 Found 2356 hits with Last Name = 'das' and Initial = 's'
Found 2356 hits with Last Name = 'das' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204090

(CHEMBL3958859)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1cc(-[#8])cc(-[#16]-c2ccccc2-[#6](-[#8])=O)c1-[#8] Show InChI InChI=1S/C33H42O4S/c1-23(2)11-8-12-24(3)13-9-14-25(4)15-10-16-26(5)19-20-27-21-28(34)22-31(32(27)35)38-30-18-7-6-17-29(30)33(36)37/h6-7,11,13,15,17-19,21-22,34-35H,8-10,12,14,16,20H2,1-5H3,(H,36,37)/b24-13+,25-15+,26-19+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204086
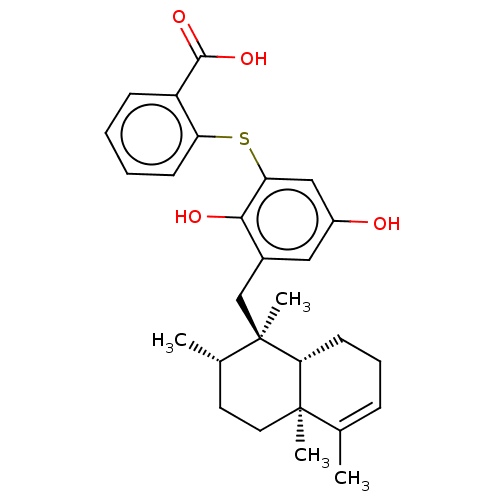
(Avarol-3''-Thiosalicylate | CHEMBL238756)Show SMILES [H][C@@]12CCC=C(C)[C@@]1(C)CC[C@H](C)[C@@]2(C)Cc1cc(O)cc(Sc2ccccc2C(O)=O)c1O |r,t:4| Show InChI InChI=1S/C28H34O4S/c1-17-8-7-11-24-27(17,3)13-12-18(2)28(24,4)16-19-14-20(29)15-23(25(19)30)33-22-10-6-5-9-21(22)26(31)32/h5-6,8-10,14-15,18,24,29-30H,7,11-13,16H2,1-4H3,(H,31,32)/t18-,24+,27+,28+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416
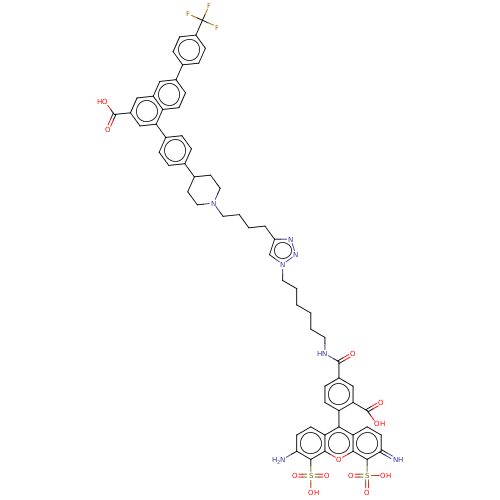
(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14R |
J Med Chem 63: 9563-9589 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00745
BindingDB Entry DOI: 10.7270/Q20R9SZP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513
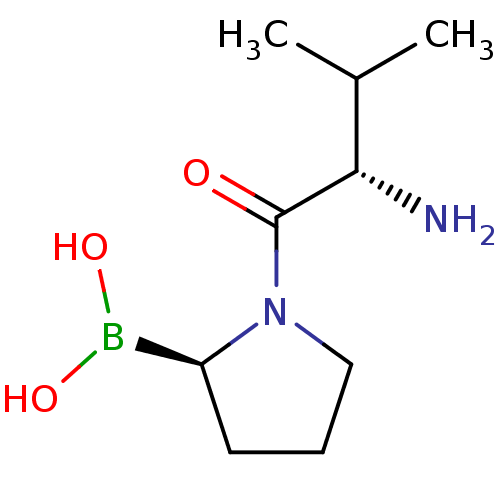
((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116748
BindingDB Entry DOI: 10.7270/Q2MG7TGW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204087
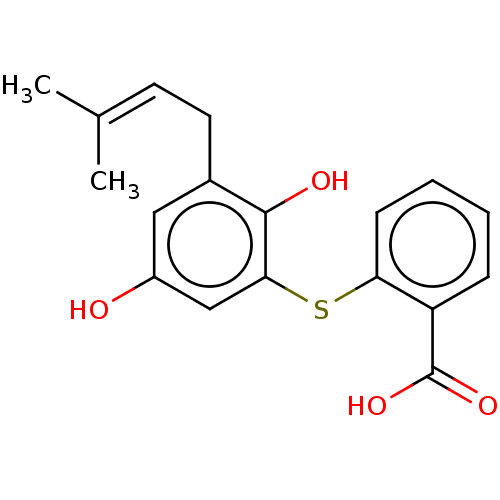
(CHEMBL3920392)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(-[#8])cc(-[#16]-c2ccccc2-[#6](-[#8])=O)c1-[#8] Show InChI InChI=1S/C18H18O4S/c1-11(2)7-8-12-9-13(19)10-16(17(12)20)23-15-6-4-3-5-14(15)18(21)22/h3-7,9-10,19-20H,8H2,1-2H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Rattus norvegicus) | BDBM50456168
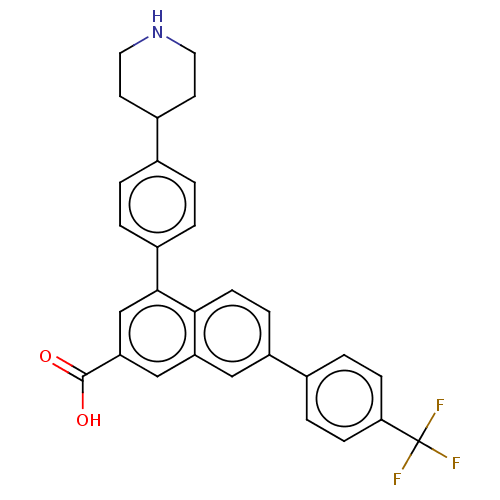
(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y14R in rat C6 cells assessed as suppression of UDP-glucose-mediated inhibition of forskolin-stimulated [3H]cyclic-AMP accum... |
J Med Chem 61: 4860-4882 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00168
BindingDB Entry DOI: 10.7270/Q2X069NH |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50592900
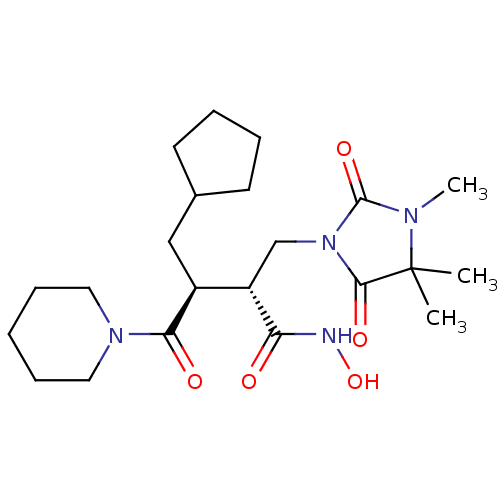
(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM13126
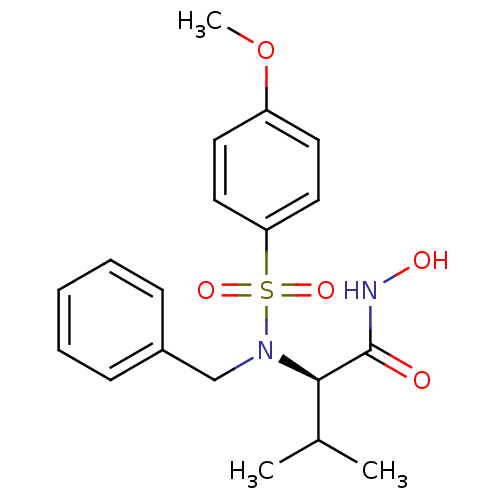
((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11548

(CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(CN2C(CCS2(=O)=O)C(=O)NO)cc1 Show InChI InChI=1S/C18H20N2O5S/c1-25-16-8-6-15(7-9-16)14-4-2-13(3-5-14)12-20-17(18(21)19-22)10-11-26(20,23)24/h2-9,17,22H,10-12H2,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50592900

(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50335495
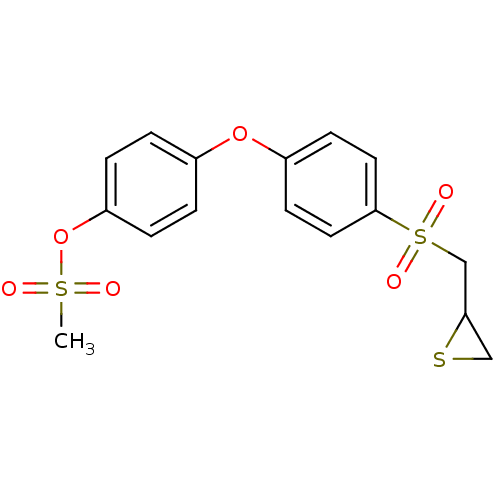
(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50247674
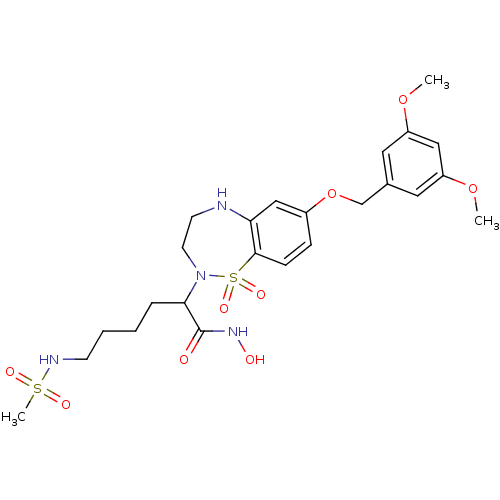
(2-[7-(3,5-Dimethoxy-benzyloxy)-1,1-dioxo-1,3,4,5-t...)Show SMILES COc1cc(COc2ccc3c(NCCN(C(CCCCNS(C)(=O)=O)C(=O)NO)S3(=O)=O)c2)cc(OC)c1 Show InChI InChI=1S/C24H34N4O9S2/c1-35-19-12-17(13-20(14-19)36-2)16-37-18-7-8-23-21(15-18)25-10-11-28(39(23,33)34)22(24(29)27-30)6-4-5-9-26-38(3,31)32/h7-8,12-15,22,25-26,30H,4-6,9-11,16H2,1-3H3,(H,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of pig TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116748
BindingDB Entry DOI: 10.7270/Q2MG7TGW |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388914
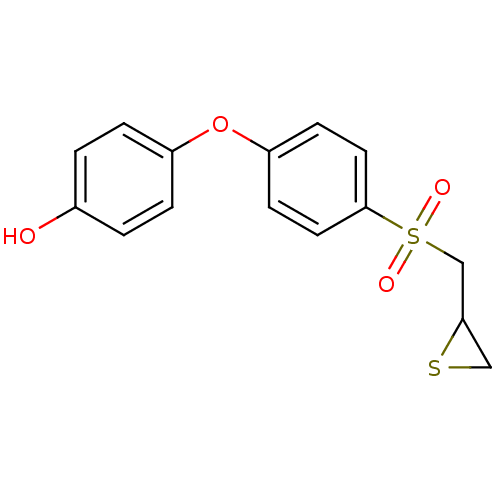
(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM82353
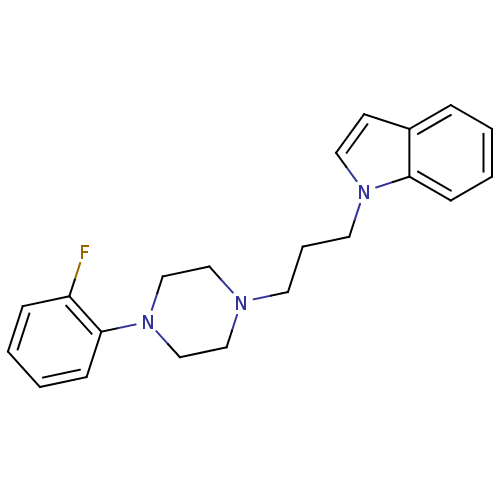
(1-{3-[4-(substitutedphenyl)piperazin1-yl]propyl}-1...)Show InChI InChI=1S/C21H24FN3/c22-19-7-2-4-9-21(19)25-16-14-23(15-17-25)11-5-12-24-13-10-18-6-1-3-8-20(18)24/h1-4,6-10,13H,5,11-12,14-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-di-o-tolylguanidine from sigma-2 receptor in rat liver membranes after 180 mins by scintillation counting method |
Eur J Med Chem 150: 9-29 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.065
BindingDB Entry DOI: 10.7270/Q2X92DXX |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50084661

(4-(4'-Chloro-biphenyl-4-yl)-4-oxo-2-phenylsulfanyl...)Show SMILES OC(=O)[C@@H](CSc1ccccc1)CC(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H19ClO3S/c24-20-12-10-17(11-13-20)16-6-8-18(9-7-16)22(25)14-19(23(26)27)15-28-21-4-2-1-3-5-21/h1-13,19H,14-15H2,(H,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM82351
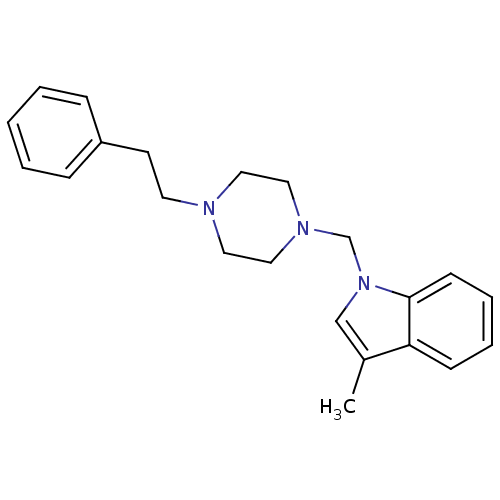
(1-{[4-(substitutedphenyl/phenylethyl)piperazin1-yl...)Show InChI InChI=1S/C22H27N3/c1-19-17-25(22-10-6-5-9-21(19)22)18-24-15-13-23(14-16-24)12-11-20-7-3-2-4-8-20/h2-10,17H,11-16,18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in guinea pig brain membranes after 180 mins by scintillation counting method |
Eur J Med Chem 150: 9-29 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.065
BindingDB Entry DOI: 10.7270/Q2X92DXX |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809
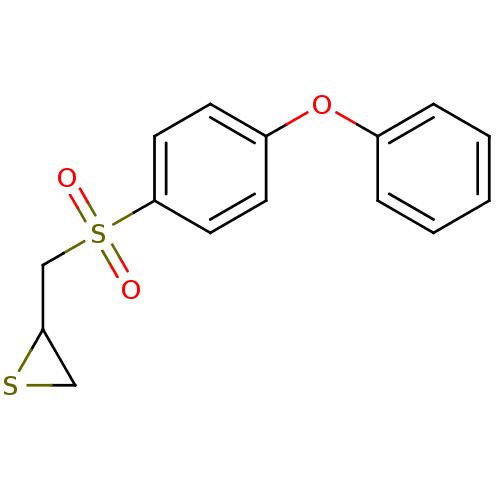
(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM21006
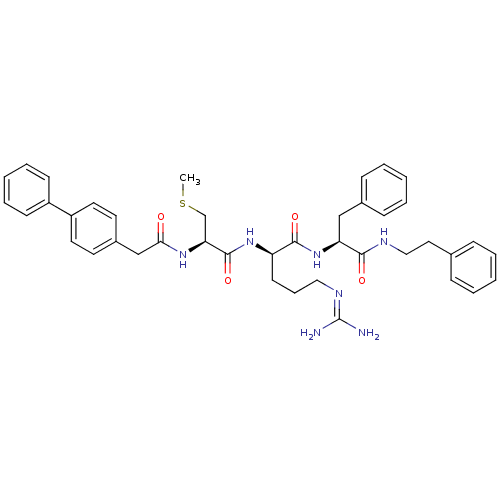
((2R)-5-[(diaminomethylidene)amino]-2-[(2R)-3-(meth...)Show SMILES [#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C41H49N7O4S/c1-53-28-36(46-37(49)27-31-19-21-33(22-20-31)32-16-9-4-10-17-32)40(52)47-34(18-11-24-45-41(42)43)39(51)48-35(26-30-14-7-3-8-15-30)38(50)44-25-23-29-12-5-2-6-13-29/h2-10,12-17,19-22,34-36H,11,18,23-28H2,1H3,(H,44,50)(H,46,49)(H,47,52)(H,48,51)(H4,42,43,45)/t34-,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20998
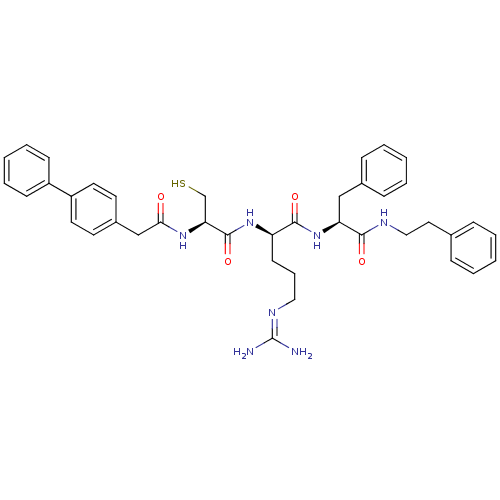
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-pheny...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16])-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C40H47N7O4S/c41-40(42)44-23-10-17-33(38(50)47-34(25-29-13-6-2-7-14-29)37(49)43-24-22-28-11-4-1-5-12-28)46-39(51)35(27-52)45-36(48)26-30-18-20-32(21-19-30)31-15-8-3-9-16-31/h1-9,11-16,18-21,33-35,52H,10,17,22-27H2,(H,43,49)(H,45,48)(H,46,51)(H,47,50)(H4,41,42,44)/t33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | -43.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20997
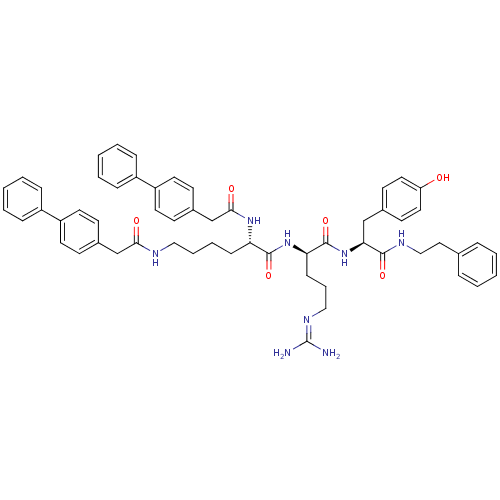
((2S)-N-[(1R)-4-[(diaminomethylidene)amino]-1-{[(1S...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C57H64N8O6/c58-57(59)62-35-12-20-50(56(71)65-51(37-41-25-31-48(66)32-26-41)54(69)61-36-33-40-13-4-1-5-14-40)64-55(70)49(63-53(68)39-43-23-29-47(30-24-43)45-17-8-3-9-18-45)19-10-11-34-60-52(67)38-42-21-27-46(28-22-42)44-15-6-2-7-16-44/h1-9,13-18,21-32,49-51,66H,10-12,19-20,33-39H2,(H,60,67)(H,61,69)(H,63,68)(H,64,70)(H,65,71)(H4,58,59,62)/t49-,50+,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | -43.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20999
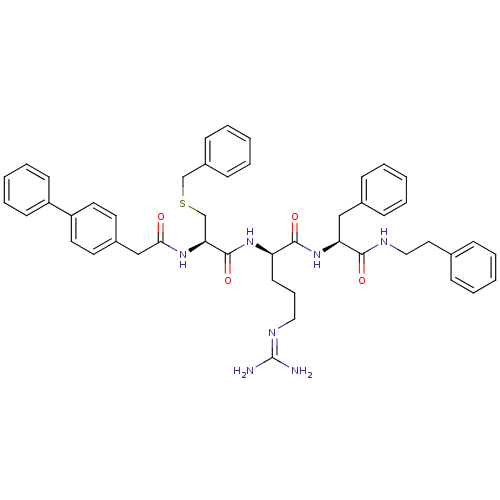
((2R)-2-[(2R)-3-(benzylsulfanyl)-2-[1-(4-phenylphen...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C47H53N7O4S/c48-47(49)51-28-13-22-40(45(57)54-41(30-35-16-7-2-8-17-35)44(56)50-29-27-34-14-5-1-6-15-34)53-46(58)42(33-59-32-37-18-9-3-10-19-37)52-43(55)31-36-23-25-39(26-24-36)38-20-11-4-12-21-38/h1-12,14-21,23-26,40-42H,13,22,27-33H2,(H,50,56)(H,52,55)(H,53,58)(H,54,57)(H4,48,49,51)/t40-,41+,42+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354624
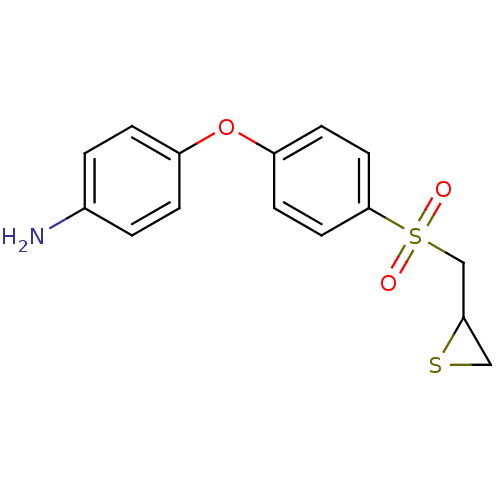
(CHEMBL1834422 | US10357546, ND-322 | US9951035, ND...)Show InChI InChI=1S/C15H15NO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Homo sapiens (Human)) | BDBM50292713

(5'-phospho-2'-deoxyuridine 3-pyrophosphate (P'->5'...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H](C[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(30)15(9(4-27)38-18)39-42(34,35)40-41(32,33)36-5-10-8(28)3-12(37-10)25-2-1-11(29)24-19(25)31/h1-2,6-10,12,14-15,18,27-28,30H,3-5H2,(H,32,33)(H,34,35)(H2,20,21,22)(H,24,29,31)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of RNase A |
J Med Chem 52: 932-42 (2009)
Article DOI: 10.1021/jm800724t
BindingDB Entry DOI: 10.7270/Q2H99570 |
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Bison bison (American bison)) | BDBM50342010
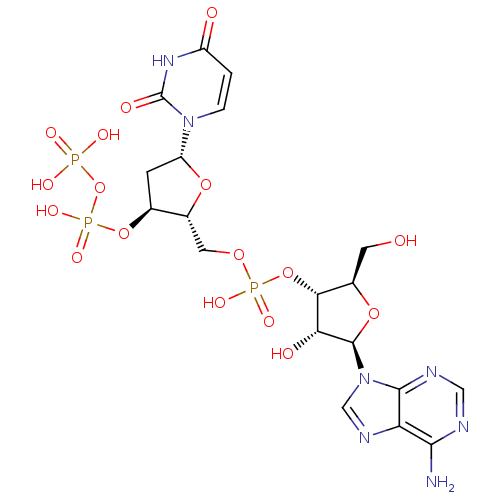
(5'-phospho-2'-deoxyuridine-3'-pyrophosphate(P'->5'...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](OP(O)(=O)OC[C@H]2O[C@H](C[C@@H]2OP(O)(=O)OP(O)(O)=O)n2ccc(=O)[nH]c2=O)[C@H]1O |r| Show InChI InChI=1S/C19H26N7O17P3/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(29)15(9(4-27)40-18)42-45(34,35)38-5-10-8(41-46(36,37)43-44(31,32)33)3-12(39-10)25-2-1-11(28)24-19(25)30/h1-2,6-10,12,14-15,18,27,29H,3-5H2,(H,34,35)(H,36,37)(H2,20,21,22)(H,24,28,30)(H2,31,32,33)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | 5.9 | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of bovine pancreatic RNase A at pH 5.9 by spectrophotometric method |
Bioorg Med Chem 19: 2478-84 (2011)
Article DOI: 10.1016/j.bmc.2010.08.059
BindingDB Entry DOI: 10.7270/Q2736R7B |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50456166

(CHEMBL4216870)Show SMILES NCCCCCCn1cc(CCCCN2CCC(CC2)c2ccc(cc2)-c2cc(cc3cc(ccc23)-c2ccc(cc2)C(F)(F)F)C(O)=O)nn1 Show InChI InChI=1S/C41H46F3N5O2/c42-41(43,44)36-15-12-30(13-16-36)33-14-17-38-34(25-33)26-35(40(50)51)27-39(38)32-10-8-29(9-11-32)31-18-23-48(24-19-31)21-6-3-7-37-28-49(47-46-37)22-5-2-1-4-20-45/h8-17,25-28,31H,1-7,18-24,45H2,(H,50,51) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]Rauwolscine from human adrenergic alpha2A receptor expressed in MDCK cell membranes after 90 mins by scintillation counting metho... |
J Med Chem 61: 4860-4882 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00168
BindingDB Entry DOI: 10.7270/Q2X069NH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556
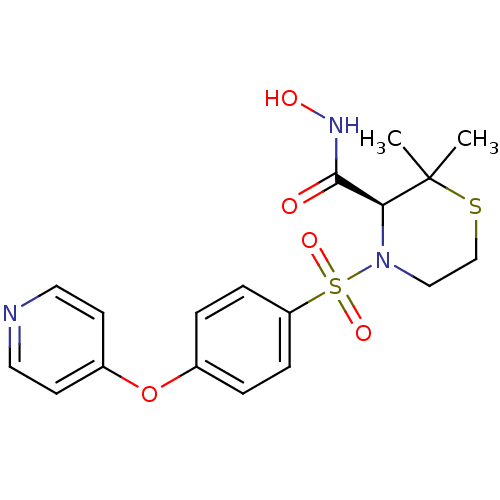
((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Bison bison (American bison)) | BDBM50292721
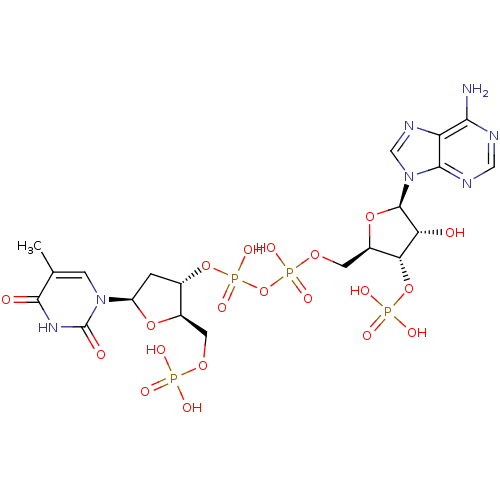
(CHEMBL504858 | [({[(2R,3S,4R,5R)-5-(6-amino-9H-pur...)Show SMILES Cc1cn([C@H]2C[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]3O[C@H]([C@H](O)[C@@H]3OP(O)(O)=O)n3cnc4c(N)ncnc34)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C20H29N7O20P4/c1-8-3-26(20(30)25-18(8)29)12-2-9(10(43-12)4-41-48(31,32)33)45-51(39,40)47-50(37,38)42-5-11-15(46-49(34,35)36)14(28)19(44-11)27-7-24-13-16(21)22-6-23-17(13)27/h3,6-7,9-12,14-15,19,28H,2,4-5H2,1H3,(H,37,38)(H,39,40)(H2,21,22,23)(H,25,29,30)(H2,31,32,33)(H2,34,35,36)/t9-,10+,11+,12+,14+,15+,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of bovine pancreatic RNase A by spectrophotometric method |
J Med Chem 52: 932-42 (2009)
Article DOI: 10.1021/jm800724t
BindingDB Entry DOI: 10.7270/Q2H99570 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dihydrofolate reductase
(Escherichia coli) | BDBM50498871

(CHEMBL97313)Show SMILES CC1(C)N=C(N)N=C(N)N1c1cccc(CCCCc2ccc(cc2)S(F)(=O)=O)c1 |t:3,6| Show InChI InChI=1S/C21H26FN5O2S/c1-21(2)26-19(23)25-20(24)27(21)17-9-5-8-16(14-17)7-4-3-6-15-10-12-18(13-11-15)30(22,28)29/h5,8-14H,3-4,6-7H2,1-2H3,(H4,23,24,25,26) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Competitive inhibition of Escherichia coli DHFR by Lineweaver-Burk plot analysis in presence of H2F |
Eur J Med Chem 103: 600-14 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.021
BindingDB Entry DOI: 10.7270/Q2DR2ZGJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20993
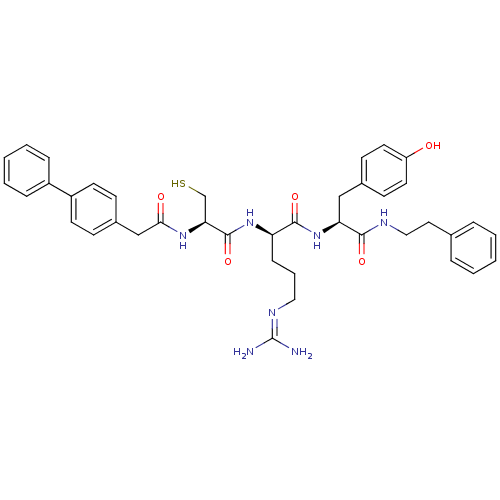
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16])-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C40H47N7O5S/c41-40(42)44-22-7-12-33(46-39(52)35(26-53)45-36(49)25-29-13-17-31(18-14-29)30-10-5-2-6-11-30)38(51)47-34(24-28-15-19-32(48)20-16-28)37(50)43-23-21-27-8-3-1-4-9-27/h1-6,8-11,13-20,33-35,48,53H,7,12,21-26H2,(H,43,50)(H,45,49)(H,46,52)(H,47,51)(H4,41,42,44)/t33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 45 | -41.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11548

(CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(CN2C(CCS2(=O)=O)C(=O)NO)cc1 Show InChI InChI=1S/C18H20N2O5S/c1-25-16-8-6-15(7-9-16)14-4-2-13(3-5-14)12-20-17(18(21)19-22)10-11-26(20,23)24/h2-9,17,22H,10-12H2,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50498871

(CHEMBL97313)Show SMILES CC1(C)N=C(N)N=C(N)N1c1cccc(CCCCc2ccc(cc2)S(F)(=O)=O)c1 |t:3,6| Show InChI InChI=1S/C21H26FN5O2S/c1-21(2)26-19(23)25-20(24)27(21)17-9-5-8-16(14-17)7-4-3-6-15-10-12-18(13-11-15)30(22,28)29/h5,8-14H,3-4,6-7H2,1-2H3,(H4,23,24,25,26) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli DHFR assessed as NADP formation by quadratic Morrison plot analysis |
Eur J Med Chem 103: 600-14 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.021
BindingDB Entry DOI: 10.7270/Q2DR2ZGJ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11548

(CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(CN2C(CCS2(=O)=O)C(=O)NO)cc1 Show InChI InChI=1S/C18H20N2O5S/c1-25-16-8-6-15(7-9-16)14-4-2-13(3-5-14)12-20-17(18(21)19-22)10-11-26(20,23)24/h2-9,17,22H,10-12H2,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM82351

(1-{[4-(substitutedphenyl/phenylethyl)piperazin1-yl...)Show InChI InChI=1S/C22H27N3/c1-19-17-25(22-10-6-5-9-21(19)22)18-24-15-13-23(14-16-24)12-11-20-7-3-2-4-8-20/h2-10,17H,11-16,18H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-di-o-tolylguanidine from sigma-2 receptor in rat liver membranes after 180 mins by scintillation counting method |
Eur J Med Chem 150: 9-29 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.065
BindingDB Entry DOI: 10.7270/Q2X92DXX |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50592900

(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335500
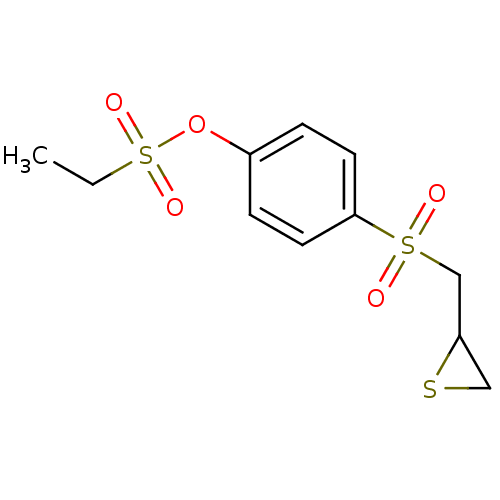
(4-(Thiiran-2-ylmethylsulfonyl)phenyl ethanesulfona...)Show InChI InChI=1S/C11H14O5S3/c1-2-19(14,15)16-9-3-5-11(6-4-9)18(12,13)8-10-7-17-10/h3-6,10H,2,7-8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50498878

(CHEMBL97567)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(CCCCCCc2ccc(cc2)S(F)(=O)=O)cc1 |t:3,6| Show InChI InChI=1S/C23H30FN5O2S/c1-23(2)28-21(25)27-22(26)29(23)19-13-9-17(10-14-19)7-5-3-4-6-8-18-11-15-20(16-12-18)32(24,30)31/h9-16H,3-8H2,1-2H3,(H4,25,26,27,28) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli DHFR assessed as NADP formation by quadratic Morrison plot analysis |
Eur J Med Chem 103: 600-14 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.021
BindingDB Entry DOI: 10.7270/Q2DR2ZGJ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50498878

(CHEMBL97567)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(CCCCCCc2ccc(cc2)S(F)(=O)=O)cc1 |t:3,6| Show InChI InChI=1S/C23H30FN5O2S/c1-23(2)28-21(25)27-22(26)29(23)19-13-9-17(10-14-19)7-5-3-4-6-8-18-11-15-20(16-12-18)32(24,30)31/h9-16H,3-8H2,1-2H3,(H4,25,26,27,28) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Competitive inhibition of Escherichia coli DHFR by Lineweaver-Burk plot analysis in presence of H2F |
Eur J Med Chem 103: 600-14 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.021
BindingDB Entry DOI: 10.7270/Q2DR2ZGJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20996
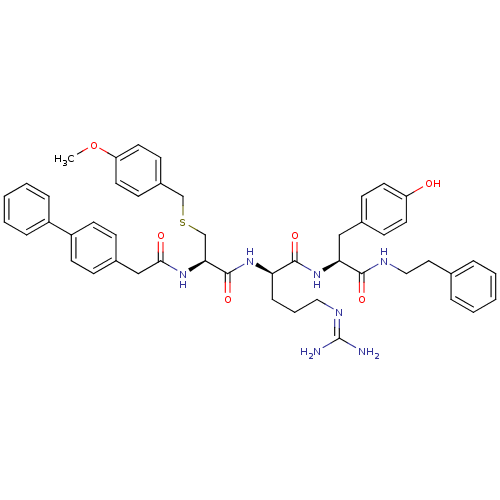
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c2ccc(cc2)-c2ccccc2)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6]-c2ccccc2)cc1 |r| Show InChI InChI=1S/C48H55N7O6S/c1-61-40-24-18-36(19-25-40)31-62-32-43(53-44(57)30-35-14-20-38(21-15-35)37-11-6-3-7-12-37)47(60)54-41(13-8-27-52-48(49)50)46(59)55-42(29-34-16-22-39(56)23-17-34)45(58)51-28-26-33-9-4-2-5-10-33/h2-7,9-12,14-25,41-43,56H,8,13,26-32H2,1H3,(H,51,58)(H,53,57)(H,54,60)(H,55,59)(H4,49,50,52)/t41-,42+,43+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | -39.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50084661

(4-(4'-Chloro-biphenyl-4-yl)-4-oxo-2-phenylsulfanyl...)Show SMILES OC(=O)[C@@H](CSc1ccccc1)CC(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H19ClO3S/c24-20-12-10-17(11-13-20)16-6-8-18(9-7-16)22(25)14-19(23(26)27)15-28-21-4-2-1-3-5-21/h1-13,19H,14-15H2,(H,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data