 Found 239 hits with Last Name = 'davies' and Initial = 'k'
Found 239 hits with Last Name = 'davies' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50416472

(CHEMBL1209708)Show SMILES COc1cccc2c(cn(CC3CCCCC3)c12)C(=O)N1C[C@H](C)N(C)[C@H](C)C1 |r| Show InChI InChI=1S/C24H35N3O2/c1-17-13-27(14-18(2)25(17)3)24(28)21-16-26(15-19-9-6-5-7-10-19)23-20(21)11-8-12-22(23)29-4/h8,11-12,16-19H,5-7,9-10,13-15H2,1-4H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50416472

(CHEMBL1209708)Show SMILES COc1cccc2c(cn(CC3CCCCC3)c12)C(=O)N1C[C@H](C)N(C)[C@H](C)C1 |r| Show InChI InChI=1S/C24H35N3O2/c1-17-13-27(14-18(2)25(17)3)24(28)21-16-26(15-19-9-6-5-7-10-19)23-20(21)11-8-12-22(23)29-4/h8,11-12,16-19H,5-7,9-10,13-15H2,1-4H3/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50380719
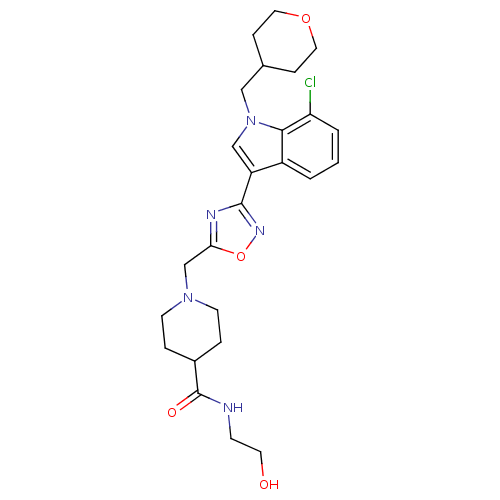
(CHEMBL1682275)Show SMILES OCCNC(=O)C1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C25H32ClN5O4/c26-21-3-1-2-19-20(15-31(23(19)21)14-17-6-12-34-13-7-17)24-28-22(35-29-24)16-30-9-4-18(5-10-30)25(33)27-8-11-32/h1-3,15,17-18,32H,4-14,16H2,(H,27,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50380717

(CHEMBL2017678)Show SMILES COc1cccc2c(cn(CC3CCOCC3)c12)-c1nsc(CN2CCN(CC(N)=O)CC2)n1 Show InChI InChI=1S/C24H32N6O3S/c1-32-20-4-2-3-18-19(14-30(23(18)20)13-17-5-11-33-12-6-17)24-26-22(34-27-24)16-29-9-7-28(8-10-29)15-21(25)31/h2-4,14,17H,5-13,15-16H2,1H3,(H2,25,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50380717

(CHEMBL2017678)Show SMILES COc1cccc2c(cn(CC3CCOCC3)c12)-c1nsc(CN2CCN(CC(N)=O)CC2)n1 Show InChI InChI=1S/C24H32N6O3S/c1-32-20-4-2-3-18-19(14-30(23(18)20)13-17-5-11-33-12-6-17)24-26-22(34-27-24)16-29-9-7-28(8-10-29)15-21(25)31/h2-4,14,17H,5-13,15-16H2,1H3,(H2,25,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50380719

(CHEMBL1682275)Show SMILES OCCNC(=O)C1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C25H32ClN5O4/c26-21-3-1-2-19-20(15-31(23(19)21)14-17-6-12-34-13-7-17)24-28-22(35-29-24)16-30-9-4-18(5-10-30)25(33)27-8-11-32/h1-3,15,17-18,32H,4-14,16H2,(H,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50380720
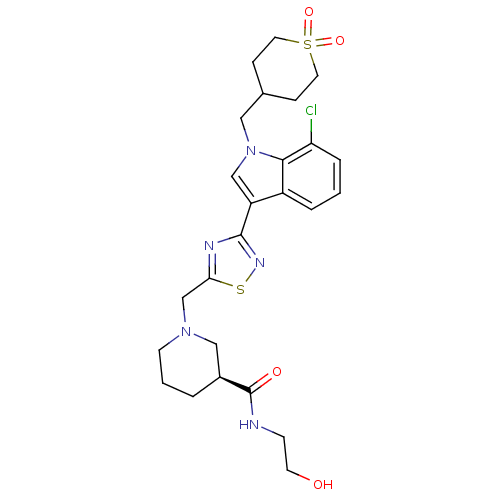
(CHEMBL2017682)Show SMILES OCCNC(=O)[C@H]1CCCN(Cc2nc(ns2)-c2cn(CC3CCS(=O)(=O)CC3)c3c(Cl)cccc23)C1 |r| Show InChI InChI=1S/C25H32ClN5O4S2/c26-21-5-1-4-19-20(15-31(23(19)21)13-17-6-11-37(34,35)12-7-17)24-28-22(36-29-24)16-30-9-2-3-18(14-30)25(33)27-8-10-32/h1,4-5,15,17-18,32H,2-3,6-14,16H2,(H,27,33)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50380718
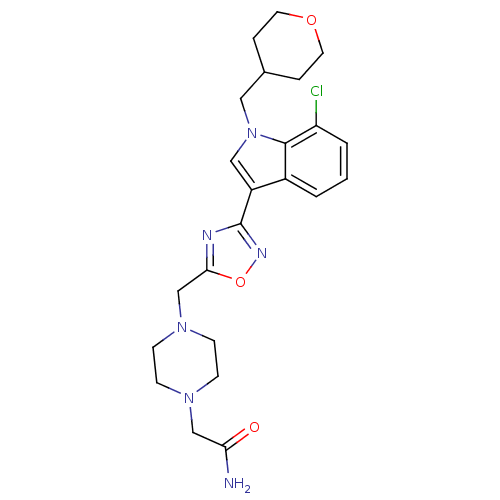
(CHEMBL2017684)Show SMILES NC(=O)CN1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C23H29ClN6O3/c24-19-3-1-2-17-18(13-30(22(17)19)12-16-4-10-32-11-5-16)23-26-21(33-27-23)15-29-8-6-28(7-9-29)14-20(25)31/h1-3,13,16H,4-12,14-15H2,(H2,25,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50380720

(CHEMBL2017682)Show SMILES OCCNC(=O)[C@H]1CCCN(Cc2nc(ns2)-c2cn(CC3CCS(=O)(=O)CC3)c3c(Cl)cccc23)C1 |r| Show InChI InChI=1S/C25H32ClN5O4S2/c26-21-5-1-4-19-20(15-31(23(19)21)13-17-6-11-37(34,35)12-7-17)24-28-22(36-29-24)16-30-9-2-3-18(14-30)25(33)27-8-10-32/h1,4-5,15,17-18,32H,2-3,6-14,16H2,(H,27,33)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50380718

(CHEMBL2017684)Show SMILES NC(=O)CN1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C23H29ClN6O3/c24-19-3-1-2-17-18(13-30(22(17)19)12-16-4-10-32-11-5-16)23-26-21(33-27-23)15-29-8-6-28(7-9-29)14-20(25)31/h1-3,13,16H,4-12,14-15H2,(H2,25,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50380718

(CHEMBL2017684)Show SMILES NC(=O)CN1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C23H29ClN6O3/c24-19-3-1-2-17-18(13-30(22(17)19)12-16-4-10-32-11-5-16)23-26-21(33-27-23)15-29-8-6-28(7-9-29)14-20(25)31/h1-3,13,16H,4-12,14-15H2,(H2,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG channel in HEK293 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464067

(CHEMBL4251359)Show SMILES COC(=O)c1ccc(N2CCCCCC2)c(NS(=O)(=O)c2ccc(CCCCC3CCN(C)CC3)cc2)c1 Show InChI InChI=1S/C30H43N3O4S/c1-32-21-17-25(18-22-32)10-6-5-9-24-11-14-27(15-12-24)38(35,36)31-28-23-26(30(34)37-2)13-16-29(28)33-19-7-3-4-8-20-33/h11-16,23,25,31H,3-10,17-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50423653
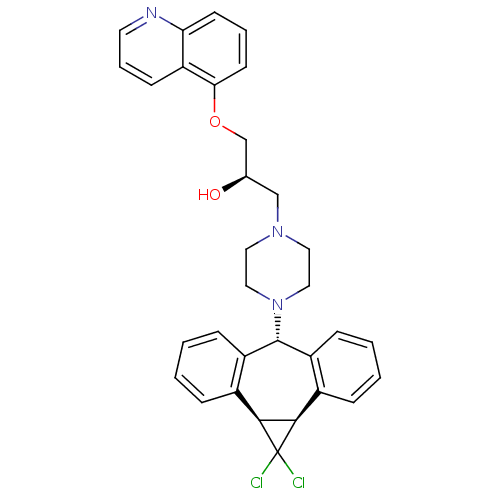
(CHEMBL2087786)Show SMILES O[C@@H](COc1cccc2ncccc12)CN1CCN(CC1)[C@@H]1c2ccccc2[C@@H]2[C@H](c3ccccc13)C2(Cl)Cl |r| Show InChI InChI=1S/C32H31Cl2N3O2/c33-32(34)29-22-7-1-3-9-24(22)31(25-10-4-2-8-23(25)30(29)32)37-17-15-36(16-18-37)19-21(38)20-39-28-13-5-12-27-26(28)11-6-14-35-27/h1-14,21,29-31,38H,15-20H2/t21-,29-,30+,31-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York
Curated by ChEMBL
| Assay Description
Inhibition of P-gp (unknown origin) |
J Med Chem 57: 8622-34 (2014)
Article DOI: 10.1021/jm501259v
BindingDB Entry DOI: 10.7270/Q2154M12 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464069
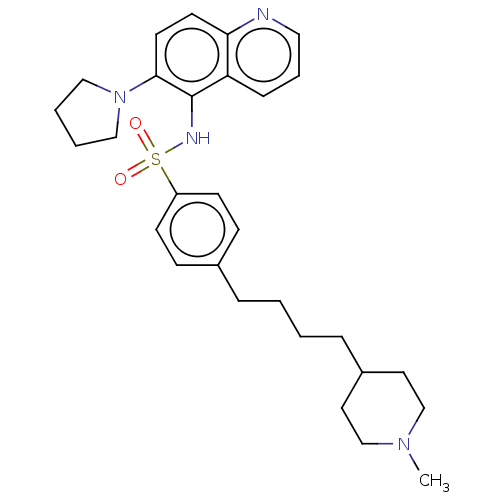
(CHEMBL4237767)Show SMILES CN1CCC(CCCCc2ccc(cc2)S(=O)(=O)Nc2c(ccc3ncccc23)N2CCCC2)CC1 Show InChI InChI=1S/C29H38N4O2S/c1-32-21-16-24(17-22-32)8-3-2-7-23-10-12-25(13-11-23)36(34,35)31-29-26-9-6-18-30-27(26)14-15-28(29)33-19-4-5-20-33/h6,9-15,18,24,31H,2-5,7-8,16-17,19-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464090

(CHEMBL4242822)Show SMILES [H][C@]12CCCN1CCN(CCCc1ccc(cc1)S(=O)(=O)Nc1c(CC(C)C)ccc3ncccc13)C2 |r| Show InChI InChI=1S/C29H38N4O2S/c1-22(2)20-24-11-14-28-27(8-3-15-30-28)29(24)31-36(34,35)26-12-9-23(10-13-26)6-4-16-32-18-19-33-17-5-7-25(33)21-32/h3,8-15,22,25,31H,4-7,16-21H2,1-2H3/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464077
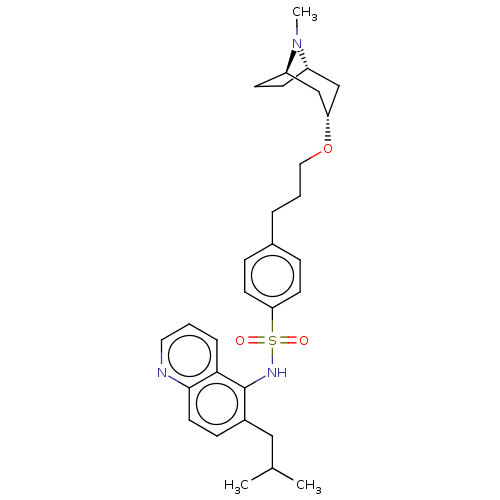
(CHEMBL4237244)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)OCCCc1ccc(cc1)S(=O)(=O)Nc1c(CC(C)C)ccc3ncccc13)N2C |r,THB:38:37:7.6.8:3.2| Show InChI InChI=1S/C30H39N3O3S/c1-21(2)18-23-10-15-29-28(7-4-16-31-29)30(23)32-37(34,35)27-13-8-22(9-14-27)6-5-17-36-26-19-24-11-12-25(20-26)33(24)3/h4,7-10,13-16,21,24-26,32H,5-6,11-12,17-20H2,1-3H3/t24-,25+,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464053
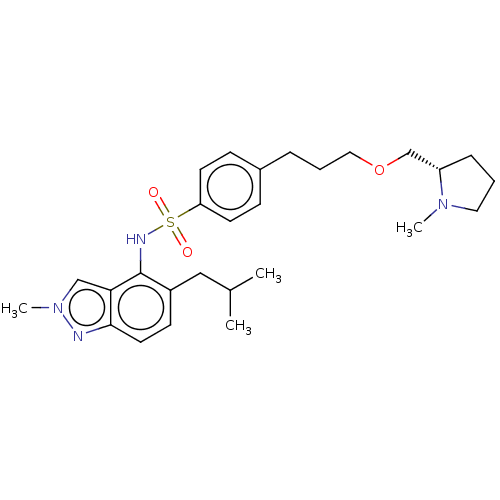
(CHEMBL4249236)Show SMILES CC(C)Cc1ccc2nn(C)cc2c1NS(=O)(=O)c1ccc(CCCOC[C@@H]2CCCN2C)cc1 |r| Show InChI InChI=1S/C27H38N4O3S/c1-20(2)17-22-11-14-26-25(18-31(4)28-26)27(22)29-35(32,33)24-12-9-21(10-13-24)7-6-16-34-19-23-8-5-15-30(23)3/h9-14,18,20,23,29H,5-8,15-17,19H2,1-4H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464095
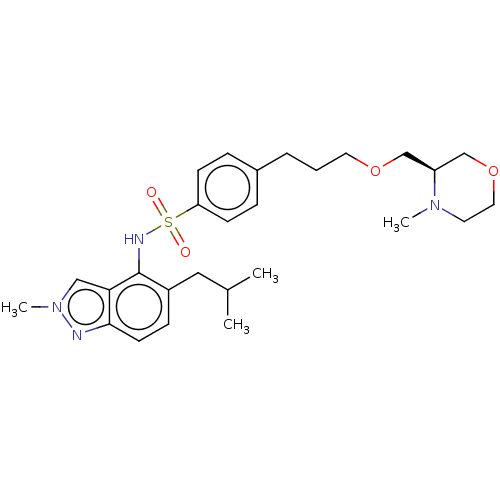
(CHEMBL4247729)Show SMILES CC(C)Cc1ccc2nn(C)cc2c1NS(=O)(=O)c1ccc(CCCOC[C@H]2COCCN2C)cc1 |r| Show InChI InChI=1S/C27H38N4O4S/c1-20(2)16-22-9-12-26-25(17-31(4)28-26)27(22)29-36(32,33)24-10-7-21(8-11-24)6-5-14-34-18-23-19-35-15-13-30(23)3/h7-12,17,20,23,29H,5-6,13-16,18-19H2,1-4H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464049
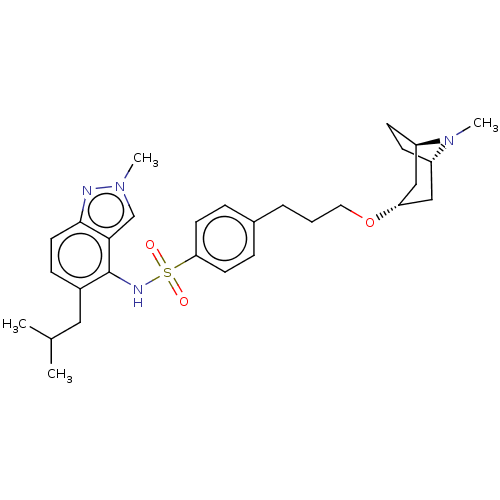
(CHEMBL4243933)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)OCCCc1ccc(cc1)S(=O)(=O)Nc1c(CC(C)C)ccc3nn(C)cc13)N2C |r,THB:38:37:7.6.8:3.2| Show InChI InChI=1S/C29H40N4O3S/c1-20(2)16-22-9-14-28-27(19-32(3)30-28)29(22)31-37(34,35)26-12-7-21(8-13-26)6-5-15-36-25-17-23-10-11-24(18-25)33(23)4/h7-9,12-14,19-20,23-25,31H,5-6,10-11,15-18H2,1-4H3/t23-,24+,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464082

(CHEMBL4240867)Show SMILES CC(C)Cc1ccc2nn(C)cc2c1NS(=O)(=O)c1ccc(CCCCC2CCN(C)CC2)cc1 Show InChI InChI=1S/C28H40N4O2S/c1-21(2)19-24-11-14-27-26(20-32(4)29-27)28(24)30-35(33,34)25-12-9-22(10-13-25)7-5-6-8-23-15-17-31(3)18-16-23/h9-14,20-21,23,30H,5-8,15-19H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50519637

(CHEMBL4550375)Show SMILES CCS(=O)(=O)c1ccc2oc(nc2c1)-c1ccc2[C@H](O)[C@@H](O)C=Cc2c1 |r,c:24| Show InChI InChI=1S/C19H17NO5S/c1-2-26(23,24)13-5-8-17-15(10-13)20-19(25-17)12-3-6-14-11(9-12)4-7-16(21)18(14)22/h3-10,16,18,21-22H,2H2,1H3/t16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of monoamino oxidase B (unknown origin) |
J Med Chem 63: 2547-2556 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01547
BindingDB Entry DOI: 10.7270/Q24B34P2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50519636

(BMN 195 | BMN-195 | Ezutromid | SMT-C1100 | SMTC-1...)Show InChI InChI=1S/C19H15NO3S/c1-2-24(21,22)16-9-10-18-17(12-16)20-19(23-18)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes |
J Med Chem 63: 2547-2556 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01547
BindingDB Entry DOI: 10.7270/Q24B34P2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464085
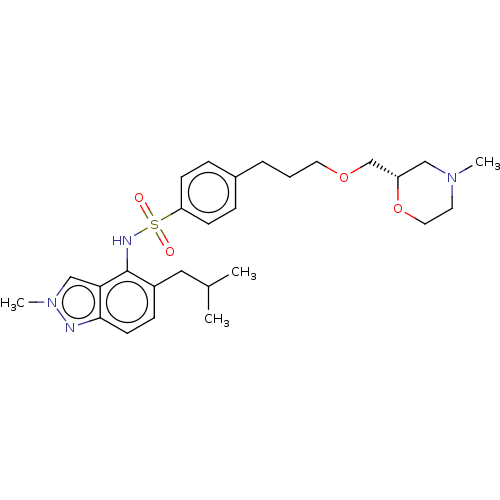
(CHEMBL4243499)Show SMILES CC(C)Cc1ccc2nn(C)cc2c1NS(=O)(=O)c1ccc(CCCOC[C@@H]2CN(C)CCO2)cc1 |r| Show InChI InChI=1S/C27H38N4O4S/c1-20(2)16-22-9-12-26-25(18-31(4)28-26)27(22)29-36(32,33)24-10-7-21(8-11-24)6-5-14-34-19-23-17-30(3)13-15-35-23/h7-12,18,20,23,29H,5-6,13-17,19H2,1-4H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464054

(CHEMBL4246475)Show SMILES CC(C)Cc1ccc2nn(C)cc2c1N(C(F)F)S(=O)(=O)c1ccc(CCCCC2CCN(C)CC2)cc1 Show InChI InChI=1S/C29H40F2N4O2S/c1-21(2)19-24-11-14-27-26(20-34(4)32-27)28(24)35(29(30)31)38(36,37)25-12-9-22(10-13-25)7-5-6-8-23-15-17-33(3)18-16-23/h9-14,20-21,23,29H,5-8,15-19H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464048
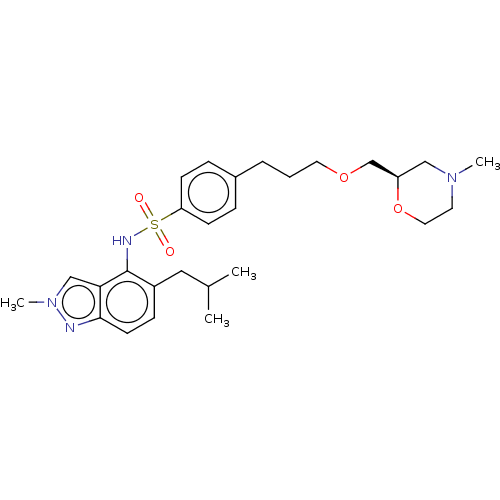
(CHEMBL4250740)Show SMILES CC(C)Cc1ccc2nn(C)cc2c1NS(=O)(=O)c1ccc(CCCOC[C@H]2CN(C)CCO2)cc1 |r| Show InChI InChI=1S/C27H38N4O4S/c1-20(2)16-22-9-12-26-25(18-31(4)28-26)27(22)29-36(32,33)24-10-7-21(8-11-24)6-5-14-34-19-23-17-30(3)13-15-35-23/h7-12,18,20,23,29H,5-6,13-17,19H2,1-4H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Multidrug and toxin extrusion protein 1
(Homo sapiens (Human)) | BDBM50519637

(CHEMBL4550375)Show SMILES CCS(=O)(=O)c1ccc2oc(nc2c1)-c1ccc2[C@H](O)[C@@H](O)C=Cc2c1 |r,c:24| Show InChI InChI=1S/C19H17NO5S/c1-2-26(23,24)13-5-8-17-15(10-13)20-19(25-17)12-3-6-14-11(9-12)4-7-16(21)18(14)22/h3-10,16,18,21-22H,2H2,1H3/t16-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of MATE1 (unknown origin) |
J Med Chem 63: 2547-2556 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01547
BindingDB Entry DOI: 10.7270/Q24B34P2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 63: 2547-2556 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01547
BindingDB Entry DOI: 10.7270/Q24B34P2 |
More data for this
Ligand-Target Pair | |
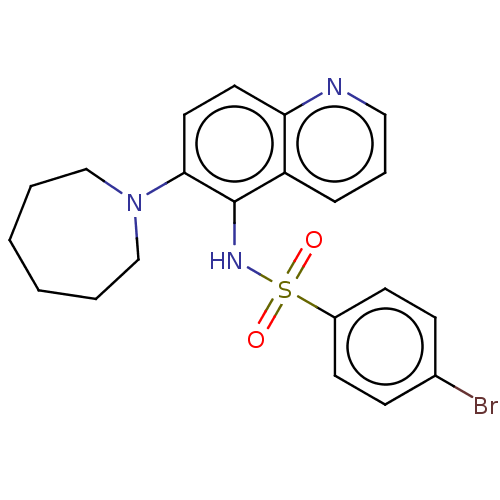
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464096
(CHEMBL4241923)Show SMILES Brc1ccc(cc1)S(=O)(=O)Nc1c(ccc2ncccc12)N1CCCCCC1 Show InChI InChI=1S/C21H22BrN3O2S/c22-16-7-9-17(10-8-16)28(26,27)24-21-18-6-5-13-23-19(18)11-12-20(21)25-14-3-1-2-4-15-25/h5-13,24H,1-4,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464062
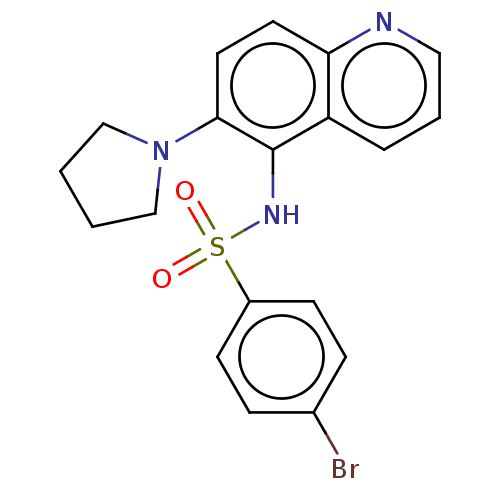
(CHEMBL4248236)Show SMILES Brc1ccc(cc1)S(=O)(=O)Nc1c(ccc2ncccc12)N1CCCC1 Show InChI InChI=1S/C19H18BrN3O2S/c20-14-5-7-15(8-6-14)26(24,25)22-19-16-4-3-11-21-17(16)9-10-18(19)23-12-1-2-13-23/h3-11,22H,1-2,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
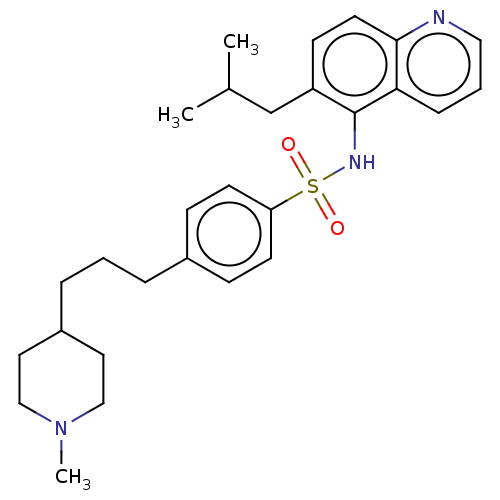
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464073
(CHEMBL4250899)Show SMILES CC(C)Cc1ccc2ncccc2c1NS(=O)(=O)c1ccc(CCCC2CCN(C)CC2)cc1 Show InChI InChI=1S/C28H37N3O2S/c1-21(2)20-24-11-14-27-26(8-5-17-29-27)28(24)30-34(32,33)25-12-9-22(10-13-25)6-4-7-23-15-18-31(3)19-16-23/h5,8-14,17,21,23,30H,4,6-7,15-16,18-20H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
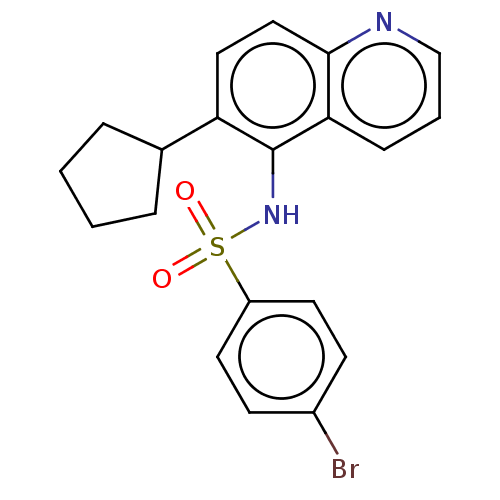
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464094
(CHEMBL4245730)Show SMILES Brc1ccc(cc1)S(=O)(=O)Nc1c(ccc2ncccc12)C1CCCC1 Show InChI InChI=1S/C20H19BrN2O2S/c21-15-7-9-16(10-8-15)26(24,25)23-20-17(14-4-1-2-5-14)11-12-19-18(20)6-3-13-22-19/h3,6-14,23H,1-2,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
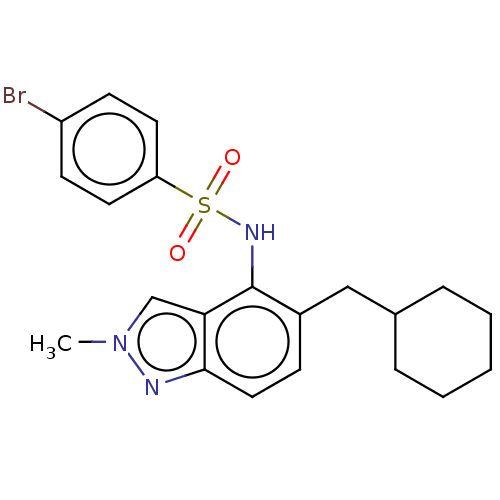
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464081
(CHEMBL4241516)Show SMILES Cn1cc2c(NS(=O)(=O)c3ccc(Br)cc3)c(CC3CCCCC3)ccc2n1 Show InChI InChI=1S/C21H24BrN3O2S/c1-25-14-19-20(23-25)12-7-16(13-15-5-3-2-4-6-15)21(19)24-28(26,27)18-10-8-17(22)9-11-18/h7-12,14-15,24H,2-6,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464080

(CHEMBL4249039)Show SMILES Cn1cc2c(NS(=O)(=O)c3ccc(Br)cc3)c(ccc2n1)C1CCCC1 Show InChI InChI=1S/C19H20BrN3O2S/c1-23-12-17-18(21-23)11-10-16(13-4-2-3-5-13)19(17)22-26(24,25)15-8-6-14(20)7-9-15/h6-13,22H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464086

(CHEMBL4249054)Show SMILES COC(=O)c1ccc(N2CCCCCC2)c(NS(=O)(=O)c2ccc(OC)cc2)c1 Show InChI InChI=1S/C21H26N2O5S/c1-27-17-8-10-18(11-9-17)29(25,26)22-19-15-16(21(24)28-2)7-12-20(19)23-13-5-3-4-6-14-23/h7-12,15,22H,3-6,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464087

(CHEMBL4240321)Show SMILES Brc1ccc(cc1)S(=O)(=O)Nc1c(Cc2ccccc2)ccc2ncccc12 Show InChI InChI=1S/C22H17BrN2O2S/c23-18-9-11-19(12-10-18)28(26,27)25-22-17(15-16-5-2-1-3-6-16)8-13-21-20(22)7-4-14-24-21/h1-14,25H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464072
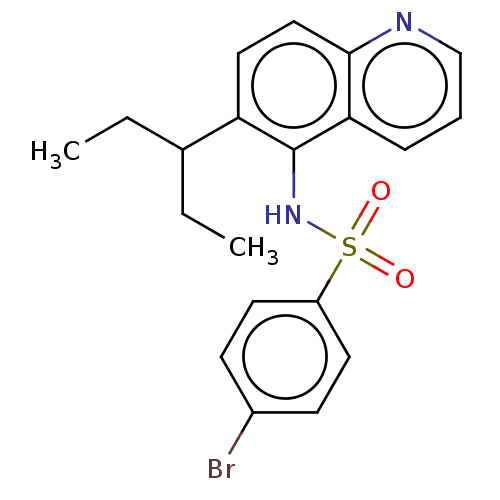
(CHEMBL4250444)Show SMILES CCC(CC)c1ccc2ncccc2c1NS(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C20H21BrN2O2S/c1-3-14(4-2)17-11-12-19-18(6-5-13-22-19)20(17)23-26(24,25)16-9-7-15(21)8-10-16/h5-14,23H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464071
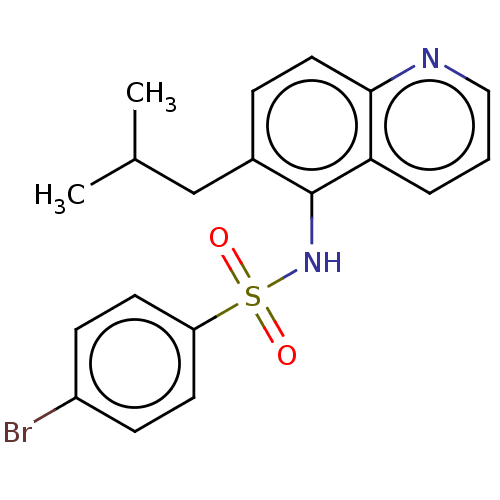
(CHEMBL4250235)Show InChI InChI=1S/C19H19BrN2O2S/c1-13(2)12-14-5-10-18-17(4-3-11-21-18)19(14)22-25(23,24)16-8-6-15(20)7-9-16/h3-11,13,22H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464070
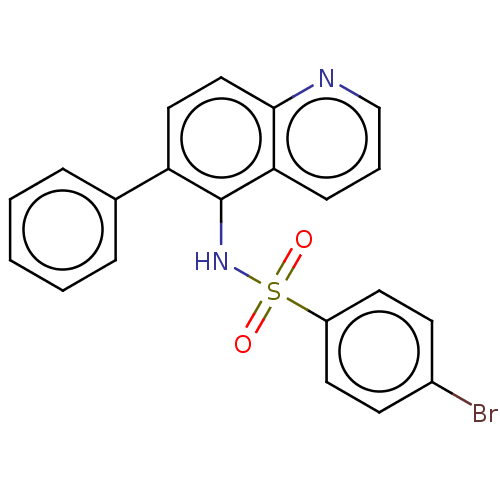
(CHEMBL4247869)Show SMILES Brc1ccc(cc1)S(=O)(=O)Nc1c(ccc2ncccc12)-c1ccccc1 Show InChI InChI=1S/C21H15BrN2O2S/c22-16-8-10-17(11-9-16)27(25,26)24-21-18(15-5-2-1-3-6-15)12-13-20-19(21)7-4-14-23-20/h1-14,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464068
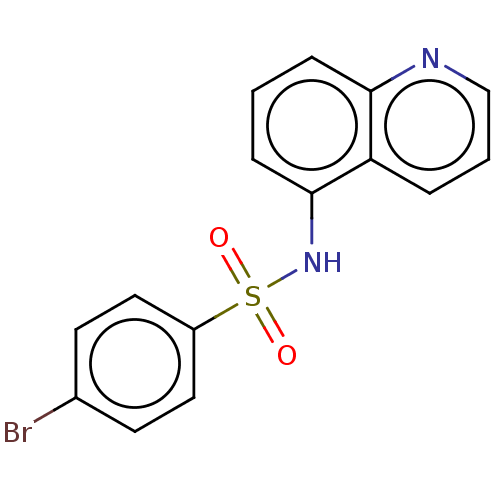
(CHEMBL4240800)Show InChI InChI=1S/C15H11BrN2O2S/c16-11-6-8-12(9-7-11)21(19,20)18-15-5-1-4-14-13(15)3-2-10-17-14/h1-10,18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464066

(CHEMBL4250077)Show SMILES COC(=O)c1ccc(N2CCCC2)c(NS(=O)(=O)c2ccc(OC)cc2)c1 Show InChI InChI=1S/C19H22N2O5S/c1-25-15-6-8-16(9-7-15)27(23,24)20-17-13-14(19(22)26-2)5-10-18(17)21-11-3-4-12-21/h5-10,13,20H,3-4,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464060

(CHEMBL4249159)Show SMILES Cn1cc2c(NS(=O)(=O)c3ccc(Br)cc3)c(ccc2n1)C1CCCCC1 Show InChI InChI=1S/C20H22BrN3O2S/c1-24-13-18-19(22-24)12-11-17(14-5-3-2-4-6-14)20(18)23-27(25,26)16-9-7-15(21)8-10-16/h7-14,23H,2-6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464047

(CHEMBL4244163)Show SMILES CC(C)Cc1ccc2nn(C)cc2c1NS(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C18H20BrN3O2S/c1-12(2)10-13-4-9-17-16(11-22(3)20-17)18(13)21-25(23,24)15-7-5-14(19)6-8-15/h4-9,11-12,21H,10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50464057

(CHEMBL4239867)Show SMILES Brc1ccc(cc1)S(=O)(=O)Nc1c(CC2CCCCC2)ccc2ncccc12 Show InChI InChI=1S/C22H23BrN2O2S/c23-18-9-11-19(12-10-18)28(26,27)25-22-17(15-16-5-2-1-3-6-16)8-13-21-20(22)7-4-14-24-21/h4,7-14,16,25H,1-3,5-6,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assay |
J Med Chem 61: 8374-8389 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00884
BindingDB Entry DOI: 10.7270/Q28K7CR1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data