

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50030448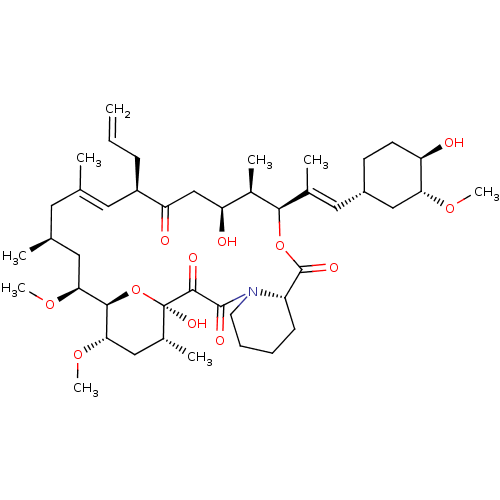 (8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM36609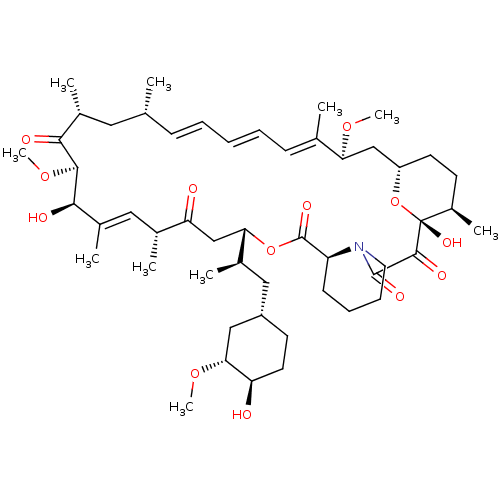 (Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217619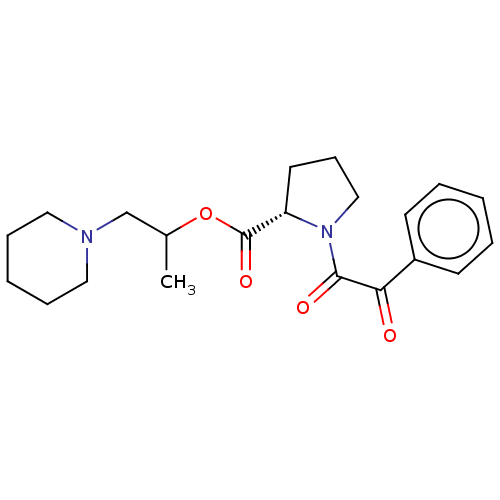 (CHEMBL6683) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217621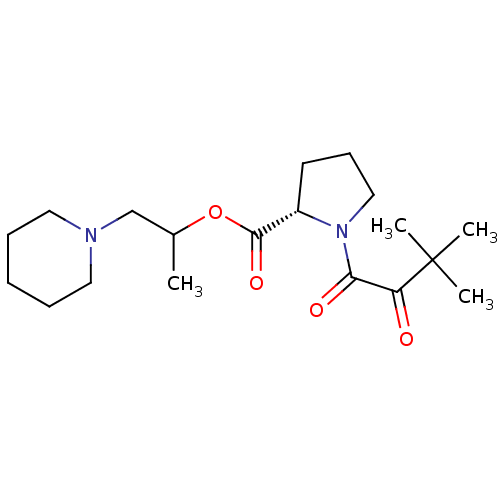 (CHEMBL269775) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217620 (CHEMBL6833) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217614 (CHEMBL269154) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217617 (CHEMBL7237) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217618 (CHEMBL266941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217615 (CHEMBL266660) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217616 (CHEMBL6809) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50217613 (CHEMBL6449) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay. | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM23334 (3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Curated by ChEMBL | Assay Description The inhibitory activity by using FK506 binding protein 12 SPA binding assay | Bioorg Med Chem Lett 10: 1007-10 (2000) BindingDB Entry DOI: 10.7270/Q28K7BCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001603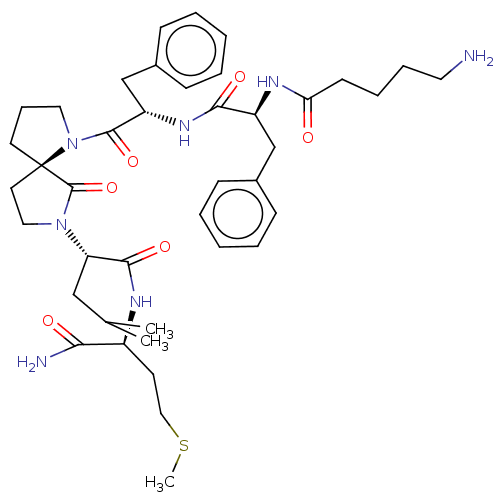 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 