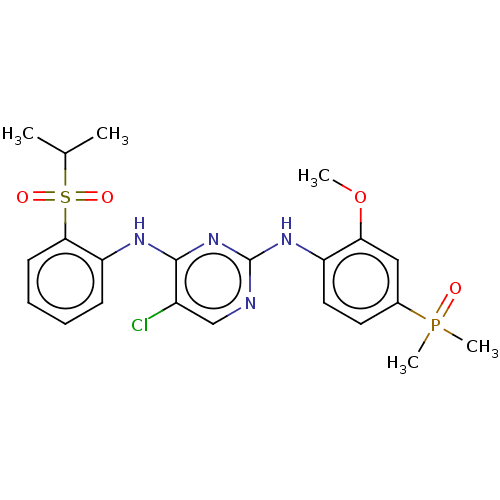
(CHEMBL3824304)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)P(C)(C)=O Show InChI InChI=1S/C22H26ClN4O4PS/c1-14(2)33(29,30)20-9-7-6-8-18(20)25-21-16(23)13-24-22(27-21)26-17-11-10-15(32(4,5)28)12-19(17)31-3/h6-14H,1-5H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185283

(CHEMBL3823603)Show SMILES COc1cc(OC2CCN(C)C2)ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C24H29ClN5O3P/c1-30-12-11-17(15-30)33-16-9-10-19(21(13-16)32-2)28-24-26-14-18(25)23(29-24)27-20-7-5-6-8-22(20)34(3,4)31/h5-10,13-14,17H,11-12,15H2,1-4H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185272
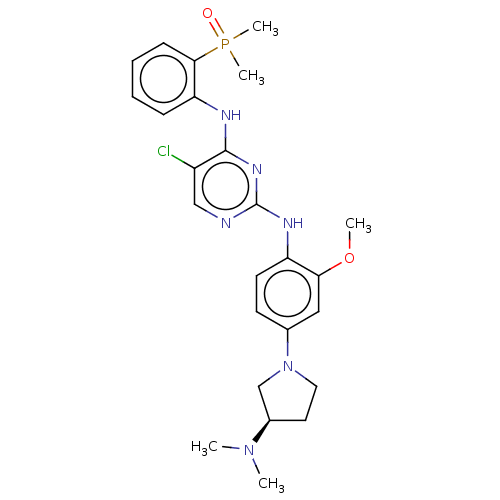
(CHEMBL3823107)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CC[C@H](C1)N(C)C |r| Show InChI InChI=1S/C25H32ClN6O2P/c1-31(2)18-12-13-32(16-18)17-10-11-20(22(14-17)34-3)29-25-27-15-19(26)24(30-25)28-21-8-6-7-9-23(21)35(4,5)33/h6-11,14-15,18H,12-13,16H2,1-5H3,(H2,27,28,29,30)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185286
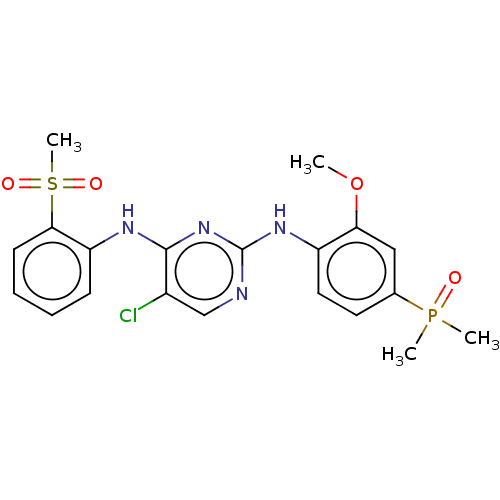
(CHEMBL3823256)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(C)(=O)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H22ClN4O4PS/c1-29-17-11-13(30(2,3)26)9-10-15(17)24-20-22-12-14(21)19(25-20)23-16-7-5-6-8-18(16)31(4,27)28/h5-12H,1-4H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM482158

(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185278

(CHEMBL3823017)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(O)CC1 Show InChI InChI=1S/C24H29ClN5O3P/c1-33-21-14-16(30-12-10-17(31)11-13-30)8-9-19(21)28-24-26-15-18(25)23(29-24)27-20-6-4-5-7-22(20)34(2,3)32/h4-9,14-15,17,31H,10-13H2,1-3H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535
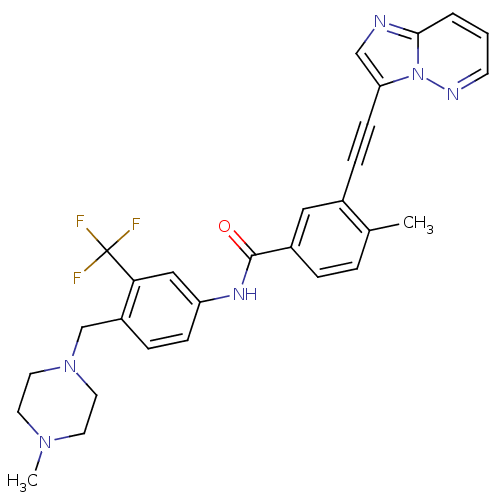
(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ABL autophosphorylation |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185140
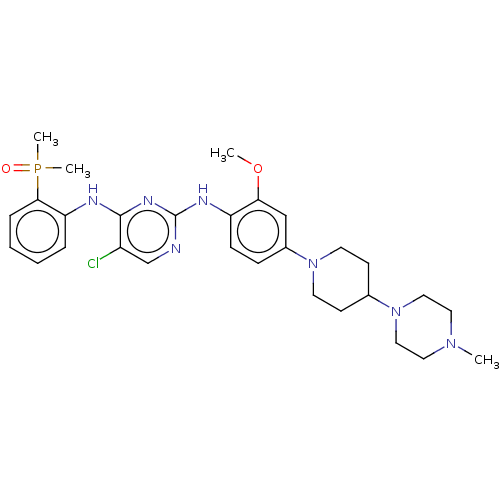
(AP-26113 | Brigatinib | US11248003, Example Brigat...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185245

(CHEMBL3823190)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)C1CCN(C)CC1 Show InChI InChI=1S/C25H31ClN5O2P/c1-31-13-11-17(12-14-31)18-9-10-20(22(15-18)33-2)29-25-27-16-19(26)24(30-25)28-21-7-5-6-8-23(21)34(3,4)32/h5-10,15-17H,11-14H2,1-4H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185239
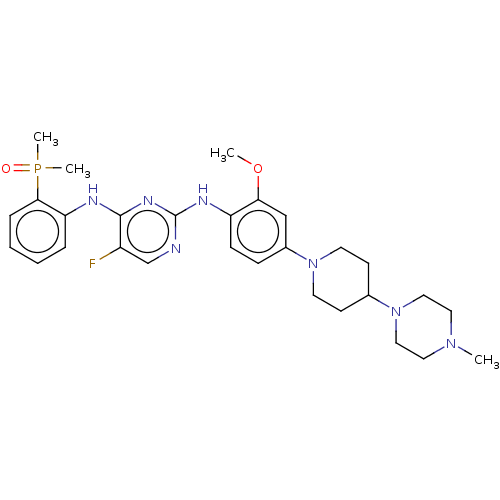
(CHEMBL3823296)Show SMILES COc1cc(ccc1Nc1ncc(F)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39FN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185286

(CHEMBL3823256)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(C)(=O)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H22ClN4O4PS/c1-29-17-11-13(30(2,3)26)9-10-15(17)24-20-22-12-14(21)19(25-20)23-16-7-5-6-8-18(16)31(4,27)28/h5-12H,1-4H3,(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185238

(CHEMBL3823577)Show SMILES COc1cc(ccc1Nc1ncc(C)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H42N7O2P/c1-22-21-31-30(34-29(22)32-26-8-6-7-9-28(26)40(4,5)38)33-25-11-10-24(20-27(25)39-3)36-14-12-23(13-15-36)37-18-16-35(2)17-19-37/h6-11,20-21,23H,12-19H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074
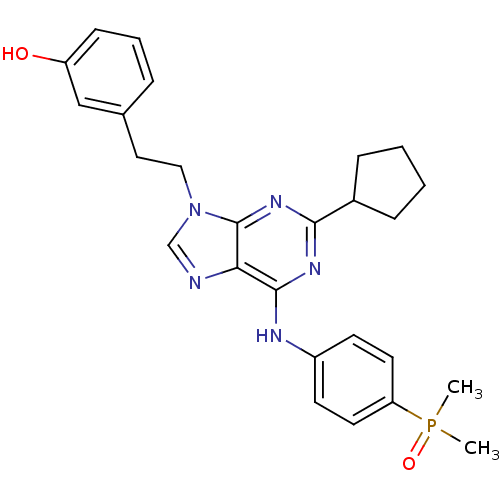
(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals
| Assay Description
Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... |
Chem Biol Drug Des 67: 46-57 (2006)
Article DOI: 10.1111/j.1747-0285.2005.00316.x
BindingDB Entry DOI: 10.7270/Q2M61HRQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074

(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 6x-His-tagged human Src kinase domain (T250 to L536 residues) expressed in Sf9 cells incubated for 2 hrs in presence of biotinylated cd... |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50025052
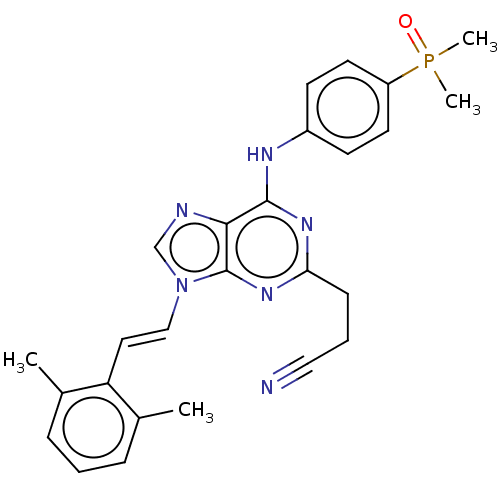
(CHEMBL517256)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(CCC#N)nc12 Show InChI InChI=1S/C26H27N6OP/c1-18-7-5-8-19(2)22(18)14-16-32-17-28-24-25(30-23(9-6-15-27)31-26(24)32)29-20-10-12-21(13-11-20)34(3,4)33/h5,7-8,10-14,16-17H,6,9H2,1-4H3,(H,29,30,31)/b16-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244664
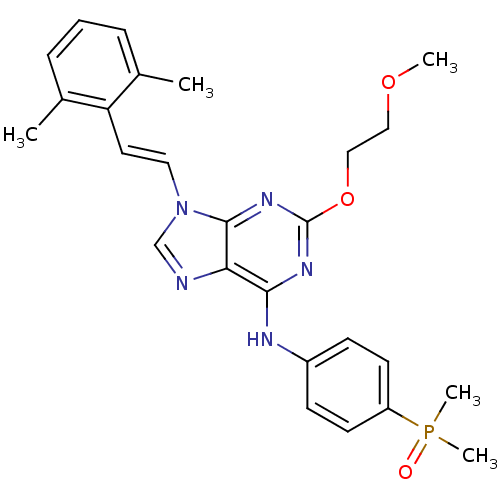
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES COCCOc1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C26H30N5O3P/c1-18-7-6-8-19(2)22(18)13-14-31-17-27-23-24(29-26(30-25(23)31)34-16-15-33-3)28-20-9-11-21(12-10-20)35(4,5)32/h6-14,17H,15-16H2,1-5H3,(H,28,29,30)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244663
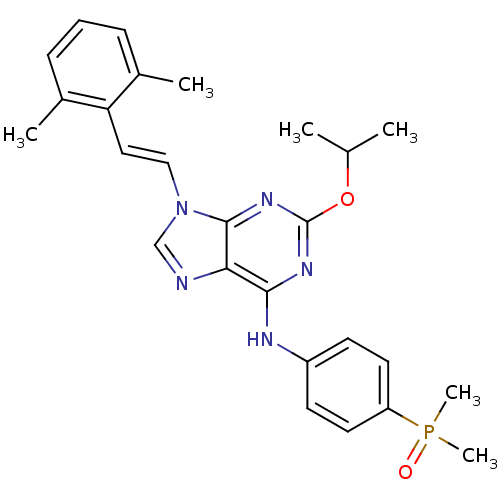
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES CC(C)Oc1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C26H30N5O2P/c1-17(2)33-26-29-24(28-20-10-12-21(13-11-20)34(5,6)32)23-25(30-26)31(16-27-23)15-14-22-18(3)8-7-9-19(22)4/h7-17H,1-6H3,(H,28,29,30)/b15-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
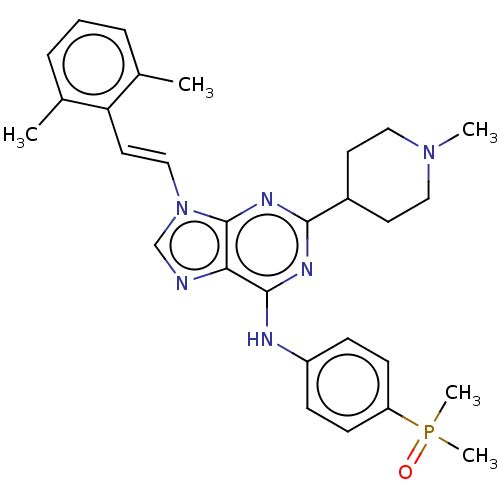
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50025051
(CHEMBL461768)Show SMILES CN1CCC(CC1)c1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C29H35N6OP/c1-20-7-6-8-21(2)25(20)15-18-35-19-30-26-28(31-23-9-11-24(12-10-23)37(4,5)36)32-27(33-29(26)35)22-13-16-34(3)17-14-22/h6-12,15,18-19,22H,13-14,16-17H2,1-5H3,(H,31,32,33)/b18-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244666
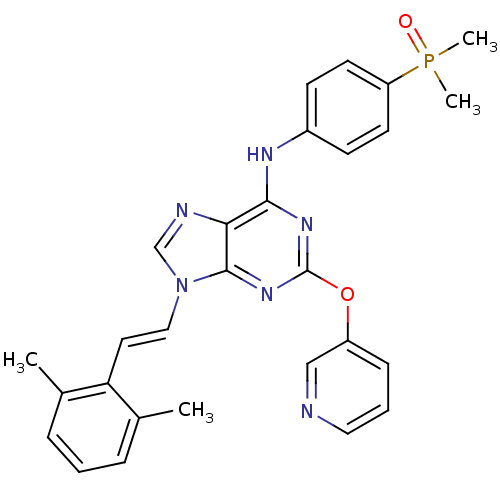
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(Oc3cccnc3)nc12 Show InChI InChI=1S/C28H27N6O2P/c1-19-7-5-8-20(2)24(19)14-16-34-18-30-25-26(31-21-10-12-23(13-11-21)37(3,4)35)32-28(33-27(25)34)36-22-9-6-15-29-17-22/h5-18H,1-4H3,(H,31,32,33)/b16-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50025051

(CHEMBL461768)Show SMILES CN1CCC(CC1)c1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C29H35N6OP/c1-20-7-6-8-21(2)25(20)15-18-35-19-30-26-28(31-23-9-11-24(12-10-23)37(4,5)36)32-27(33-29(26)35)22-13-16-34(3)17-14-22/h6-12,15,18-19,22H,13-14,16-17H2,1-5H3,(H,31,32,33)/b18-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244624
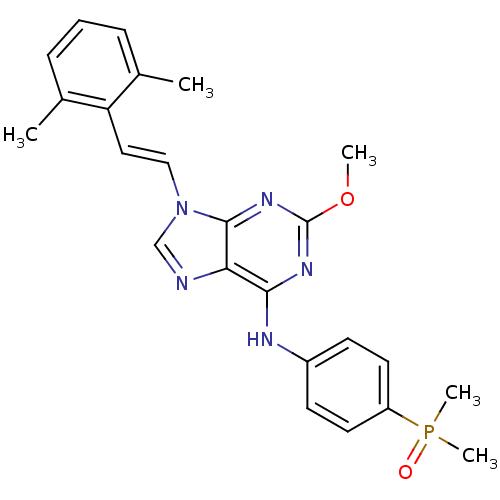
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES COc1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C24H26N5O2P/c1-16-7-6-8-17(2)20(16)13-14-29-15-25-21-22(27-24(31-3)28-23(21)29)26-18-9-11-19(12-10-18)32(4,5)30/h6-15H,1-5H3,(H,26,27,28)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244623
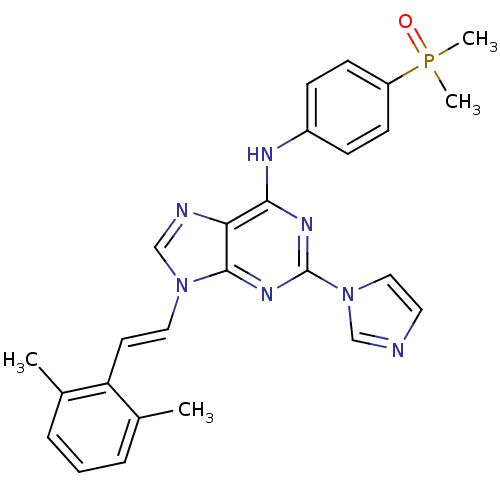
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(nc12)-n1ccnc1 Show InChI InChI=1S/C26H26N7OP/c1-18-6-5-7-19(2)22(18)12-14-32-17-28-23-24(29-20-8-10-21(11-9-20)35(3,4)34)30-26(31-25(23)32)33-15-13-27-16-33/h5-17H,1-4H3,(H,29,30,31)/b14-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50025052

(CHEMBL517256)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(CCC#N)nc12 Show InChI InChI=1S/C26H27N6OP/c1-18-7-5-8-19(2)22(18)14-16-32-17-28-24-25(30-23(9-6-15-27)31-26(24)32)29-20-10-12-21(13-11-20)34(3,4)33/h5,7-8,10-14,16-17H,6,9H2,1-4H3,(H,29,30,31)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50244569
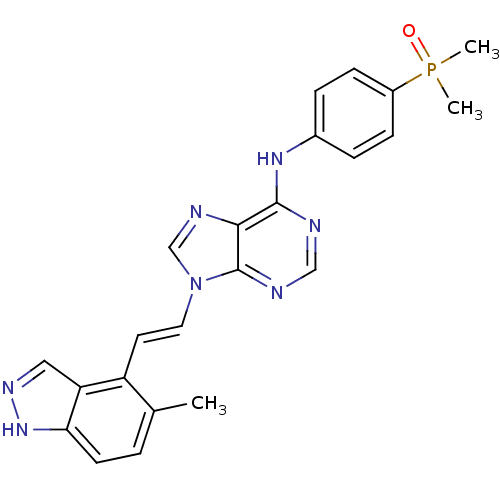
(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244569

(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185288

(CHEMBL3824326)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(C)=O)n1)P(C)(C)=O Show InChI InChI=1S/C21H22ClN4O3P/c1-13(27)15-7-5-6-8-17(15)24-20-16(22)12-23-21(26-20)25-18-10-9-14(30(3,4)28)11-19(18)29-2/h5-12H,1-4H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185265
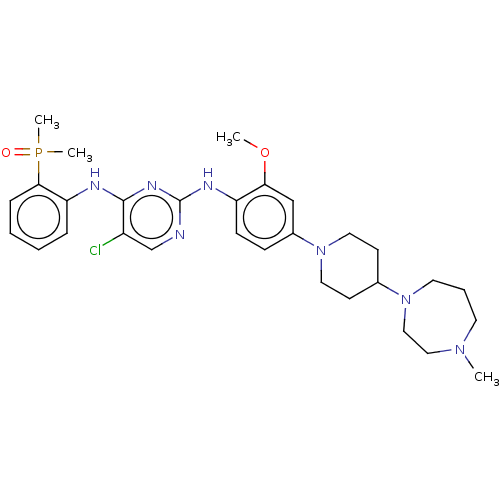
(CHEMBL3824290)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCCN(C)CC1 Show InChI InChI=1S/C30H41ClN7O2P/c1-36-14-7-15-37(19-18-36)22-12-16-38(17-13-22)23-10-11-25(27(20-23)40-2)34-30-32-21-24(31)29(35-30)33-26-8-5-6-9-28(26)41(3,4)39/h5-6,8-11,20-22H,7,12-19H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185267
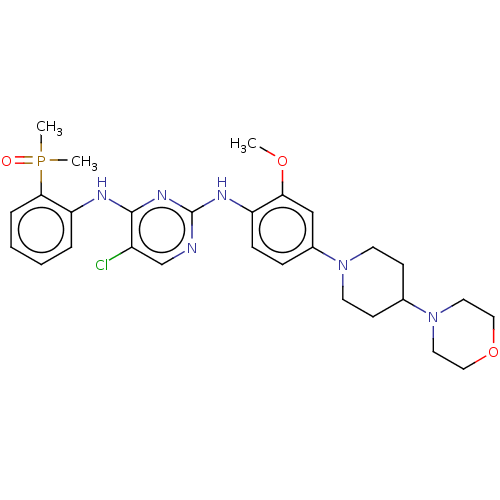
(CHEMBL3822557)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCOCC1 Show InChI InChI=1S/C28H36ClN6O3P/c1-37-25-18-21(34-12-10-20(11-13-34)35-14-16-38-17-15-35)8-9-23(25)32-28-30-19-22(29)27(33-28)31-24-6-4-5-7-26(24)39(2,3)36/h4-9,18-20H,10-17H2,1-3H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50244569

(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM82130
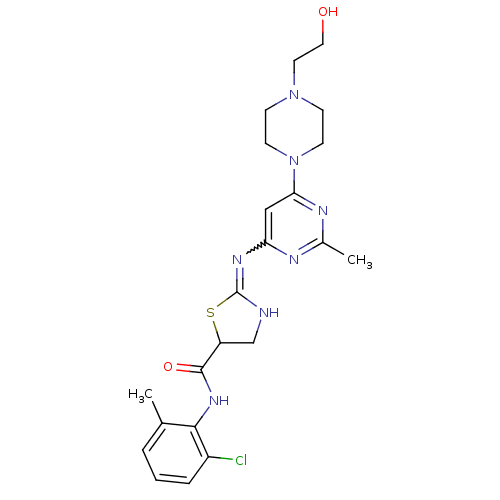
(Dasatinib)Show SMILES Cc1nc(cc(n1)N1CCN(CCO)CC1)N=C1NCC(S1)C(=O)Nc1c(C)cccc1Cl |w:16.17| Show InChI InChI=1S/C22H28ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12,17,31H,6-11,13H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM82130

(Dasatinib)Show SMILES Cc1nc(cc(n1)N1CCN(CCO)CC1)N=C1NCC(S1)C(=O)Nc1c(C)cccc1Cl |w:16.17| Show InChI InChI=1S/C22H28ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12,17,31H,6-11,13H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Isoform 1 of Fibronectin (1)
(Homo sapiens (Human)) | BDBM50244569

(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data