

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50397290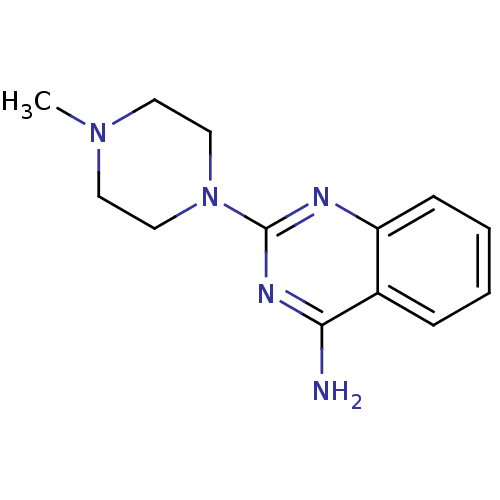 (CHEMBL475331 | VUF-10147) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine | J Med Chem 55: 8603-14 (2012) Article DOI: 10.1021/jm300801u BindingDB Entry DOI: 10.7270/Q2RF5W5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546794 (CHEMBL4777335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01803 BindingDB Entry DOI: 10.7270/Q2KD22XQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50079527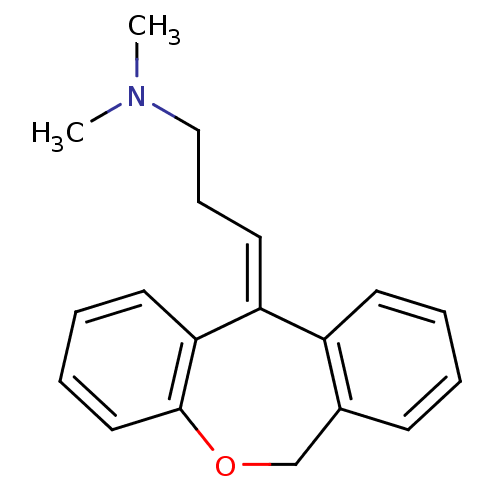 ((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8195-206 (2011) Article DOI: 10.1021/jm2011589 BindingDB Entry DOI: 10.7270/Q2QF8T85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50099623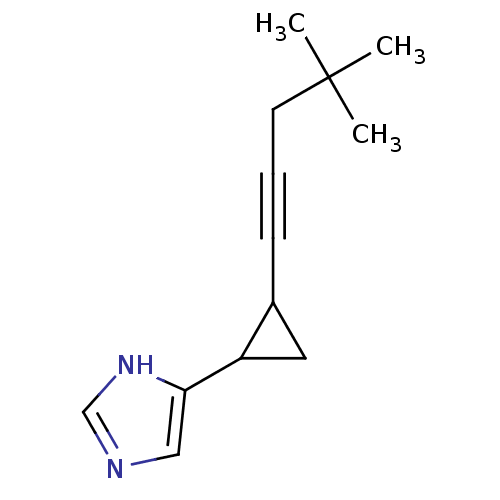 (4-[2-(4,4-Dimethyl-pent-1-ynyl)-cyclopropyl]-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex | J Med Chem 44: 1666-74 (2001) BindingDB Entry DOI: 10.7270/Q2WH2QP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50079527 ((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human wild type N-terminal hemagglutinin-tagged histamine H1 receptor expressed in HEK293T cells after 4 hrs by m... | J Med Chem 59: 9047-9061 (2016) Article DOI: 10.1021/acs.jmedchem.6b00981 BindingDB Entry DOI: 10.7270/Q2G44S8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.339 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam | Assay Description Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... | J Med Chem 51: 2944-53 (2008) Article DOI: 10.1021/jm7014149 BindingDB Entry DOI: 10.7270/Q24F1P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50031228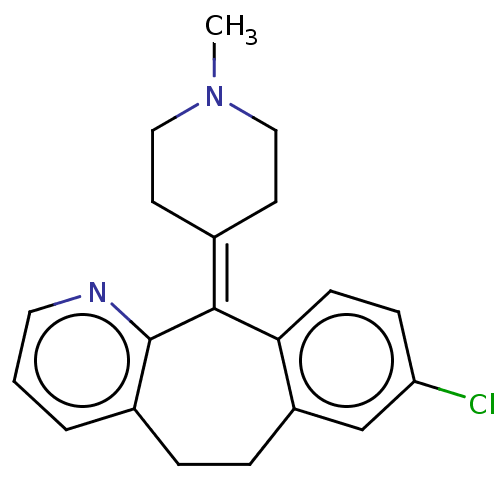 (CHEMBL420316) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774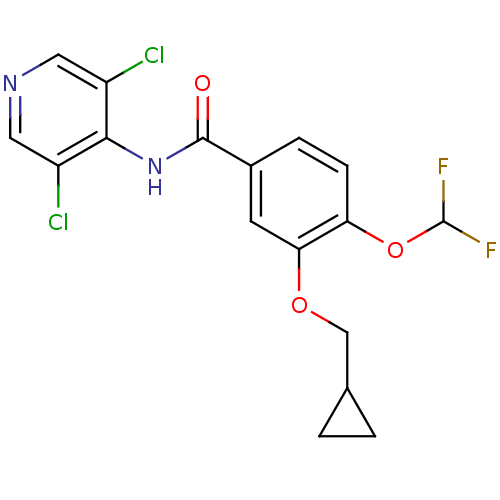 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50099622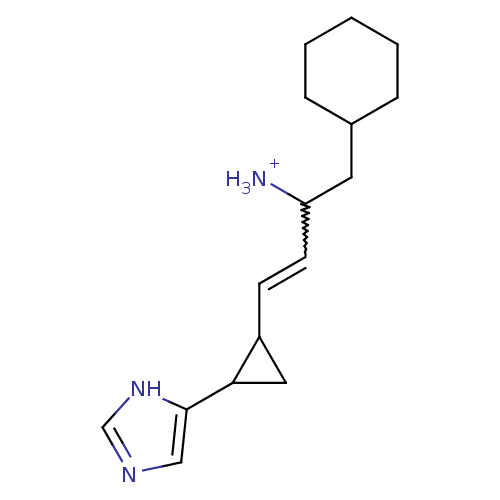 (1-Cyclohexylmethyl-3-[2-(1H-imidazol-4-yl)-cyclopr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex | J Med Chem 44: 1666-74 (2001) BindingDB Entry DOI: 10.7270/Q2WH2QP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542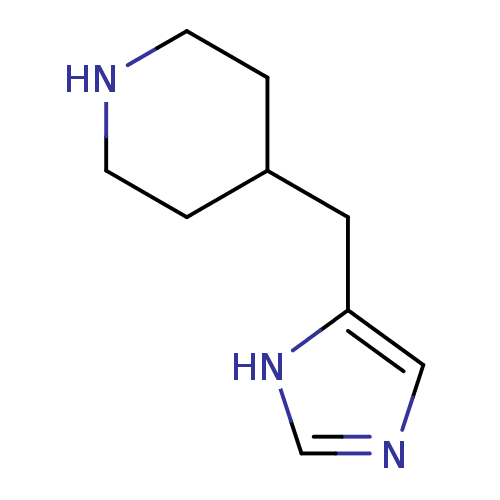 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.407 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam | Assay Description Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... | J Med Chem 51: 2944-53 (2008) Article DOI: 10.1021/jm7014149 BindingDB Entry DOI: 10.7270/Q24F1P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516710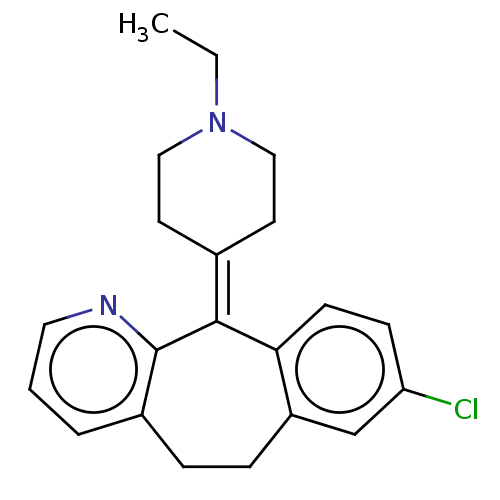 (CHEMBL4469024) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50053631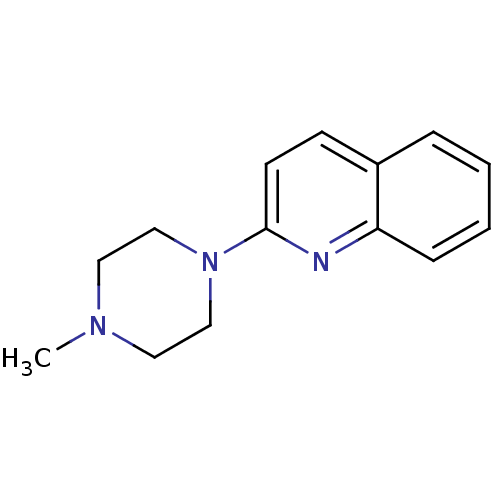 (2-(4-Methyl-piperazin-1-yl)-quinoline | 2-(4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine | J Med Chem 55: 8603-14 (2012) Article DOI: 10.1021/jm300801u BindingDB Entry DOI: 10.7270/Q2RF5W5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50150945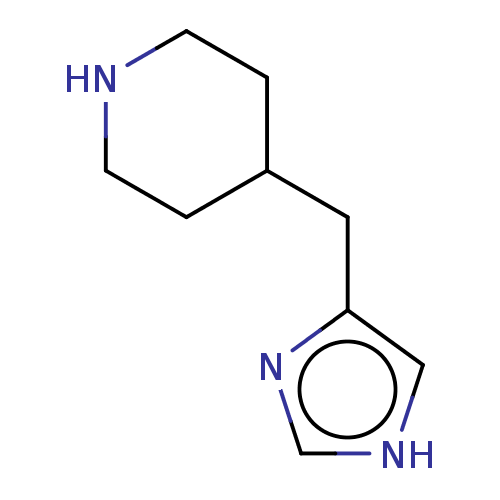 (CHEBI:81390 | Immepip) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50150945 (CHEBI:81390 | Immepip) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-R-methylhistamine binding to SK-N-MC cell membranes expressing human H3 receptor | J Med Chem 48: 2100-7 (2005) Article DOI: 10.1021/jm049475h BindingDB Entry DOI: 10.7270/Q28K7CVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50335557 (CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516713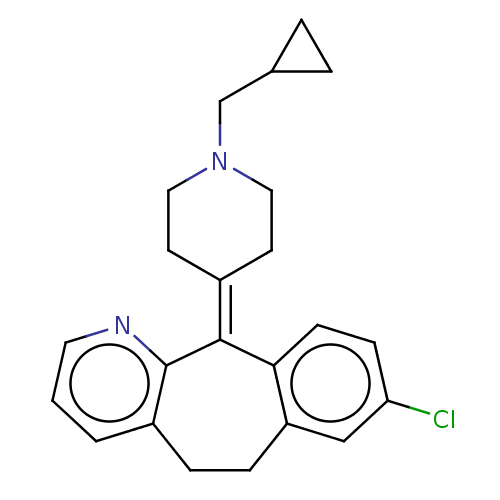 (CHEMBL4448535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50427452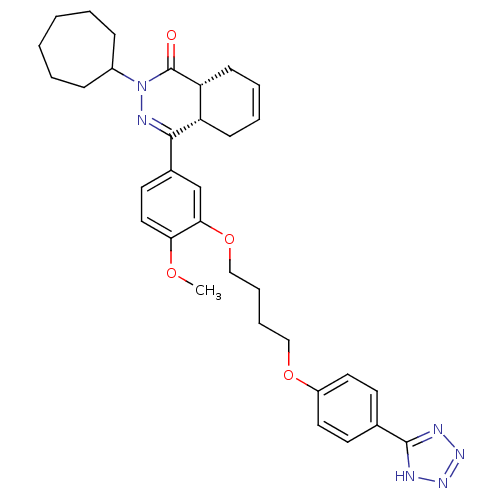 (CHEMBL2326941) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22916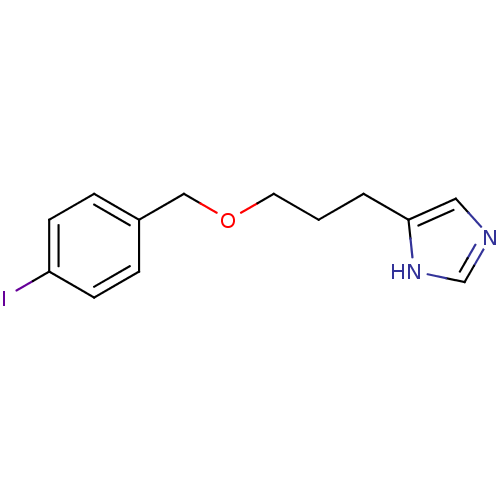 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516707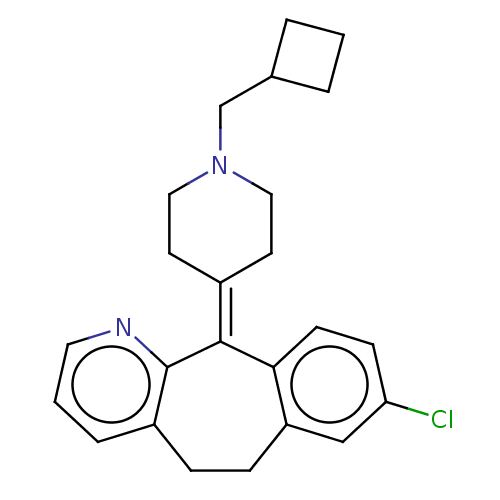 (CHEMBL4588983) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22548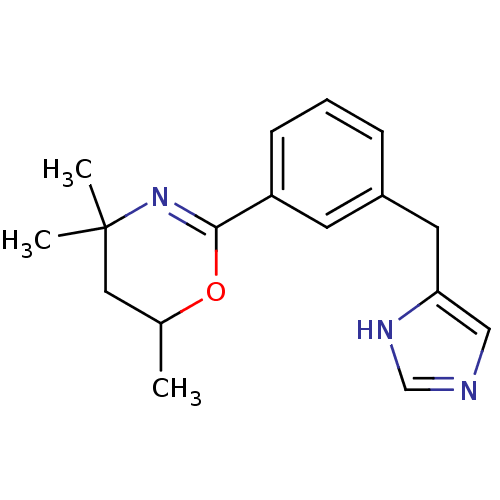 (2-[3-(1H-imidazol-4-ylmethyl)phenyl]-4,4,6-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -51.4 | n/a | n/a | 2 | n/a | n/a | 7.4 | 25 |
VU University Amsterdam | Assay Description Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... | J Med Chem 51: 2944-53 (2008) Article DOI: 10.1021/jm7014149 BindingDB Entry DOI: 10.7270/Q24F1P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22564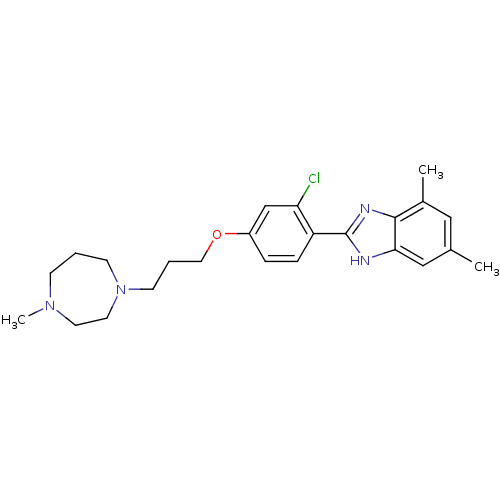 (2-arylbenzimidazole derivative, 10 | 2-{2-chloro-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in HEK cells | J Med Chem 51: 7855-65 (2008) Article DOI: 10.1021/jm800876b BindingDB Entry DOI: 10.7270/Q29Z964R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516717 (CHEMBL4582006) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50514105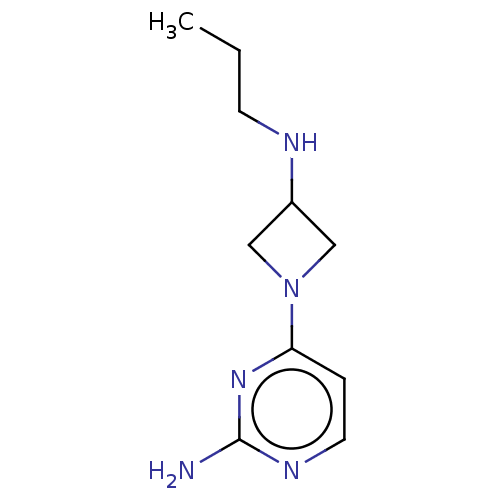 (CHEMBL4441731) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from mouse H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis | J Med Chem 62: 10848-10866 (2019) Article DOI: 10.1021/acs.jmedchem.9b01462 BindingDB Entry DOI: 10.7270/Q2M61PKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50475340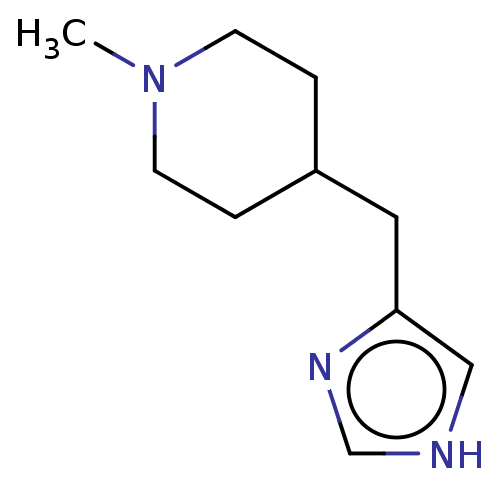 (Methimepip) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-R-methylhistamine binding to SK-N-MC cell membranes expressing human H3 receptor | J Med Chem 48: 2100-7 (2005) Article DOI: 10.1021/jm049475h BindingDB Entry DOI: 10.7270/Q28K7CVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419448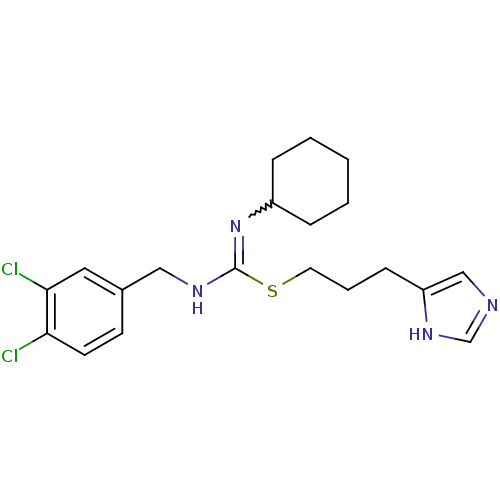 (CHEMBL1923026) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419444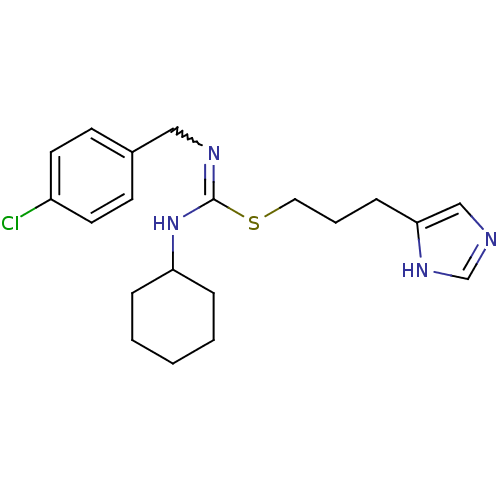 (CHEMBL43934 | VUF-5228) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50397307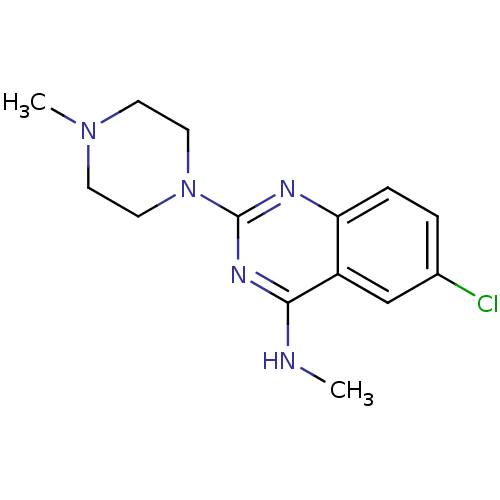 (CHEMBL489062 | VUF-10349) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine | J Med Chem 55: 8603-14 (2012) Article DOI: 10.1021/jm300801u BindingDB Entry DOI: 10.7270/Q2RF5W5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419456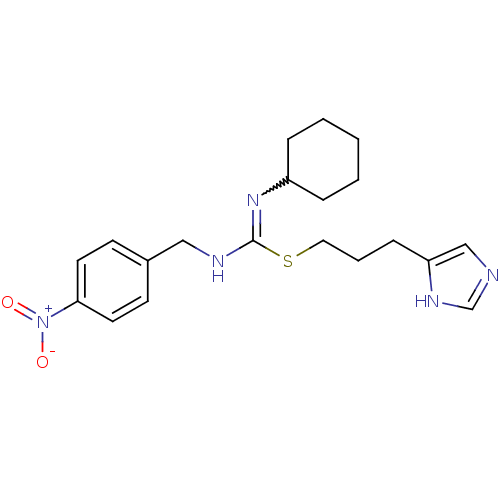 (CHEMBL1923034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567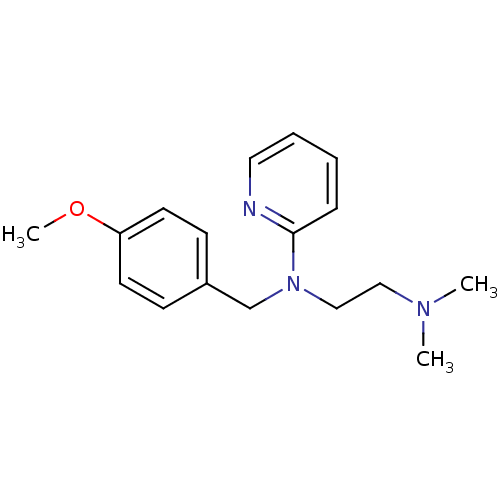 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human wild type N-terminal hemagglutinin-tagged histamine H1 receptor expressed in HEK293T cells after 4 hrs by m... | J Med Chem 59: 9047-9061 (2016) Article DOI: 10.1021/acs.jmedchem.6b00981 BindingDB Entry DOI: 10.7270/Q2G44S8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516716 (CHEMBL4474226) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50397291 (CHEMBL443764 | VUF-10249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine | J Med Chem 55: 8603-14 (2012) Article DOI: 10.1021/jm300801u BindingDB Entry DOI: 10.7270/Q2RF5W5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50474424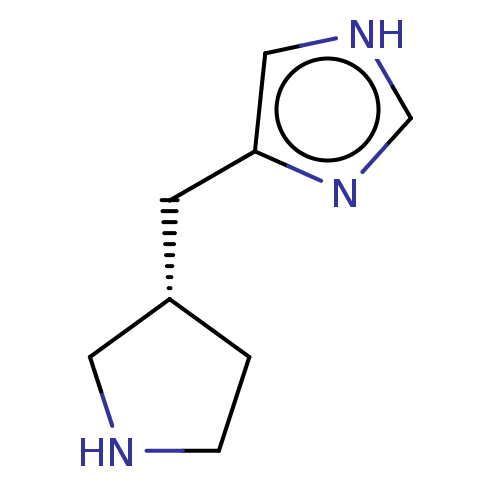 (CHEMBL153051) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50397284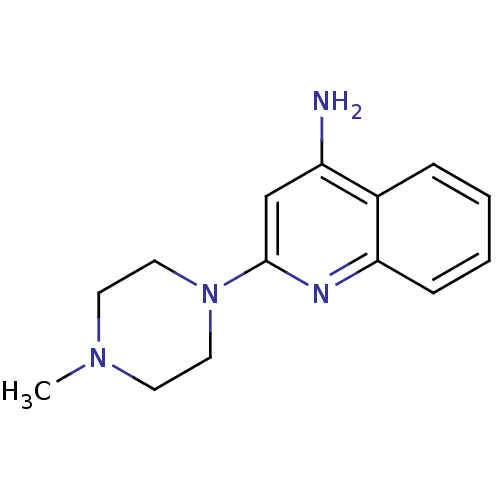 (CHEMBL2169970) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine | J Med Chem 55: 8603-14 (2012) Article DOI: 10.1021/jm300801u BindingDB Entry DOI: 10.7270/Q2RF5W5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM50361015 (CHEMBL592379) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Binding affinity to rat histamine H4 receptor | J Med Chem 53: 2390-400 (2010) Article DOI: 10.1021/jm901379s BindingDB Entry DOI: 10.7270/Q2G44RJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911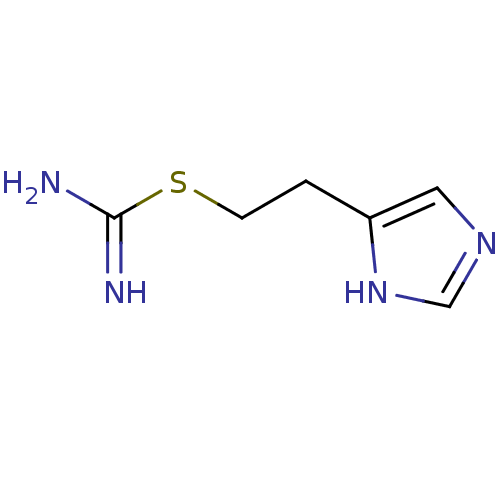 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50419048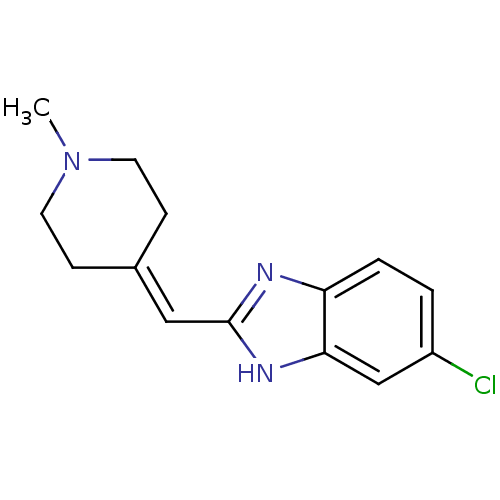 (CHEMBL1824048) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5-HT3AR expressed in HEK293 cells after 24 hrs by liquid scintillation counting | Bioorg Med Chem Lett 21: 5460-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.123 BindingDB Entry DOI: 10.7270/Q2ZC844K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50414391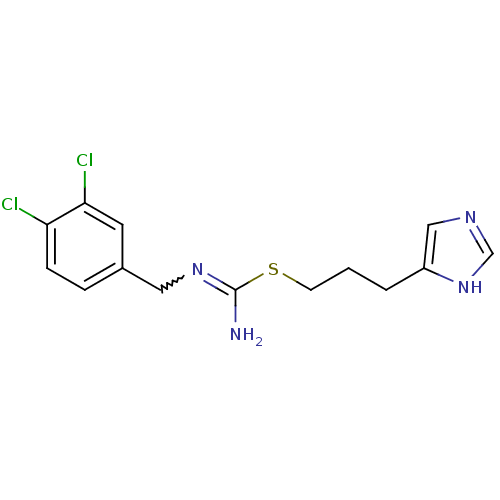 (CHEMBL1202332 | CHEMBL553423) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Agonistic activity at histamine H4 receptor | J Med Chem 54: 1693-703 (2011) Article DOI: 10.1021/jm1013488 BindingDB Entry DOI: 10.7270/Q20G3MDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50414391 (CHEMBL1202332 | CHEMBL553423) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50073179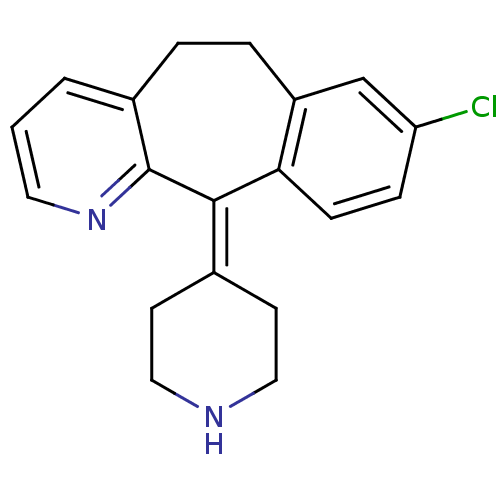 (8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50397288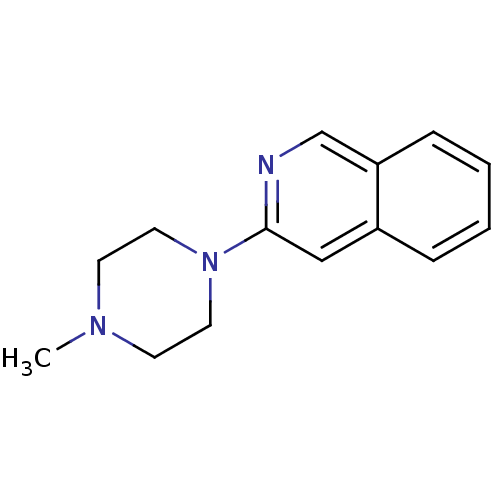 (CHEMBL2169966) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine | J Med Chem 55: 8603-14 (2012) Article DOI: 10.1021/jm300801u BindingDB Entry DOI: 10.7270/Q2RF5W5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419455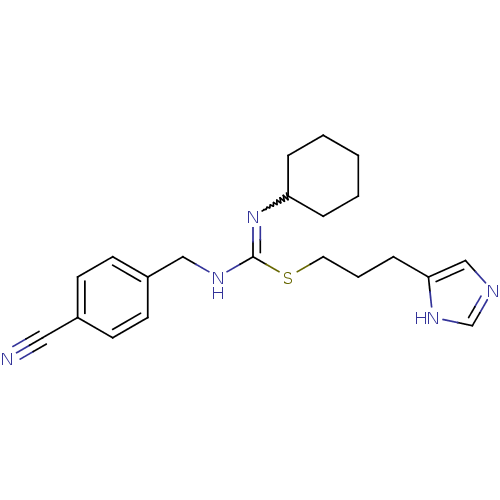 (CHEMBL1923033) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50127605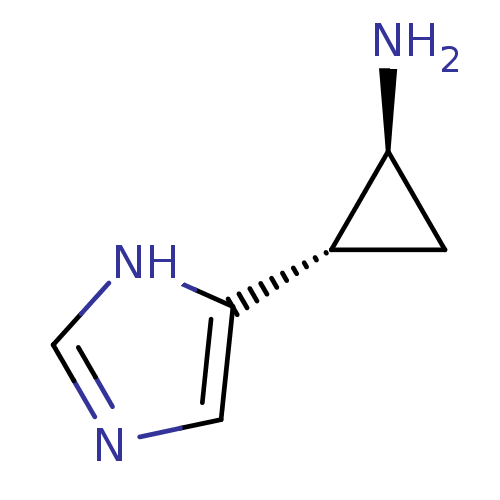 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description In vitro binding affinity was measured against Histamine H3 receptor on rat cerebral cortex. | J Med Chem 42: 1115-22 (1999) Article DOI: 10.1021/jm9810912 BindingDB Entry DOI: 10.7270/Q24Q7XPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516706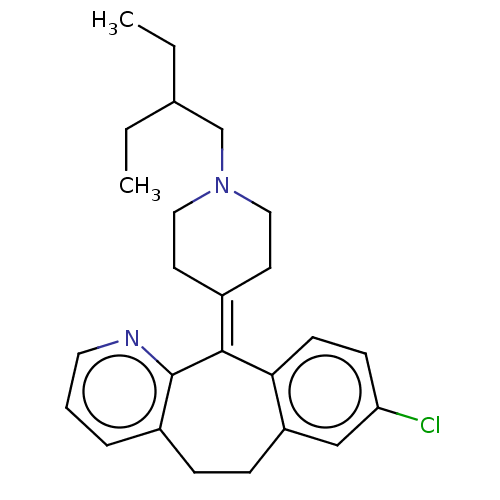 (CHEMBL4514700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22538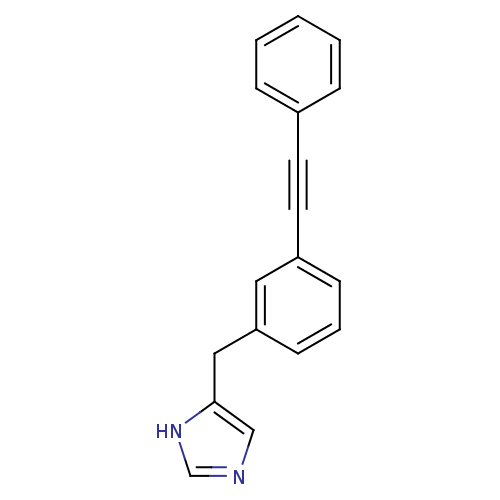 (4-Benzyl-1H-imidazole derivative, 19 | 4-{[3-(2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam | Assay Description Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... | J Med Chem 51: 2944-53 (2008) Article DOI: 10.1021/jm7014149 BindingDB Entry DOI: 10.7270/Q24F1P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50202722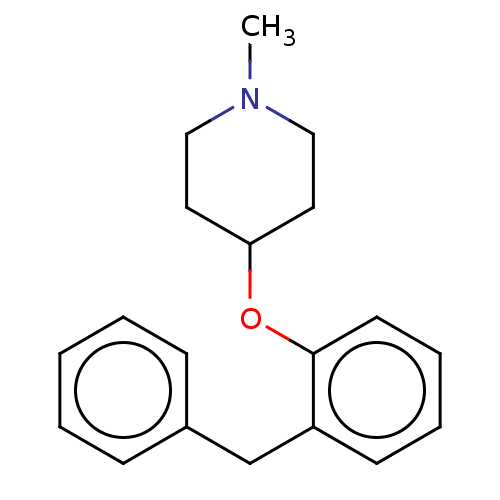 (CHEMBL3906929) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human wild type N-terminal hemagglutinin-tagged histamine H1 receptor expressed in HEK293T cells after 4 hrs by m... | J Med Chem 59: 9047-9061 (2016) Article DOI: 10.1021/acs.jmedchem.6b00981 BindingDB Entry DOI: 10.7270/Q2G44S8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8195-206 (2011) Article DOI: 10.1021/jm2011589 BindingDB Entry DOI: 10.7270/Q2QF8T85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516708 (CHEMBL4581343) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516719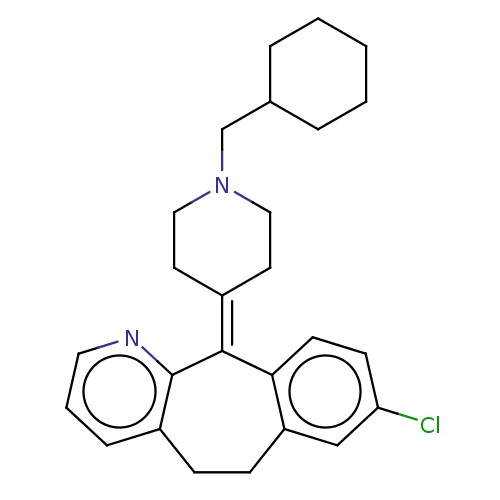 (CHEMBL4554533) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1989 total ) | Next | Last >> |