307 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001593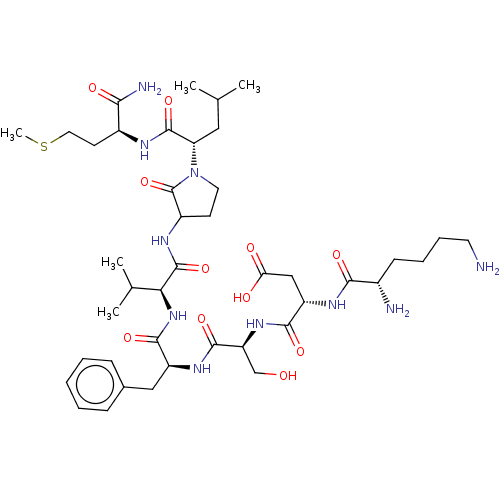 (CHEMBL131872 | N-{1-[1-(1-{1-[1-(1-Carbamoyl-3-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001594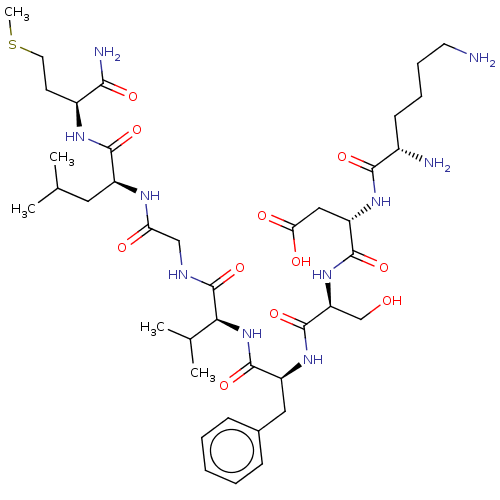 (CHEMBL335054 | N-(1-{1-[1-({[1-(1-Carbamoyl-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001602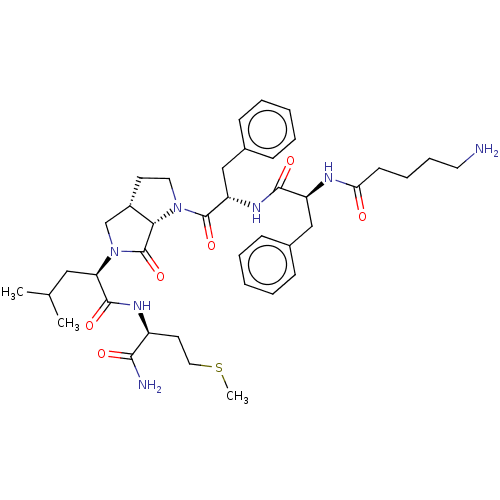 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.73E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001606 (2-(2-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001595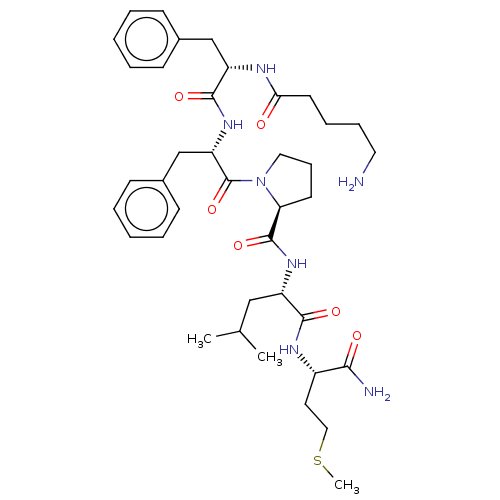 (1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propiony...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001607 (1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propiony...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 830 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001604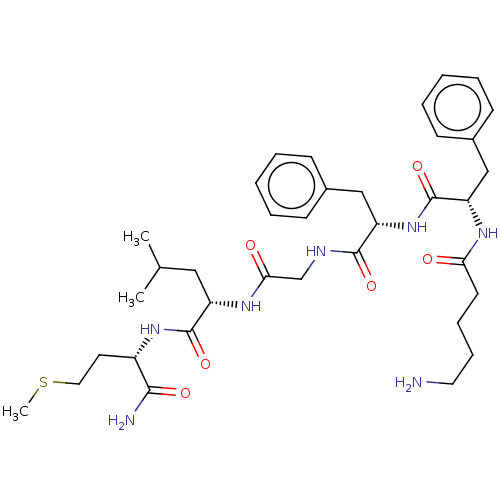 (2-(2-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001600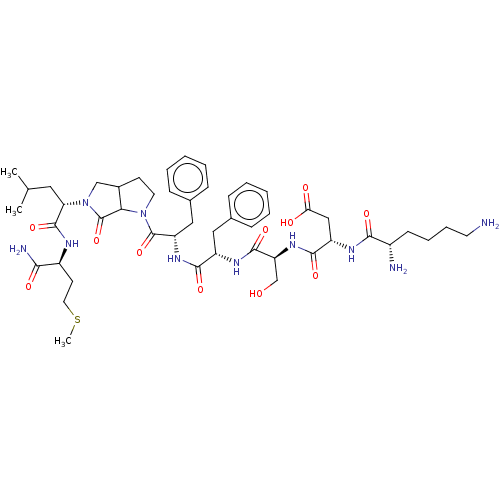 (CHEMBL267712 | N-{1-[1-(1-Benzyl-2-{5-[1-(1-carbam...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001447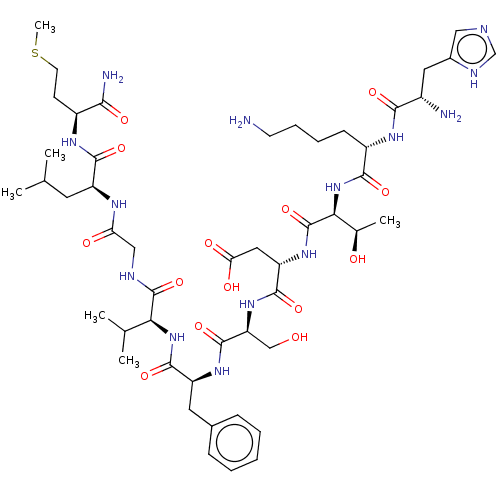 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001596 (3-(2-Acetylamino-6-amino-hexanoylamino)-N-{1-[1-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001598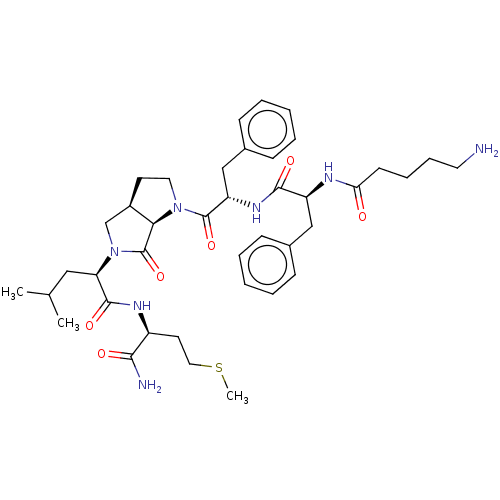 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001592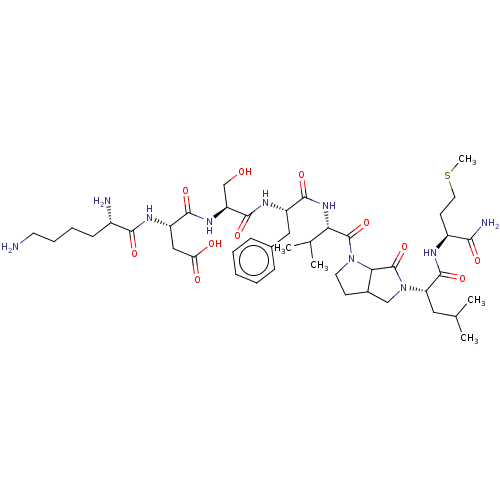 (CHEMBL131923 | N-{1-[1-(1-{5-[1-(1-Carbamoyl-3-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001599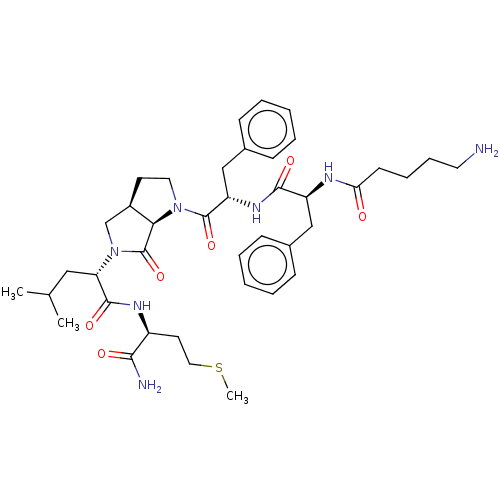 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 545 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50079412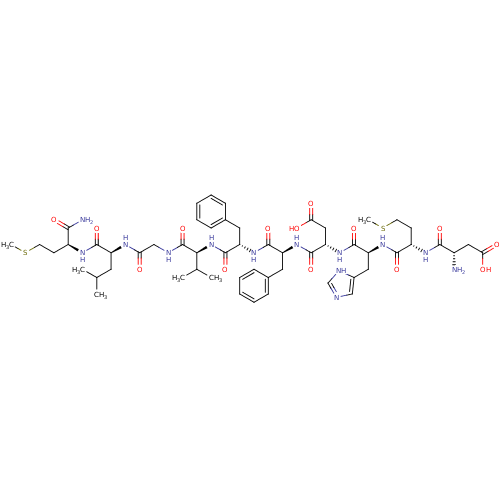 ((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001601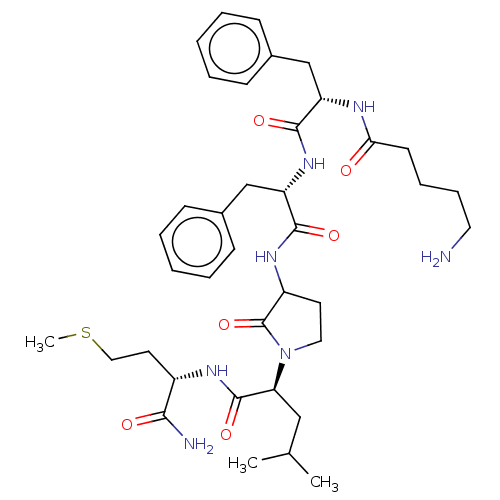 (2-(3-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001605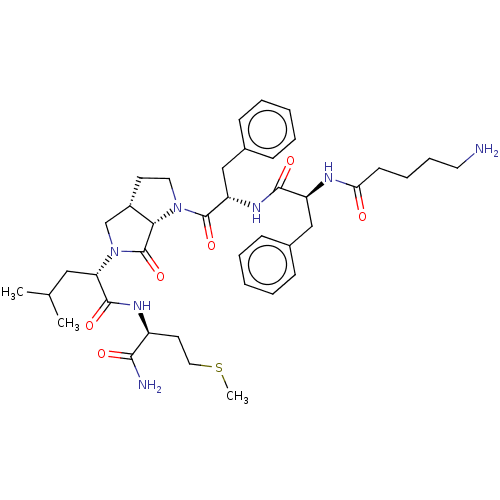 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001597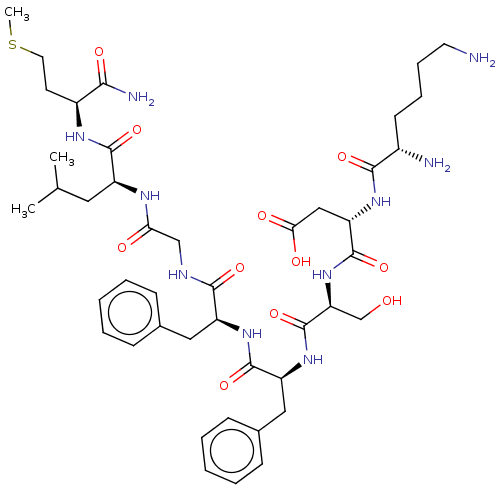 (CHEMBL134481 | N-(1-{1-[1-({[1-(1-Carbamoyl-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 1 of guinea pig ileum longitudinal smooth muscle. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001592 (CHEMBL131923 | N-{1-[1-(1-{5-[1-(1-Carbamoyl-3-met...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50001593 (CHEMBL131872 | N-{1-[1-(1-{1-[1-(1-Carbamoyl-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50001594 (CHEMBL335054 | N-(1-{1-[1-({[1-(1-Carbamoyl-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001595 (1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propiony...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001596 (3-(2-Acetylamino-6-amino-hexanoylamino)-N-{1-[1-(1...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001597 (CHEMBL134481 | N-(1-{1-[1-({[1-(1-Carbamoyl-3-meth...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50001599 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50001592 (CHEMBL131923 | N-{1-[1-(1-{5-[1-(1-Carbamoyl-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001594 (CHEMBL335054 | N-(1-{1-[1-({[1-(1-Carbamoyl-3-meth...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001600 (CHEMBL267712 | N-{1-[1-(1-Benzyl-2-{5-[1-(1-carbam...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50001595 (1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propiony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001601 (2-(3-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001598 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50001598 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >4.70E+4 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001602 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 566 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 167 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50001605 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >8.22E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50079412 ((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50001604 (2-(2-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 3 of everted rat protal vein. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |