

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM50482873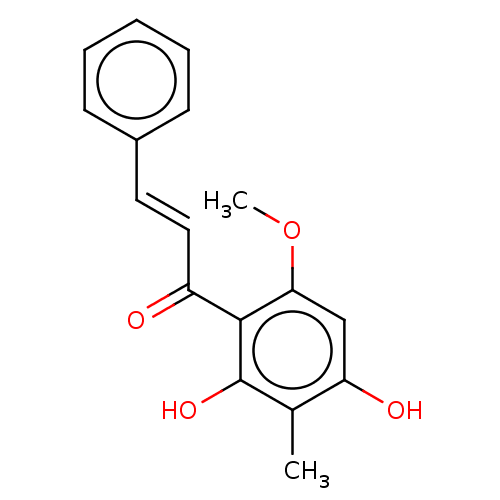 (CHEBI:70659 | CHEMBL1271362) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482872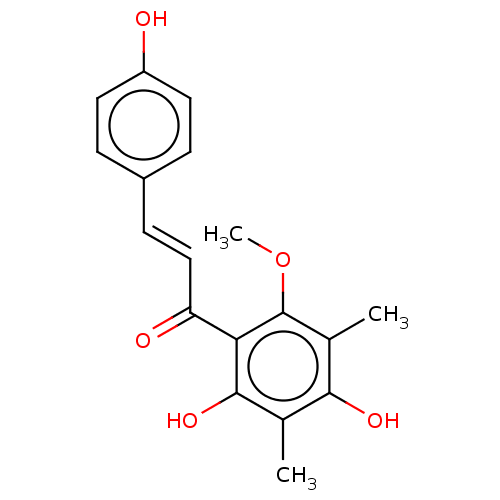 (CHEBI:70655 | CHEMBL1271157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50483016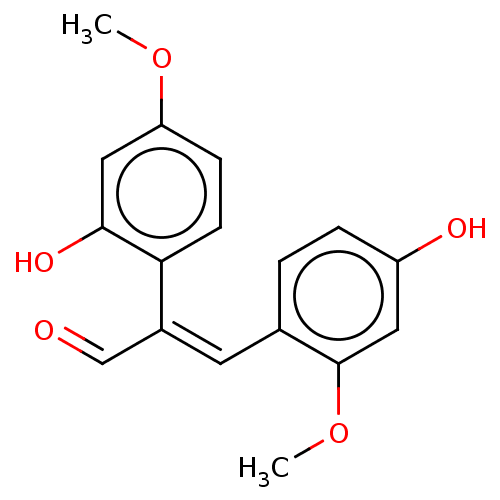 (ERYTHRADDISON B) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482871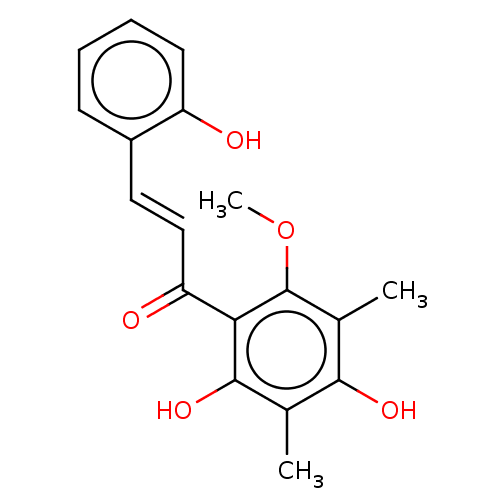 (CHEBI:66265 | CHEMBL509947) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482882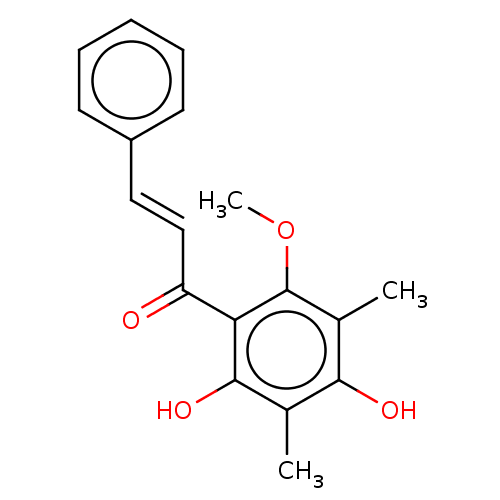 (CHEBI:70658 | CHEMBL463095) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50483015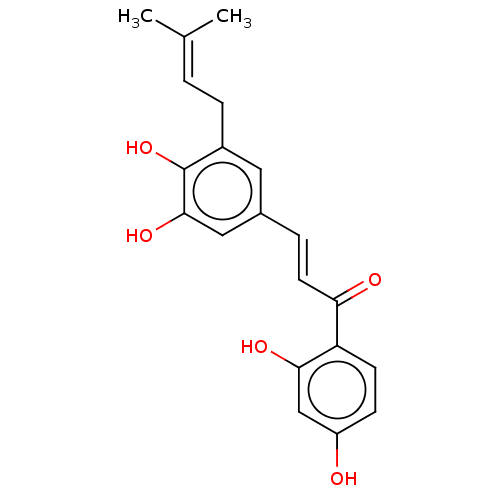 (5''-Prenylbutein | 5'-PRENYLBUTEIN) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50212400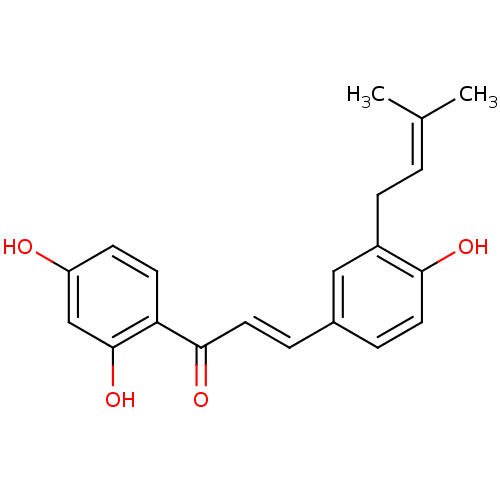 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50370984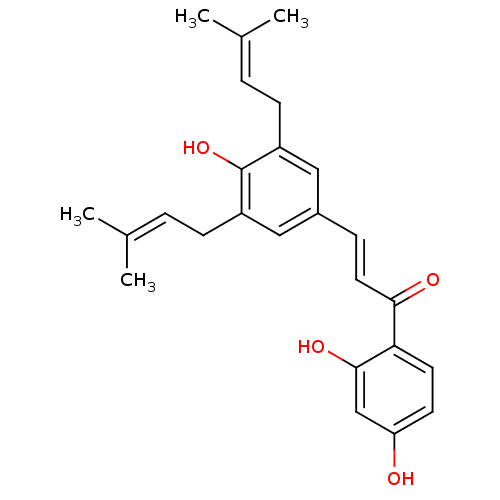 (Abyssinone Vi | CHEMBL508727) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of influenza A virus H9N2 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate substrate | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H9N2 neuraminidase using 4-MU-NANA as substrate by fluorescence assay | Bioorg Med Chem Lett 22: 3688-92 (2012) Article DOI: 10.1016/j.bmcl.2012.04.028 BindingDB Entry DOI: 10.7270/Q27947JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A H9N2 virus neuraminidase activity after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of wild type H1N1 swine influenza virus neuraminidase activity expressed in HEK293T cells after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of wild type H1N1 swine influenza virus neuraminidase using 4-MU-NANA as substrate by fluorescence assay | Bioorg Med Chem Lett 22: 3688-92 (2012) Article DOI: 10.1016/j.bmcl.2012.04.028 BindingDB Entry DOI: 10.7270/Q27947JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate substrate | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H1N1 neuraminidase using 4-MU-NANA as substrate by fluorescence assay | Bioorg Med Chem Lett 22: 3688-92 (2012) Article DOI: 10.1016/j.bmcl.2012.04.028 BindingDB Entry DOI: 10.7270/Q27947JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase activity after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50253160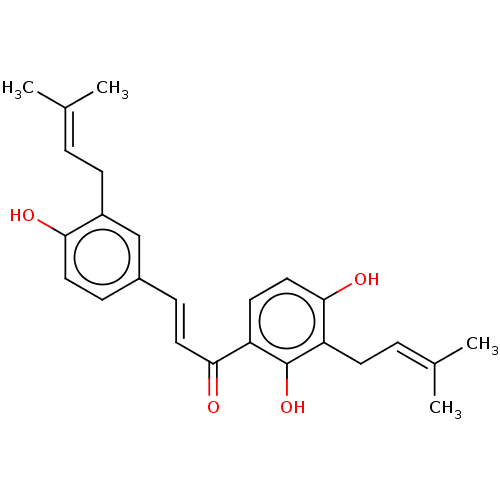 (Kanzonol C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50483310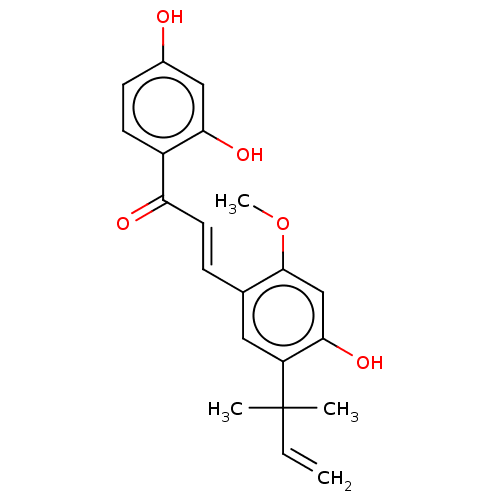 (Licochalcone G) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482871 (CHEBI:66265 | CHEMBL509947) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins | J Nat Prod 71: 1775-8 (2008) Article DOI: 10.1021/np800298w BindingDB Entry DOI: 10.7270/Q2TQ62F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482872 (CHEBI:70655 | CHEMBL1271157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50483309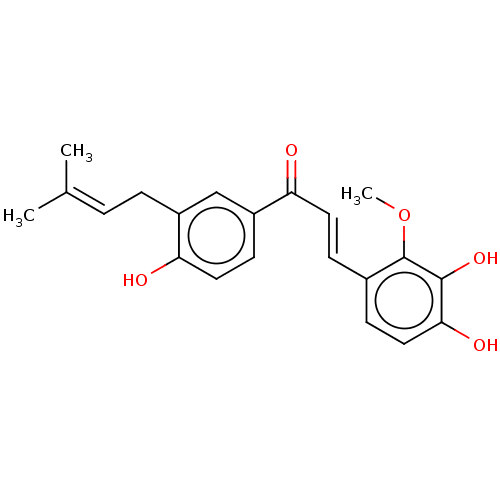 (Licochalcone D) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346613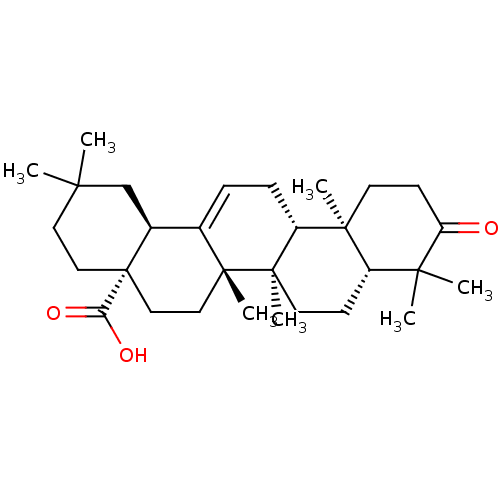 (CHEMBL470029 | Oleanonic Acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins | J Nat Prod 71: 1775-8 (2008) Article DOI: 10.1021/np800298w BindingDB Entry DOI: 10.7270/Q2TQ62F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 20: 6459-64 (2012) Article DOI: 10.1016/j.bmc.2012.08.024 BindingDB Entry DOI: 10.7270/Q29C6ZHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitorphenol production after 30 mins | Bioorg Med Chem 19: 3378-83 (2011) Article DOI: 10.1016/j.bmc.2011.04.037 BindingDB Entry DOI: 10.7270/Q2DF6RKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50241553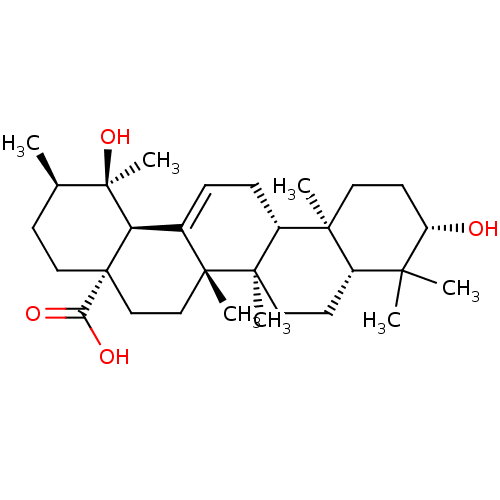 (CHEMBL486986 | pomolic acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins | J Nat Prod 71: 1775-8 (2008) Article DOI: 10.1021/np800298w BindingDB Entry DOI: 10.7270/Q2TQ62F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50104694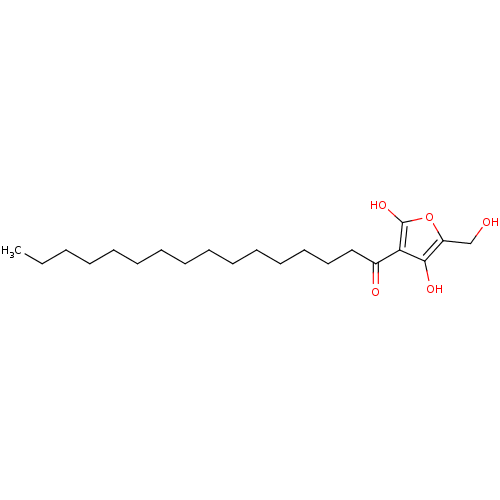 ((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins | J Nat Prod 71: 1775-8 (2008) Article DOI: 10.1021/np800298w BindingDB Entry DOI: 10.7270/Q2TQ62F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311577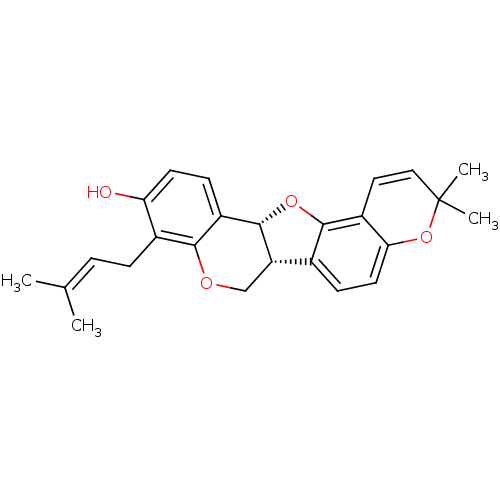 (CHEMBL561967 | Erybreadin B | erybraedin B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50104694 ((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50394207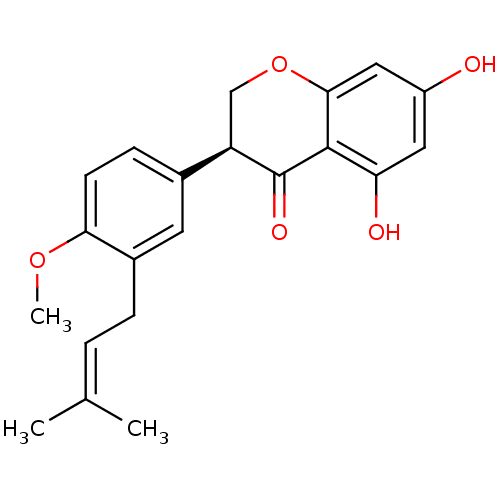 (CHEMBL2159047) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 20: 6459-64 (2012) Article DOI: 10.1016/j.bmc.2012.08.024 BindingDB Entry DOI: 10.7270/Q29C6ZHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482882 (CHEBI:70658 | CHEMBL463095) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311580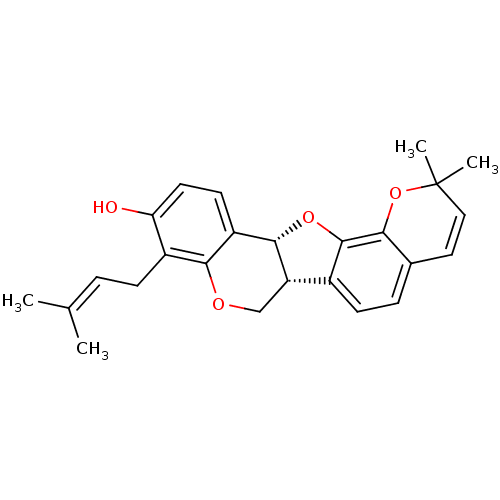 (CHEMBL1087148 | Erybreadin D) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311582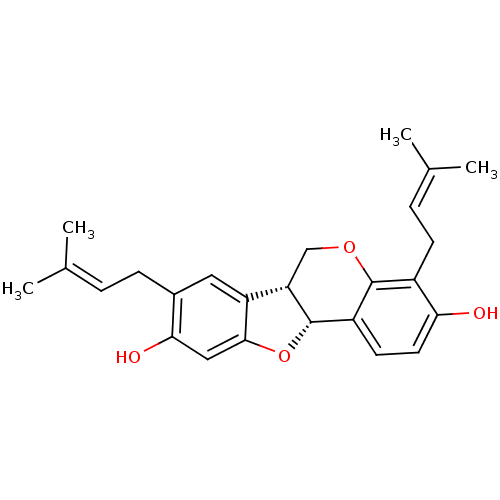 (CHEMBL1086765 | Erybreadin C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins | J Nat Prod 71: 1775-8 (2008) Article DOI: 10.1021/np800298w BindingDB Entry DOI: 10.7270/Q2TQ62F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50317432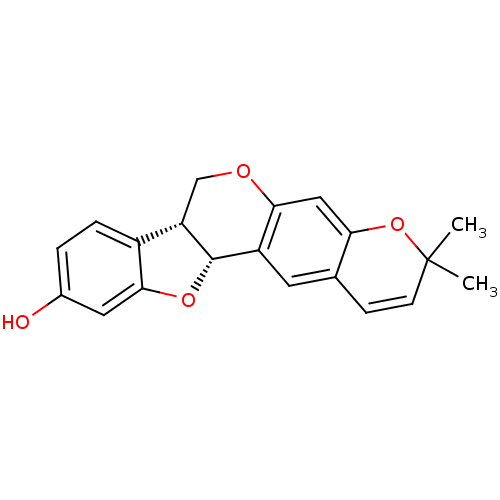 (CHEMBL1098728 | NEORAUTENOL) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50394204 (CHEMBL2159045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 20: 6459-64 (2012) Article DOI: 10.1016/j.bmc.2012.08.024 BindingDB Entry DOI: 10.7270/Q29C6ZHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311579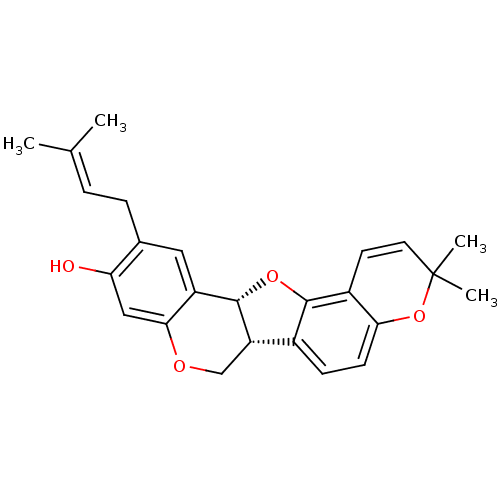 (CHEMBL551155 | folitenol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253117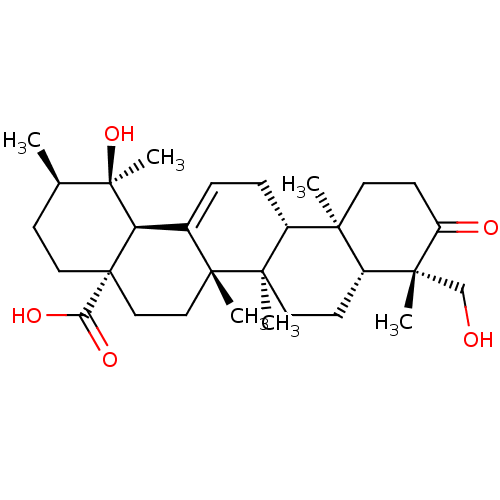 (19alpha,24-dihydroxyurs-12-en-3-on-28-oic acid | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins | J Nat Prod 71: 1775-8 (2008) Article DOI: 10.1021/np800298w BindingDB Entry DOI: 10.7270/Q2TQ62F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50068267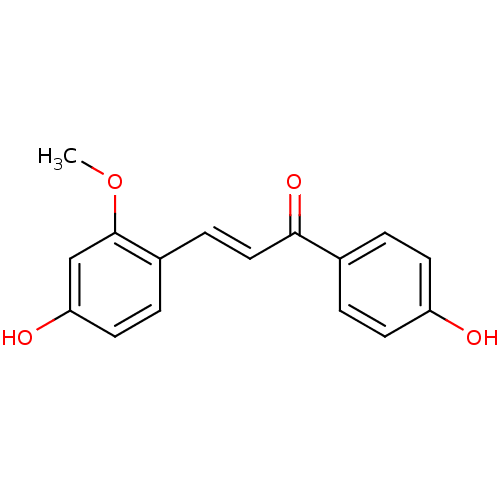 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant H1N1 swine influenza virus neuraminidase H274Y mutant activity expressed in HEK293T cells after 2 hrs by spectrof... | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482872 (CHEBI:70655 | CHEMBL1271157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) wild type neuraminidase expressed in human 293T cells after 30 mins by spectrofluorimetr... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311581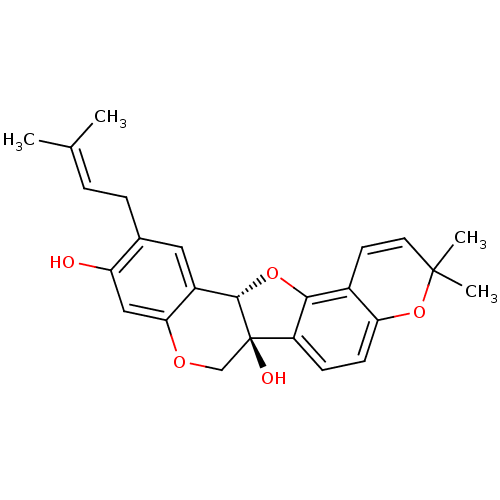 (CHEMBL1086764 | erysubin E) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of wild type H1N1 swine influenza virus neuraminidase activity expressed in HEK293T cells after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50483015 (5''-Prenylbutein | 5'-PRENYLBUTEIN) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482871 (CHEBI:66265 | CHEMBL509947) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) wild type neuraminidase expressed in human 293T cells after 30 mins by spectrofluorimetr... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Korea/01310/2001 (H9N2)) neuraminidase after 30 mins by spectrofluorimetric analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253053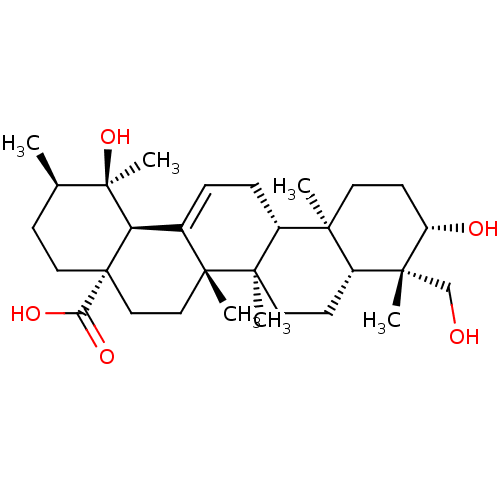 (CHEMBL493908 | rotungenic acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins | J Nat Prod 71: 1775-8 (2008) Article DOI: 10.1021/np800298w BindingDB Entry DOI: 10.7270/Q2TQ62F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50068270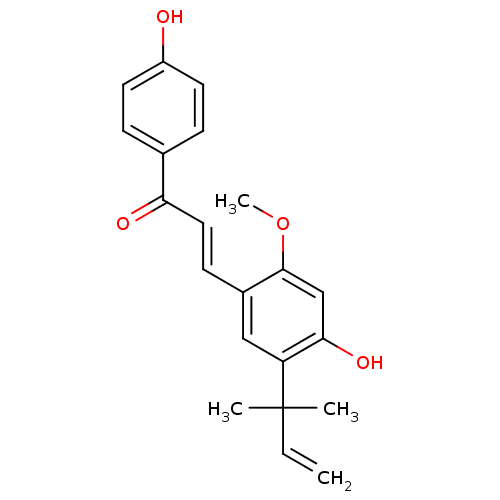 ((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant H1N1 swine influenza virus neuraminidase H274Y mutant activity expressed in HEK293T cells after 2 hrs by spectrof... | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 199 total ) | Next | Last >> |