

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291847 (7-[3-(Quinolin-2-ylmethoxy)-benzyloxy]-2-(1H-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291855 (7-[3-(Quinolin-2-ylmethoxy)-phenoxymethyl]-2-(1H-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291848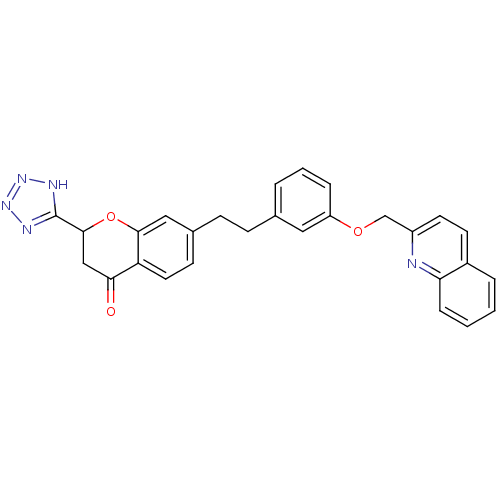 (7-{2-[3-(Quinolin-2-ylmethoxy)-phenyl]-ethyl}-2-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009075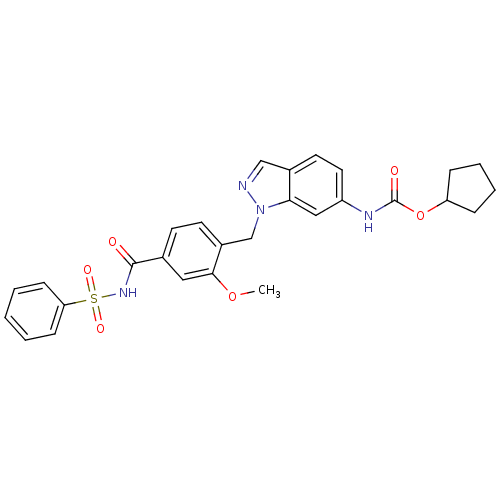 (CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291849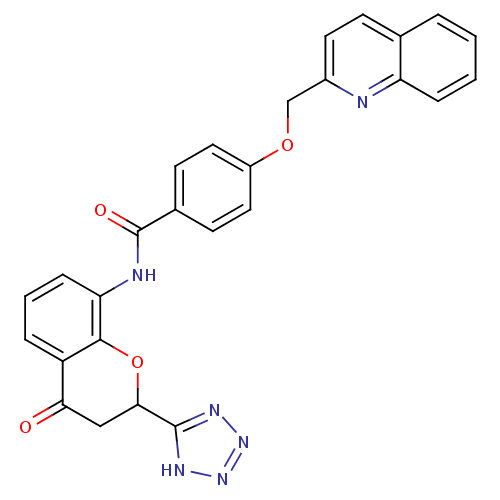 (CHEMBL47662 | N-[4-Oxo-2-(1H-tetrazol-5-yl)-chroma...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291850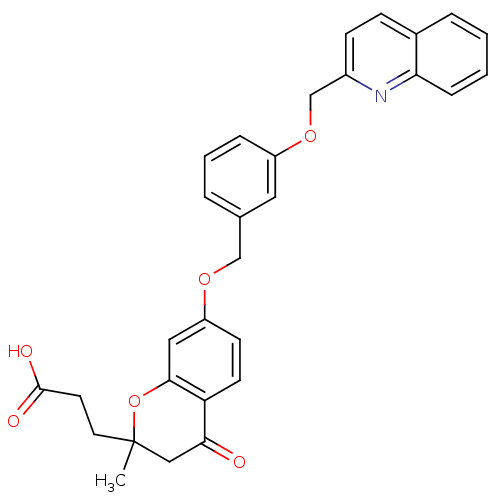 (3-{2-Methyl-4-oxo-7-[3-(quinolin-2-ylmethoxy)-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291854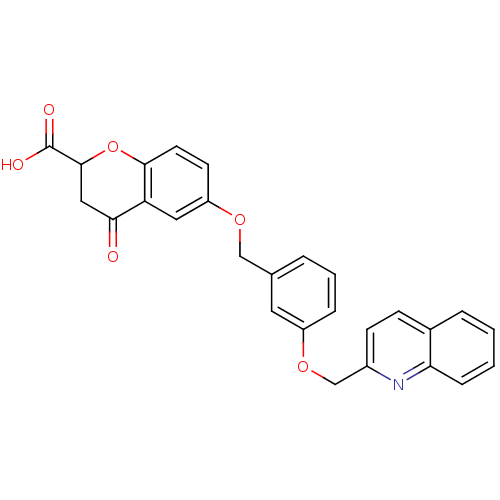 (4-Oxo-6-[3-(quinolin-2-ylmethoxy)-benzyloxy]-chrom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13304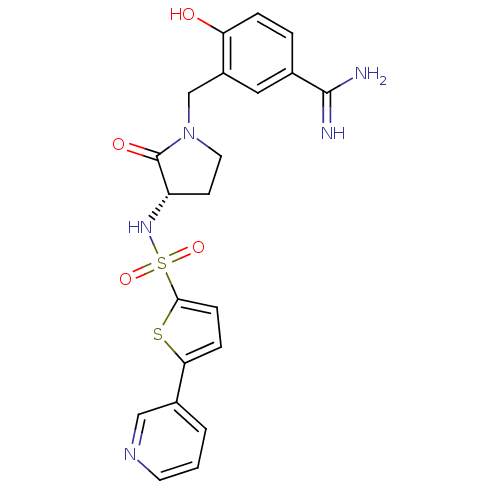 (4-hydroxy-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291856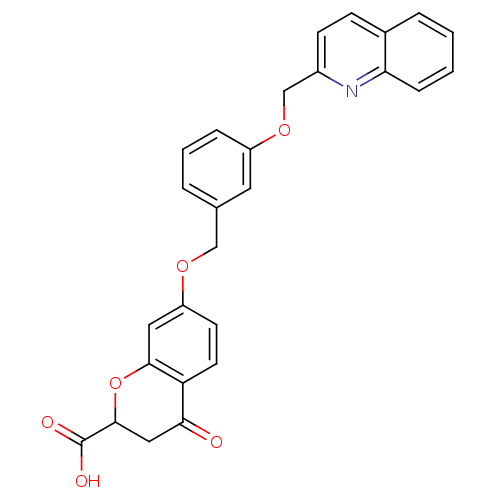 (4-Oxo-7-[3-(quinolin-2-ylmethoxy)-benzyloxy]-chrom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291858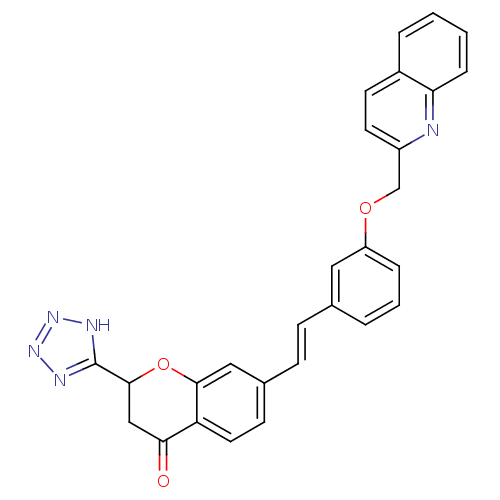 (7-{2-[3-(Quinolin-2-ylmethoxy)-phenyl]-vinyl}-2-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13306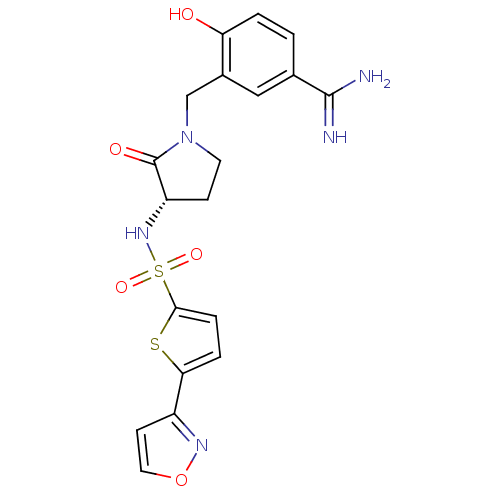 (4-Hydroxy-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-yls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13286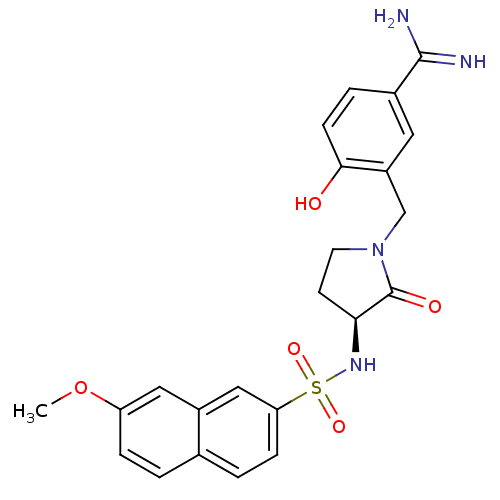 (4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50006799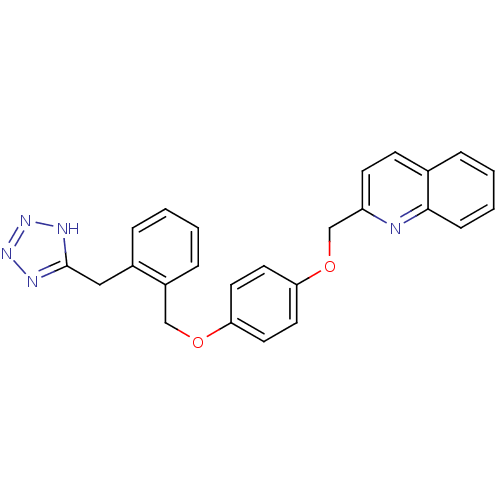 (2-{4-[2-(1H-Tetrazol-5-ylmethyl)-benzyloxy]-phenox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13283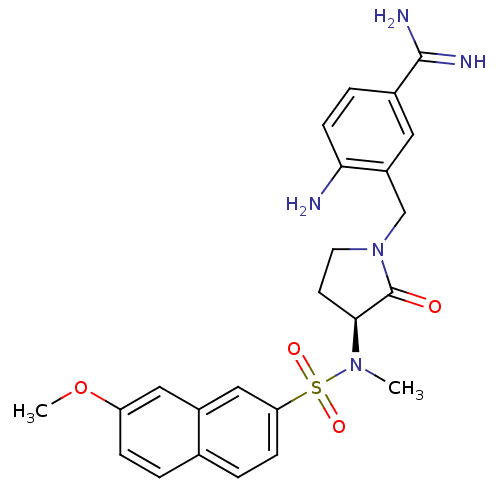 (4-amino-3-({(3S)-3-[[(7-methoxy-2-naphthyl)sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13303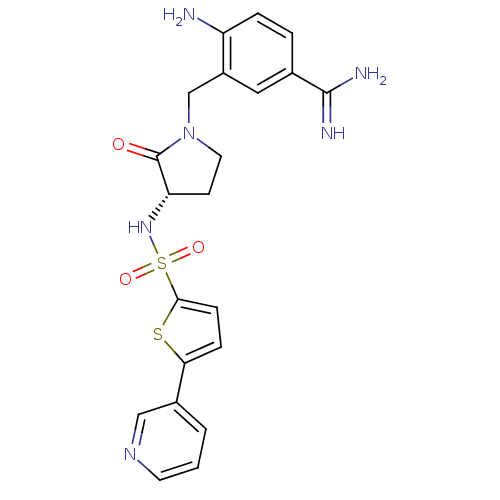 (4-Amino-3-[2-oxo-3-(S)-(5-pyridin-3-ylthiophene-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13281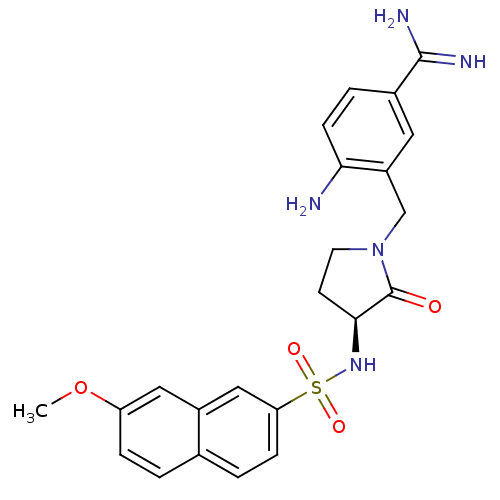 (4-Amino-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291853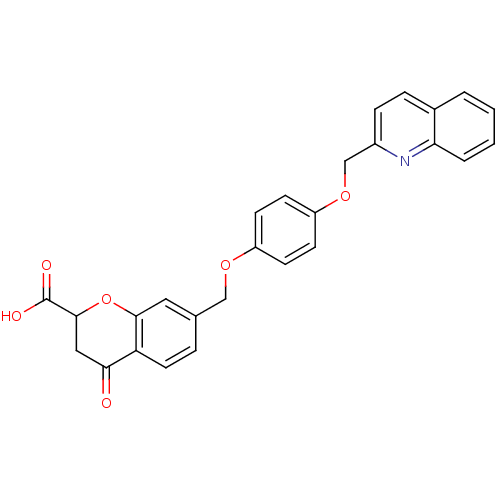 (4-Oxo-7-[4-(quinolin-2-ylmethoxy)-phenoxymethyl]-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13279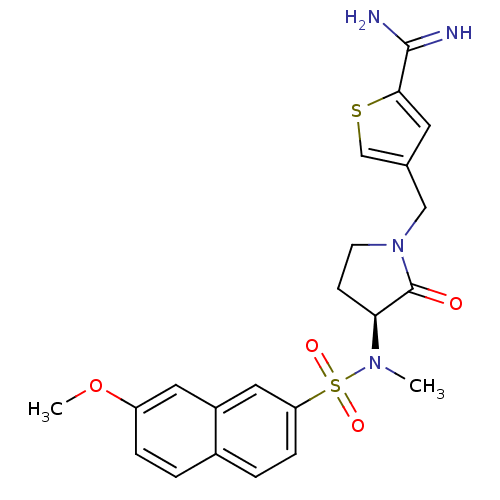 (4-{[(3S)-3-[(7-methoxynaphthalene-2-)(methyl)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13305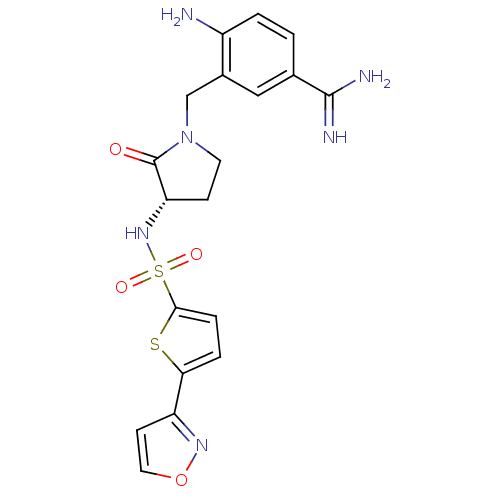 (4-Amino-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-ylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13288 (Sulfonamidopyrrolidinone 27 | methyl 2-(4-carbamim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13288 (Sulfonamidopyrrolidinone 27 | methyl 2-(4-carbamim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291859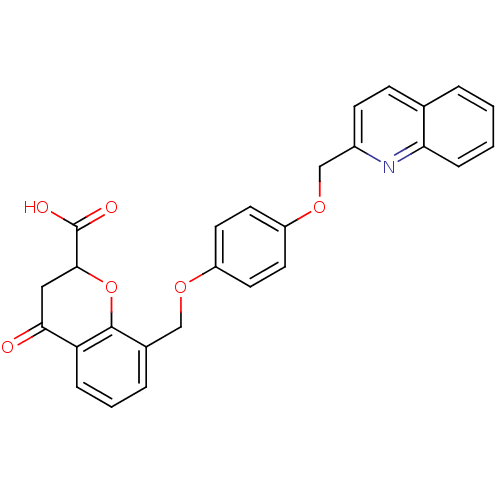 (4-Oxo-8-[4-(quinolin-2-ylmethoxy)-phenoxymethyl]-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291851 (8-[4-(Quinolin-2-ylmethoxy)-phenoxymethyl]-2-(1H-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291857 (6-Chloro-4-oxo-5-[4-(quinolin-2-ylmethoxy)-benzylo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13282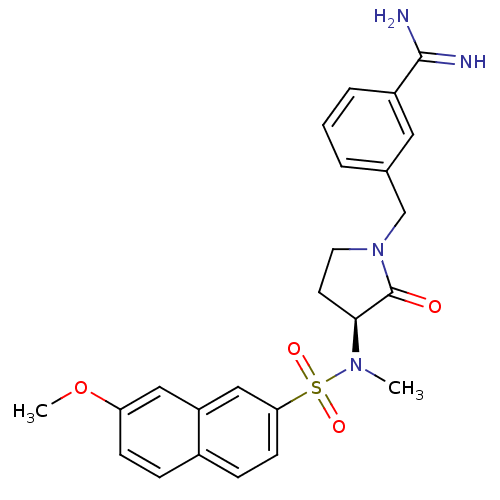 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)(methyl)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296936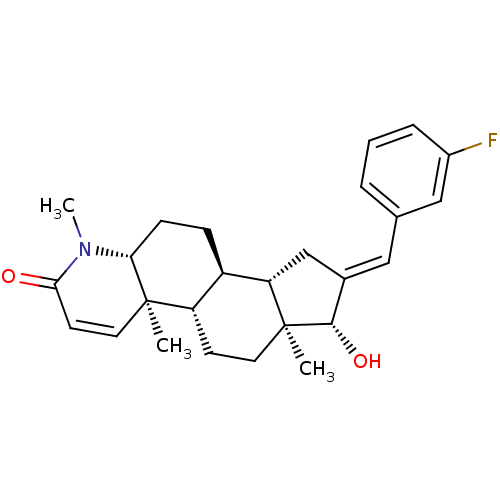 (16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291852 (4-Oxo-7-[3-(quinolin-2-ylmethoxy)-phenoxymethyl]-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13298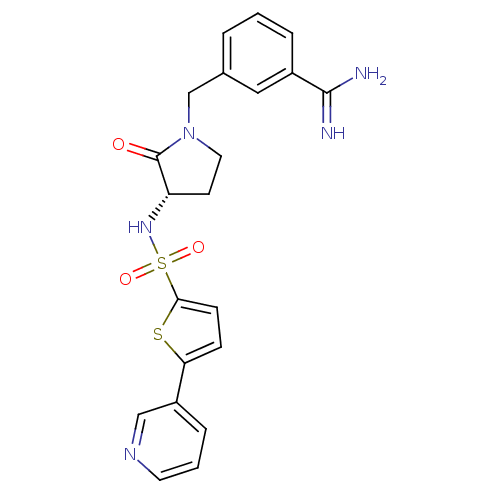 (3-[2-Oxo-3-(S)-(5-pyridin-3-ylthiophene-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296937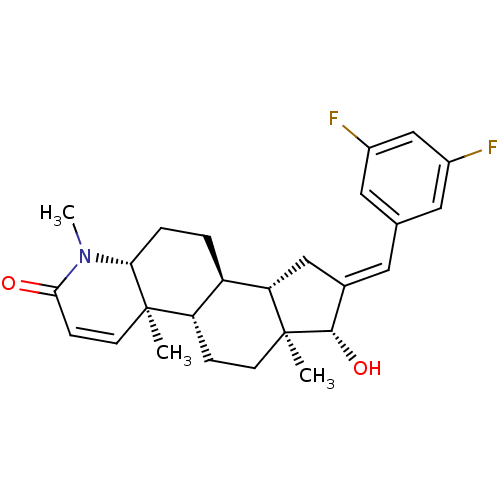 (16-[(3,5-Difluorophenyl)methylidene]-17beta-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13280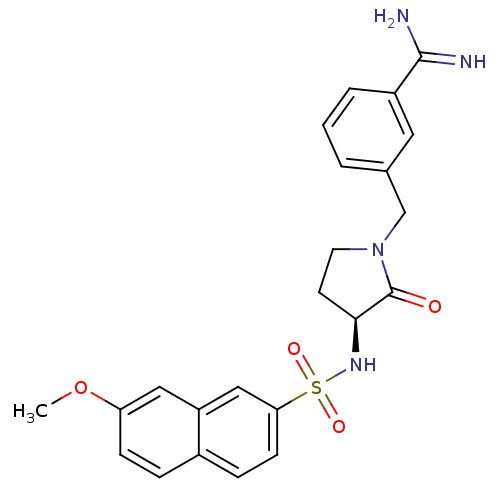 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13301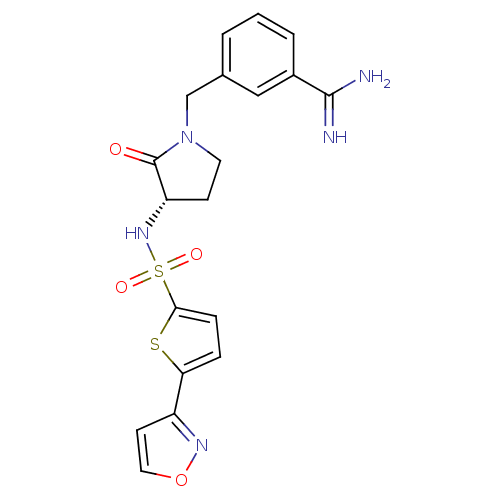 (3-[3-(S)-(5-Isoxazol-3-ylthiophene-2-ylsulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13299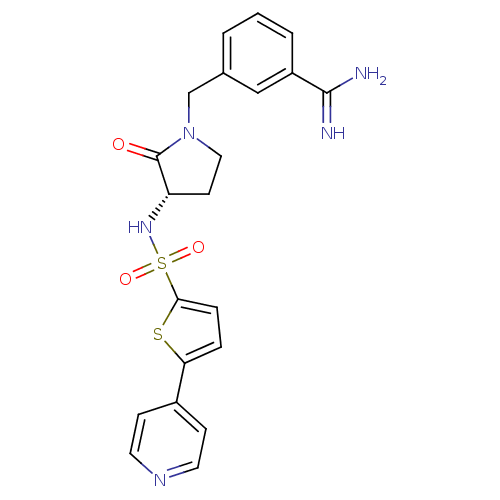 (3-[2-Oxo-3-(S)-(5-pyridin-4-ylthiophene-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13302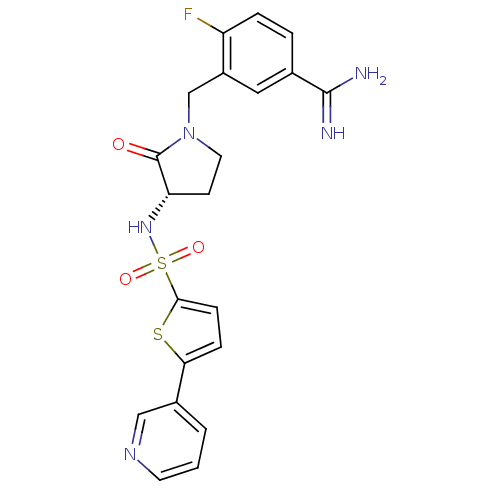 (4-fluoro-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13293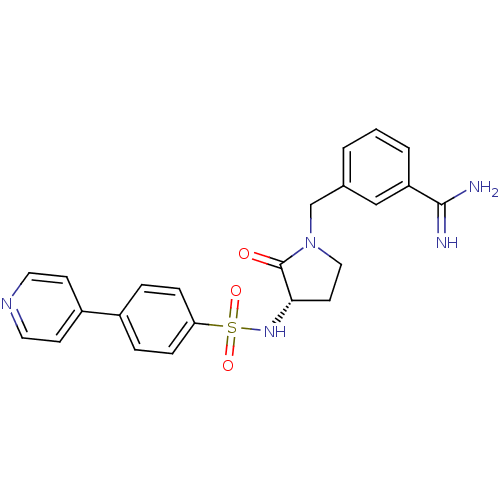 (3-{[(3S)-2-oxo-3-{[4-(pyridin-4-yl)benzene]sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13297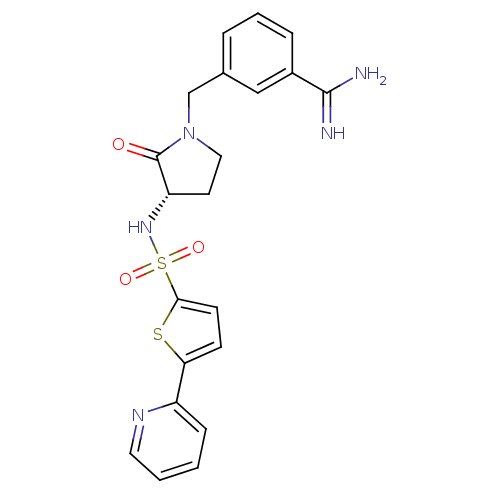 (3-{[(3S)-2-oxo-3-{[5-(pyridin-2-yl)thiophene-2-]su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13291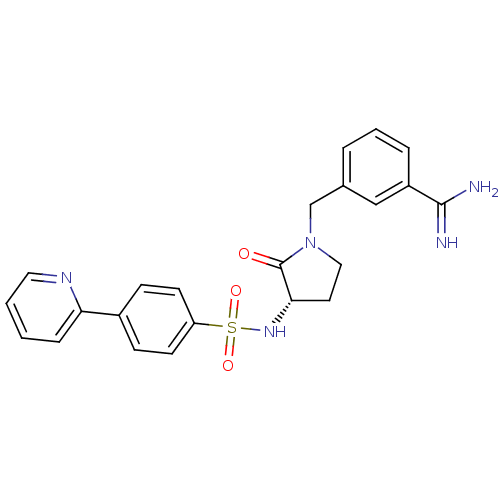 (3-{[(3S)-2-oxo-3-{[4-(pyridin-2-yl)benzene]sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13300 (3-{[(3S)-3-{[5-(2-methoxypyrimidin-4-yl)thiophene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13290 (3-{[(3S)-2-oxo-3-[(4-phenylbenzene)sulfonamido]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13283 (4-amino-3-({(3S)-3-[[(7-methoxy-2-naphthyl)sulfony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 189 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13292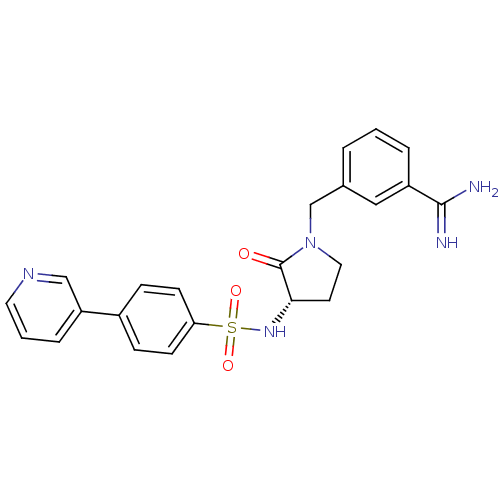 (3-{[(3S)-2-oxo-3-{[4-(pyridin-3-yl)benzene]sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296938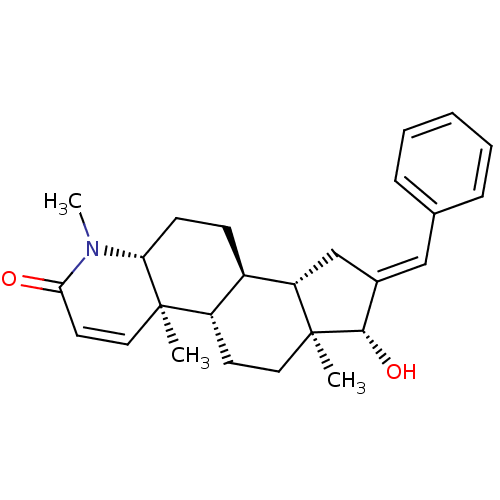 (16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296935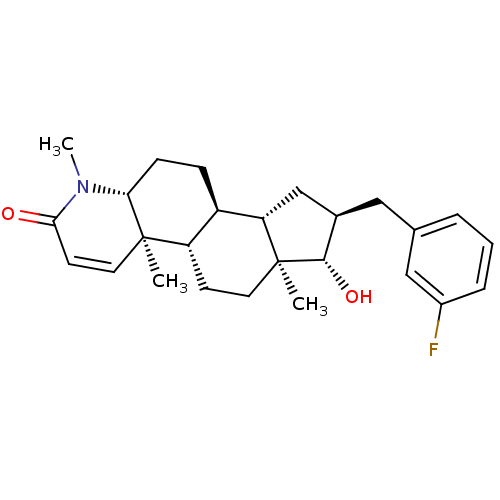 (16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13281 (4-Amino-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 299 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13286 (4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 305 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296941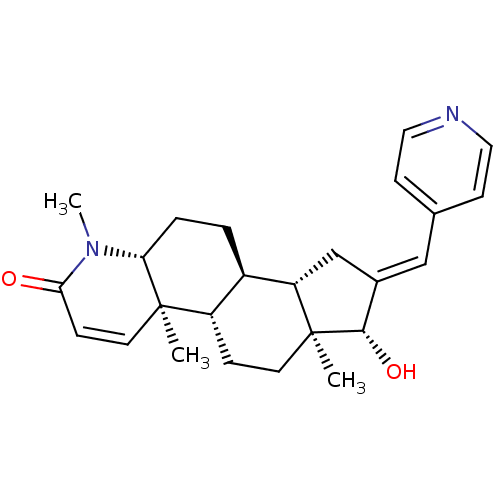 (16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13295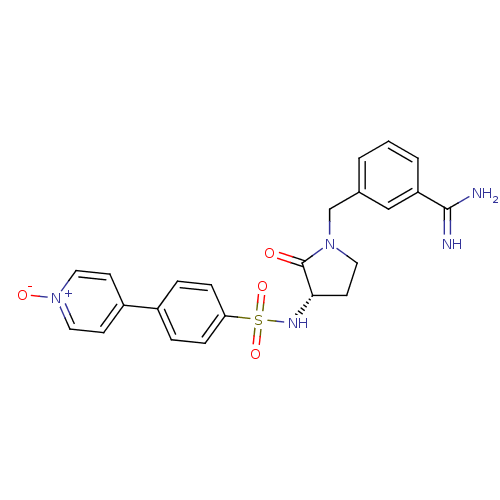 (4-(4-{[(3S)-1-[(3-carbamimidoylphenyl)methyl]-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50006812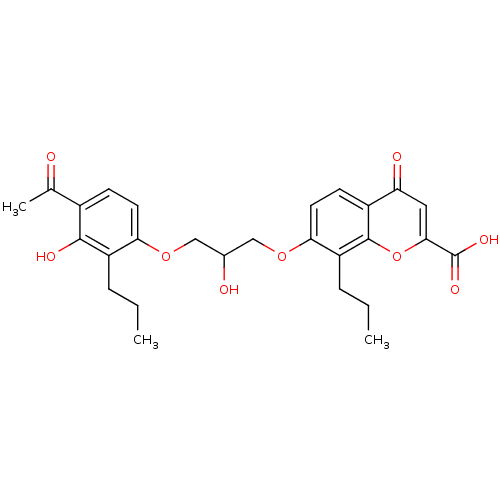 (7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13282 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)(methyl)sulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 704 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 853 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13284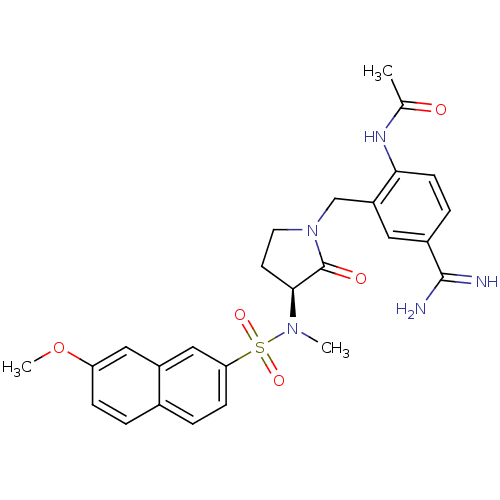 (N-(4-carbamimidoyl-2-{[(3S)-3-[(7-methoxynaphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 919 | -34.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |