 Found 591 hits with Last Name = 'de francesco' and Initial = 'r'
Found 591 hits with Last Name = 'de francesco' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110121
(3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)C(O)=O Show InChI InChI=1S/C45H56F2N6O15/c1-24(54)48-32(23-36(59)60)43(65)49-29(18-20-35(57)58)41(63)53-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)44(66)50-28(17-19-34(55)56)40(62)52-31(21-25-11-5-2-6-12-25)42(64)51-30(22-33(46)47)39(61)45(67)68/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,48,54)(H,49,65)(H,50,66)(H,51,64)(H,52,62)(H,53,63)(H,55,56)(H,57,58)(H,59,60)(H,67,68)/t28-,29-,30-,31-,32-,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110117

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C=O Show InChI InChI=1S/C44H56F2N6O13/c1-25(54)47-33(23-37(59)60)43(64)49-31(18-20-36(57)58)41(62)52-39(38(27-13-7-3-8-14-27)28-15-9-4-10-16-28)44(65)50-30(17-19-35(55)56)40(61)51-32(21-26-11-5-2-6-12-26)42(63)48-29(24-53)22-34(45)46/h3-4,7-10,13-16,24,26,29-34,38-39H,2,5-6,11-12,17-23H2,1H3,(H,47,54)(H,48,63)(H,49,64)(H,50,65)(H,51,61)(H,52,62)(H,55,56)(H,57,58)(H,59,60)/t29-,30-,31-,32-,33-,39-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110122
((S)-4-((S)-2-Acetylamino-3-carboxy-propionylamino)...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C52H63F2N7O14/c1-30(62)56-39(28-43(67)68)50(73)57-36(23-25-42(65)66)48(71)61-45(44(33-18-10-4-11-19-33)34-20-12-5-13-21-34)51(74)58-35(22-24-41(63)64)47(70)60-38(26-31-14-6-2-7-15-31)49(72)59-37(27-40(53)54)46(69)52(75)55-29-32-16-8-3-9-17-32/h3-5,8-13,16-21,31,35-40,44-45H,2,6-7,14-15,22-29H2,1H3,(H,55,75)(H,56,62)(H,57,73)(H,58,74)(H,59,72)(H,60,70)(H,61,71)(H,63,64)(H,65,66)(H,67,68)/t35-,36-,37-,38-,39-,45-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110120

(3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES COC(=O)C(=O)[C@H](CC(F)F)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C46H58F2N6O15/c1-25(55)49-33(24-37(60)61)44(66)50-30(19-21-36(58)59)42(64)54-39(38(27-14-8-4-9-15-27)28-16-10-5-11-17-28)45(67)51-29(18-20-35(56)57)41(63)53-32(22-26-12-6-3-7-13-26)43(65)52-31(23-34(47)48)40(62)46(68)69-2/h4-5,8-11,14-17,26,29-34,38-39H,3,6-7,12-13,18-24H2,1-2H3,(H,49,55)(H,50,66)(H,51,67)(H,52,65)(H,53,63)(H,54,64)(H,56,57)(H,58,59)(H,60,61)/t29-,30-,31-,32-,33-,39-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110126

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC=C)C=O Show InChI InChI=1S/C45H58N6O13/c1-3-13-31(26-52)47-43(62)34(24-28-14-7-4-8-15-28)50-41(60)32(20-22-36(54)55)49-45(64)40(39(29-16-9-5-10-17-29)30-18-11-6-12-19-30)51-42(61)33(21-23-37(56)57)48-44(63)35(25-38(58)59)46-27(2)53/h3,5-6,9-12,16-19,26,28,31-35,39-40H,1,4,7-8,13-15,20-25H2,2H3,(H,46,53)(H,47,62)(H,48,63)(H,49,64)(H,50,60)(H,51,61)(H,54,55)(H,56,57)(H,58,59)/t31-,32-,33-,34-,35-,40-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110125

(2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(O)=O Show InChI InChI=1S/C44H56F2N6O14/c1-24(53)47-31(23-36(58)59)42(63)48-29(18-20-35(56)57)40(61)52-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)43(64)49-28(17-19-34(54)55)39(60)50-30(21-25-11-5-2-6-12-25)41(62)51-32(44(65)66)22-33(45)46/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,47,53)(H,48,63)(H,49,64)(H,50,60)(H,51,62)(H,52,61)(H,54,55)(H,56,57)(H,58,59)(H,65,66)/t28-,29-,30-,31-,32-,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50084685

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C43H56N6O14S/c1-24(50)44-31(22-35(55)56)41(60)45-29(18-20-34(53)54)39(58)49-37(36(26-13-7-3-8-14-26)27-15-9-4-10-16-27)42(61)46-28(17-19-33(51)52)38(57)47-30(21-25-11-5-2-6-12-25)40(59)48-32(23-64)43(62)63/h3-4,7-10,13-16,25,28-32,36-37,64H,2,5-6,11-12,17-23H2,1H3,(H,44,50)(H,45,60)(H,46,61)(H,47,57)(H,48,59)(H,49,58)(H,51,52)(H,53,54)(H,55,56)(H,62,63)/t28-,29-,30-,31-,32-,37-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110123

(2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC=C)C(O)=O Show InChI InChI=1S/C45H58N6O14/c1-3-13-32(45(64)65)49-42(61)33(24-27-14-7-4-8-15-27)50-40(59)30(20-22-35(53)54)48-44(63)39(38(28-16-9-5-10-17-28)29-18-11-6-12-19-29)51-41(60)31(21-23-36(55)56)47-43(62)34(25-37(57)58)46-26(2)52/h3,5-6,9-12,16-19,27,30-34,38-39H,1,4,7-8,13-15,20-25H2,2H3,(H,46,52)(H,47,62)(H,48,63)(H,49,61)(H,50,59)(H,51,60)(H,53,54)(H,55,56)(H,57,58)(H,64,65)/t30-,31-,32-,33-,34-,39-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110124

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(1...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C51H59F2N7O13S/c1-28(61)54-37(27-42(66)67)49(72)55-34(22-24-41(64)65)47(70)60-44(43(30-15-7-3-8-16-30)31-17-9-4-10-18-31)50(73)56-33(21-23-40(62)63)46(69)58-36(25-29-13-5-2-6-14-29)48(71)57-35(26-39(52)53)45(68)51-59-32-19-11-12-20-38(32)74-51/h3-4,7-12,15-20,29,33-37,39,43-44H,2,5-6,13-14,21-27H2,1H3,(H,54,61)(H,55,72)(H,56,73)(H,57,71)(H,58,69)(H,60,70)(H,62,63)(H,64,65)(H,66,67)/t33-,34-,35-,36-,37-,44-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122891

(4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...)Show SMILES CC(C)COC(=O)NC(CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C29H41ClF2N4O9/c1-15(2)11-21(34-26(40)20(7-8-24(37)38)36-29(44)45-14-16(3)4)27(41)35-22(13-23(31)32)25(39)33-10-9-17-5-6-18(28(42)43)12-19(17)30/h5-6,12,15-16,20-23H,7-11,13-14H2,1-4H3,(H,33,39)(H,34,40)(H,35,41)(H,36,44)(H,37,38)(H,42,43)/t20?,21-,22?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Biinding affinity of the compound for enzyme NS3 protease wild-type |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110128

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(1...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)c1nc2ccccc2o1 Show InChI InChI=1S/C51H59F2N7O14/c1-28(61)54-37(27-42(66)67)49(72)55-34(22-24-41(64)65)47(70)60-44(43(30-15-7-3-8-16-30)31-17-9-4-10-18-31)50(73)56-33(21-23-40(62)63)46(69)58-36(25-29-13-5-2-6-14-29)48(71)57-35(26-39(52)53)45(68)51-59-32-19-11-12-20-38(32)74-51/h3-4,7-12,15-20,29,33-37,39,43-44H,2,5-6,13-14,21-27H2,1H3,(H,54,61)(H,55,72)(H,56,73)(H,57,71)(H,58,69)(H,60,70)(H,62,63)(H,64,65)(H,66,67)/t33-,34-,35-,36-,37-,44-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110127
(2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H](CC(F)F)C(O)=O Show InChI InChI=1S/C44H56F2N6O14/c1-24(53)47-31(23-36(58)59)42(63)48-29(18-20-35(56)57)40(61)52-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)43(64)49-28(17-19-34(54)55)39(60)50-30(21-25-11-5-2-6-12-25)41(62)51-32(44(65)66)22-33(45)46/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,47,53)(H,48,63)(H,49,64)(H,50,60)(H,51,62)(H,52,61)(H,54,55)(H,56,57)(H,58,59)(H,65,66)/t28-,29-,30-,31-,32+,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110118

(2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(c1ccccc1)c1ccccc1)C(O)=O Show InChI InChI=1S/C44H58N6O14/c1-3-29(44(63)64)46-41(60)32(23-26-13-7-4-8-14-26)49-39(58)30(19-21-34(52)53)48-43(62)38(37(27-15-9-5-10-16-27)28-17-11-6-12-18-28)50-40(59)31(20-22-35(54)55)47-42(61)33(24-36(56)57)45-25(2)51/h5-6,9-12,15-18,26,29-33,37-38H,3-4,7-8,13-14,19-24H2,1-2H3,(H,45,51)(H,46,60)(H,47,61)(H,48,62)(H,49,58)(H,50,59)(H,52,53)(H,54,55)(H,56,57)(H,63,64)/t29-,30-,31-,32-,33-,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122891

(4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...)Show SMILES CC(C)COC(=O)NC(CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C29H41ClF2N4O9/c1-15(2)11-21(34-26(40)20(7-8-24(37)38)36-29(44)45-14-16(3)4)27(41)35-22(13-23(31)32)25(39)33-10-9-17-5-6-18(28(42)43)12-19(17)30/h5-6,12,15-16,20-23H,7-11,13-14H2,1-4H3,(H,33,39)(H,34,40)(H,35,41)(H,36,44)(H,37,38)(H,42,43)/t20?,21-,22?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Binding affinity for enzyme K136R |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122891

(4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...)Show SMILES CC(C)COC(=O)NC(CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C29H41ClF2N4O9/c1-15(2)11-21(34-26(40)20(7-8-24(37)38)36-29(44)45-14-16(3)4)27(41)35-22(13-23(31)32)25(39)33-10-9-17-5-6-18(28(42)43)12-19(17)30/h5-6,12,15-16,20-23H,7-11,13-14H2,1-4H3,(H,33,39)(H,34,40)(H,35,41)(H,36,44)(H,37,38)(H,42,43)/t20?,21-,22?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Biinding affinity of the compound for enzyme K136M |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110119

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(3...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)c1nccs1 Show InChI InChI=1S/C47H57F2N7O13S/c1-26(57)51-34(25-38(62)63)45(68)52-31(18-20-37(60)61)43(66)56-40(39(28-13-7-3-8-14-28)29-15-9-4-10-16-29)46(69)53-30(17-19-36(58)59)42(65)55-33(23-27-11-5-2-6-12-27)44(67)54-32(24-35(48)49)41(64)47-50-21-22-70-47/h3-4,7-10,13-16,21-22,27,30-35,39-40H,2,5-6,11-12,17-20,23-25H2,1H3,(H,51,57)(H,52,68)(H,53,69)(H,54,67)(H,55,65)(H,56,66)(H,58,59)(H,60,61)(H,62,63)/t30-,31-,32-,33-,34-,40-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122883

(3-Chloro-4-(2-{4,4-difluoro-2-[2-(2-isobutoxycarbo...)Show SMILES CC(C)COC(=O)NC(C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C29H43ClF2N4O7/c1-15(2)11-21(35-27(39)24(17(5)6)36-29(42)43-14-16(3)4)26(38)34-22(13-23(31)32)25(37)33-10-9-18-7-8-19(28(40)41)12-20(18)30/h7-8,12,15-17,21-24H,9-11,13-14H2,1-6H3,(H,33,37)(H,34,38)(H,35,39)(H,36,42)(H,40,41)/t21-,22?,24?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Biinding affinity of the compound for wild type NS3 protease |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122883

(3-Chloro-4-(2-{4,4-difluoro-2-[2-(2-isobutoxycarbo...)Show SMILES CC(C)COC(=O)NC(C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C29H43ClF2N4O7/c1-15(2)11-21(35-27(39)24(17(5)6)36-29(42)43-14-16(3)4)26(38)34-22(13-23(31)32)25(37)33-10-9-18-7-8-19(28(40)41)12-20(18)30/h7-8,12,15-17,21-24H,9-11,13-14H2,1-6H3,(H,33,37)(H,34,38)(H,35,39)(H,36,42)(H,40,41)/t21-,22?,24?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Biinding affinity of the compound for NS3 protease K136R |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122883

(3-Chloro-4-(2-{4,4-difluoro-2-[2-(2-isobutoxycarbo...)Show SMILES CC(C)COC(=O)NC(C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C29H43ClF2N4O7/c1-15(2)11-21(35-27(39)24(17(5)6)36-29(42)43-14-16(3)4)26(38)34-22(13-23(31)32)25(37)33-10-9-18-7-8-19(28(40)41)12-20(18)30/h7-8,12,15-17,21-24H,9-11,13-14H2,1-6H3,(H,33,37)(H,34,38)(H,35,39)(H,36,42)(H,40,41)/t21-,22?,24?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Biinding affinity of the compound NS3 protease K136M |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122882
(4-(1-{1-[2-(2-Chloro-phenyl)-ethylcarbamoyl]-2-mer...)Show SMILES CC(C)C[C@H](NC(=O)C(CCC(O)=O)NC(=O)OC(C)(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccccc1Cl Show InChI InChI=1S/C28H41ClF2N4O7/c1-16(2)14-20(33-25(39)19(10-11-23(36)37)35-27(41)42-28(3,4)5)26(40)34-21(15-22(30)31)24(38)32-13-12-17-8-6-7-9-18(17)29/h6-9,16,19-22H,10-15H2,1-5H3,(H,32,38)(H,33,39)(H,34,40)(H,35,41)(H,36,37)/t19?,20-,21?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Biinding affinity of the compound for enzyme NS3 protease wild-type |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122882

(4-(1-{1-[2-(2-Chloro-phenyl)-ethylcarbamoyl]-2-mer...)Show SMILES CC(C)C[C@H](NC(=O)C(CCC(O)=O)NC(=O)OC(C)(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccccc1Cl Show InChI InChI=1S/C28H41ClF2N4O7/c1-16(2)14-20(33-25(39)19(10-11-23(36)37)35-27(41)42-28(3,4)5)26(40)34-21(15-22(30)31)24(38)32-13-12-17-8-6-7-9-18(17)29/h6-9,16,19-22H,10-15H2,1-5H3,(H,32,38)(H,33,39)(H,34,40)(H,35,41)(H,36,37)/t19?,20-,21?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Binding affinity for enzyme K136R |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50122882

(4-(1-{1-[2-(2-Chloro-phenyl)-ethylcarbamoyl]-2-mer...)Show SMILES CC(C)C[C@H](NC(=O)C(CCC(O)=O)NC(=O)OC(C)(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccccc1Cl Show InChI InChI=1S/C28H41ClF2N4O7/c1-16(2)14-20(33-25(39)19(10-11-23(36)37)35-27(41)42-28(3,4)5)26(40)34-21(15-22(30)31)24(38)32-13-12-17-8-6-7-9-18(17)29/h6-9,16,19-22H,10-15H2,1-5H3,(H,32,38)(H,33,39)(H,34,40)(H,35,41)(H,36,37)/t19?,20-,21?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Biinding affinity of the compound for enzyme K136M |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50122891

(4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...)Show SMILES CC(C)COC(=O)NC(CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C29H41ClF2N4O9/c1-15(2)11-21(34-26(40)20(7-8-24(37)38)36-29(44)45-14-16(3)4)27(41)35-22(13-23(31)32)25(39)33-10-9-17-5-6-18(28(42)43)12-19(17)30/h5-6,12,15-16,20-23H,7-11,13-14H2,1-4H3,(H,33,39)(H,34,40)(H,35,41)(H,36,44)(H,37,38)(H,42,43)/t20?,21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Binding affinity for enzyme human leukocyte HLE |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50122882

(4-(1-{1-[2-(2-Chloro-phenyl)-ethylcarbamoyl]-2-mer...)Show SMILES CC(C)C[C@H](NC(=O)C(CCC(O)=O)NC(=O)OC(C)(C)C)C(=O)NC(CC(F)F)C(=O)NCCc1ccccc1Cl Show InChI InChI=1S/C28H41ClF2N4O7/c1-16(2)14-20(33-25(39)19(10-11-23(36)37)35-27(41)42-28(3,4)5)26(40)34-21(15-22(30)31)24(38)32-13-12-17-8-6-7-9-18(17)29/h6-9,16,19-22H,10-15H2,1-5H3,(H,32,38)(H,33,39)(H,34,40)(H,35,41)(H,36,37)/t19?,20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Binding affinity for enzyme human leukocyte HLE |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC3 |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195108
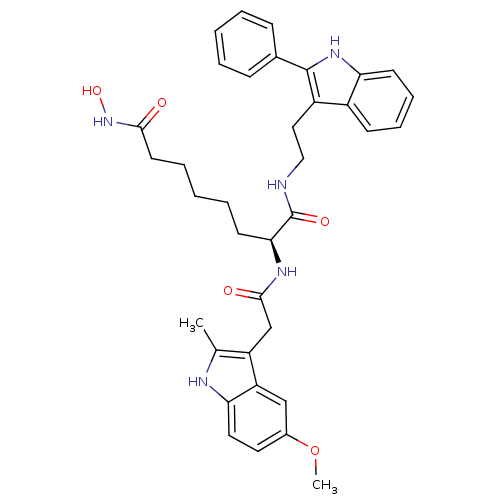
((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC4 by Biomol assay |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50238632
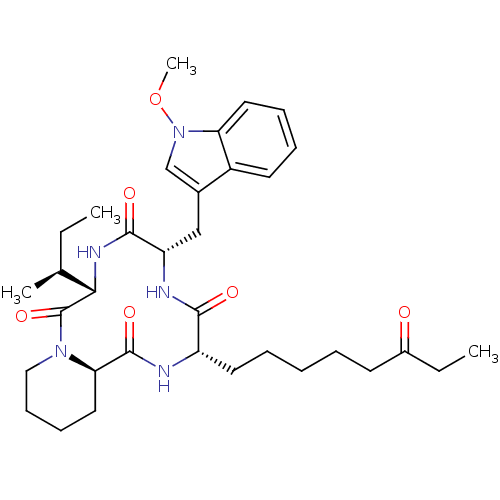
((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC4 |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC4 |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC4 by Biomol assay |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50162106

(1-Cyclohexyl-2-(2-fluoro-4-hydroxy-phenyl)-1H-benz...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(O)cc1F Show InChI InChI=1S/C20H19FN2O3/c21-16-11-14(24)7-8-15(16)19-22-17-10-12(20(25)26)6-9-18(17)23(19)13-4-2-1-3-5-13/h6-11,13,24H,1-5H2,(H,25,26) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome)
Curated by ChEMBL
| Assay Description
Inhibitory concentration against RNA dependent RNA polymerase nonstructural protein 5B of hepatitis C virus |
J Med Chem 48: 1314-7 (2005)
Article DOI: 10.1021/jm049122i
BindingDB Entry DOI: 10.7270/Q2C24VXP |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome)
Curated by ChEMBL
| Assay Description
Binding affinity to human ER beta |
J Med Chem 49: 5404-7 (2006)
Article DOI: 10.1021/jm060516e
BindingDB Entry DOI: 10.7270/Q2DB81GH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome)
Curated by ChEMBL
| Assay Description
Binding affinity to human ER alpha |
J Med Chem 49: 5404-7 (2006)
Article DOI: 10.1021/jm060516e
BindingDB Entry DOI: 10.7270/Q2DB81GH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110122

((S)-4-((S)-2-Acetylamino-3-carboxy-propionylamino)...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C52H63F2N7O14/c1-30(62)56-39(28-43(67)68)50(73)57-36(23-25-42(65)66)48(71)61-45(44(33-18-10-4-11-19-33)34-20-12-5-13-21-34)51(74)58-35(22-24-41(63)64)47(70)60-38(26-31-14-6-2-7-15-31)49(72)59-37(27-40(53)54)46(69)52(75)55-29-32-16-8-3-9-17-32/h3-5,8-13,16-21,31,35-40,44-45H,2,6-7,14-15,22-29H2,1H3,(H,55,75)(H,56,62)(H,57,73)(H,58,74)(H,59,72)(H,60,70)(H,61,71)(H,63,64)(H,65,66)(H,67,68)/t35-,36-,37-,38-,39-,45-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C NS3/NS4A protease |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50122900
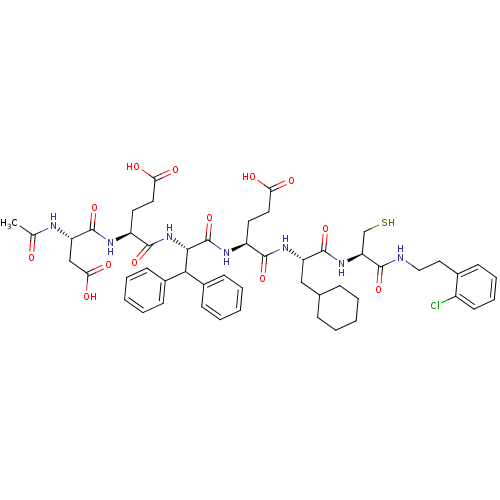
((5R,8S,11S,14S,17S,20S)-20-acetamido-14-benzhydryl...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CS)C(=O)NCCc1ccccc1Cl |r| Show InChI InChI=1S/C51H64ClN7O13S/c1-30(60)54-39(28-43(65)66)50(71)55-37(22-24-42(63)64)48(69)59-45(44(33-16-7-3-8-17-33)34-18-9-4-10-19-34)51(72)56-36(21-23-41(61)62)47(68)57-38(27-31-13-5-2-6-14-31)49(70)58-40(29-73)46(67)53-26-25-32-15-11-12-20-35(32)52/h3-4,7-12,15-20,31,36-40,44-45,73H,2,5-6,13-14,21-29H2,1H3,(H,53,67)(H,54,60)(H,55,71)(H,56,72)(H,57,68)(H,58,70)(H,59,69)(H,61,62)(H,63,64)(H,65,66)/t36-,37-,38-,39-,40-,45-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C NS3/NS4A protease |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC4 by pull-down assay |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 catalytic domain expressed in HEK293 cells by Biomol assay |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC3 |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 8.0 | 37 |
IRBM/Merck
| Assay Description
The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... |
Bioorg Med Chem Lett 18: 1814-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.025
BindingDB Entry DOI: 10.7270/Q2RJ4GSK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC4 expressed in HEK293 cells by Biomol assay |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50162110

(1-Cyclohexyl-2-(4-hydroxy-phenyl)-1H-benzoimidazol...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(O)cc1 Show InChI InChI=1S/C20H20N2O3/c23-16-9-6-13(7-10-16)19-21-17-12-14(20(24)25)8-11-18(17)22(19)15-4-2-1-3-5-15/h6-12,15,23H,1-5H2,(H,24,25) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome)
Curated by ChEMBL
| Assay Description
Inhibitory concentration against RNA dependent RNA polymerase nonstructural protein 5B of hepatitis C virus |
J Med Chem 48: 1314-7 (2005)
Article DOI: 10.1021/jm049122i
BindingDB Entry DOI: 10.7270/Q2C24VXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 8.0 | 37 |
IRBM/Merck
| Assay Description
The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... |
Bioorg Med Chem Lett 18: 1814-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.025
BindingDB Entry DOI: 10.7270/Q2RJ4GSK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Ricerche di Biologia Molecolare
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC4 by pull-down assay |
Proc Natl Acad Sci USA 104: 17335-40 (2007)
Article DOI: 10.1073/pnas.0706487104
BindingDB Entry DOI: 10.7270/Q2C82B5X |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50122902
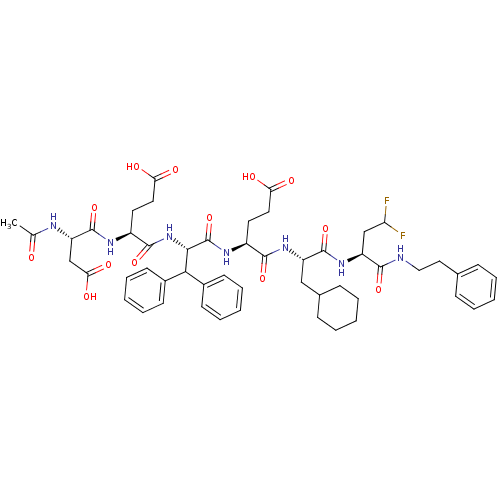
(4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{1...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C52H65F2N7O13/c1-31(62)56-40(30-44(67)68)51(73)57-37(23-25-43(65)66)49(71)61-46(45(34-18-10-4-11-19-34)35-20-12-5-13-21-35)52(74)58-36(22-24-42(63)64)48(70)59-38(28-33-16-8-3-9-17-33)50(72)60-39(29-41(53)54)47(69)55-27-26-32-14-6-2-7-15-32/h2,4-7,10-15,18-21,33,36-41,45-46H,3,8-9,16-17,22-30H2,1H3,(H,55,69)(H,56,62)(H,57,73)(H,58,74)(H,59,70)(H,60,72)(H,61,71)(H,63,64)(H,65,66)(H,67,68)/t36-,37-,38-,39-,40-,46-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C NS3/NS4A protease |
J Med Chem 46: 345-8 (2003)
Article DOI: 10.1021/jm025594q
BindingDB Entry DOI: 10.7270/Q2CF9PF1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [653-1084,H976Y]
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.5 | 37 |
IRBM/Merck
| Assay Description
The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... |
Bioorg Med Chem Lett 18: 1814-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.025
BindingDB Entry DOI: 10.7270/Q2RJ4GSK |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50162109
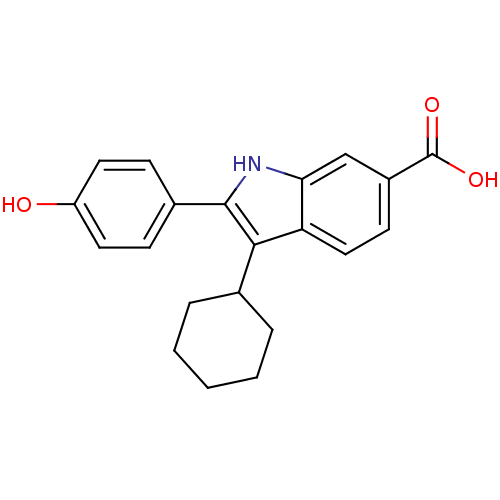
(3-Cyclohexyl-2-(4-hydroxy-phenyl)-1H-indole-6-carb...)Show SMILES OC(=O)c1ccc2c(C3CCCCC3)c([nH]c2c1)-c1ccc(O)cc1 Show InChI InChI=1S/C21H21NO3/c23-16-9-6-14(7-10-16)20-19(13-4-2-1-3-5-13)17-11-8-15(21(24)25)12-18(17)22-20/h6-13,22-23H,1-5H2,(H,24,25) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome)
Curated by ChEMBL
| Assay Description
Inhibitory concentration against RNA dependent RNA polymerase nonstructural protein 5B of hepatitis C virus |
J Med Chem 48: 1314-7 (2005)
Article DOI: 10.1021/jm049122i
BindingDB Entry DOI: 10.7270/Q2C24VXP |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50162099
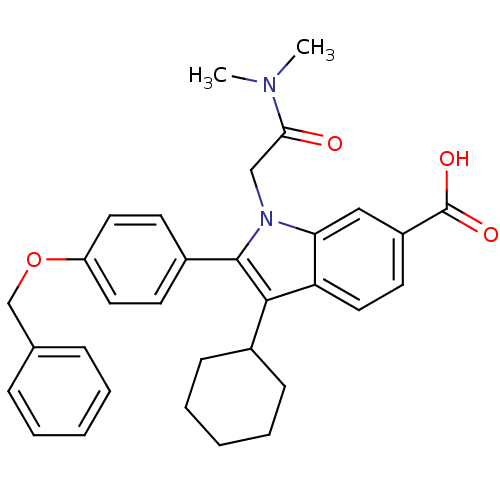
(2-(4-Benzyloxy-phenyl)-3-cyclohexyl-1-dimethylcarb...)Show SMILES CN(C)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C32H34N2O4/c1-33(2)29(35)20-34-28-19-25(32(36)37)15-18-27(28)30(23-11-7-4-8-12-23)31(34)24-13-16-26(17-14-24)38-21-22-9-5-3-6-10-22/h3,5-6,9-10,13-19,23H,4,7-8,11-12,20-21H2,1-2H3,(H,36,37) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome)
Curated by ChEMBL
| Assay Description
Inhibitory activity against RNA dependent RNA polymerase nonstructural protein 5B in hepatitis C virus; n=1 |
J Med Chem 48: 1314-7 (2005)
Article DOI: 10.1021/jm049122i
BindingDB Entry DOI: 10.7270/Q2C24VXP |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50158838

(2-{4-[2-(4-chlorophenyl)-5-(4-hydroxyhexahydro-1-p...)Show SMILES OC1CCN(CC1)C(=O)c1ccc(c(COc2ccc(-c3nc4cc(ccc4n3C3CCCCC3)C(O)=O)c(F)c2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C39H37ClFN3O5/c40-28-10-6-24(7-11-28)32-13-8-25(38(46)43-18-16-30(45)17-19-43)20-27(32)23-49-31-12-14-33(34(41)22-31)37-42-35-21-26(39(47)48)9-15-36(35)44(37)29-4-2-1-3-5-29/h6-15,20-22,29-30,45H,1-5,16-19,23H2,(H,47,48) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <6 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome)
Curated by ChEMBL
| Assay Description
Inhibitory concentration against RNA dependent RNA polymerase nonstructural protein 5B of hepatitis C virus |
J Med Chem 48: 1314-7 (2005)
Article DOI: 10.1021/jm049122i
BindingDB Entry DOI: 10.7270/Q2C24VXP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data