

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444496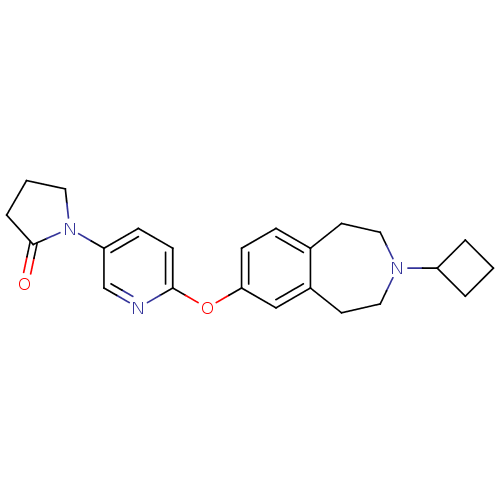 (CHEMBL3092650) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444491 (CHEMBL3092823) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444496 (CHEMBL3092650) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287729 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444500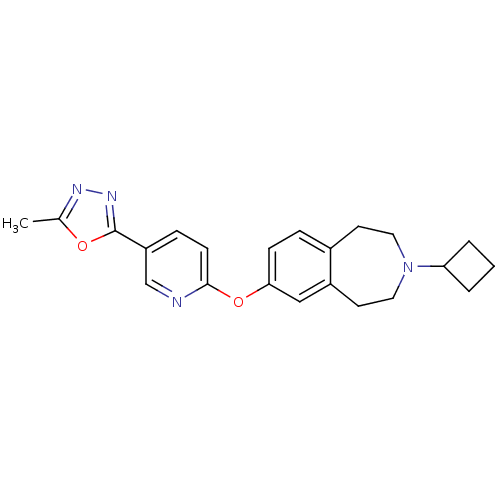 (CHEMBL3092834) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444509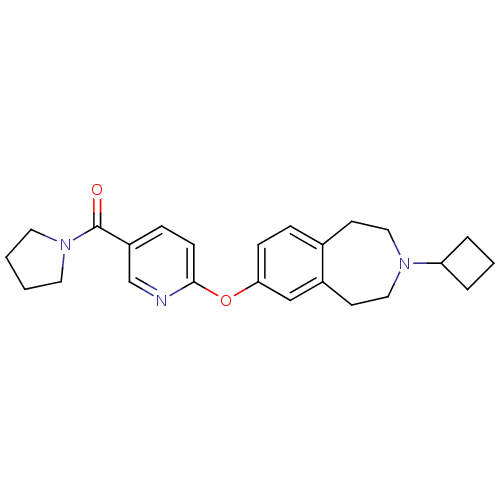 (CHEMBL3092840) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444507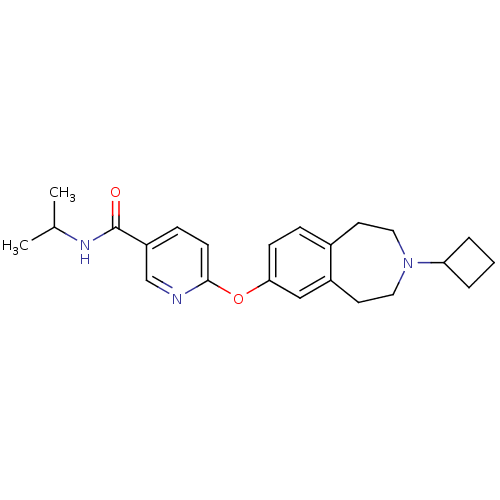 (CHEMBL3092826) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444505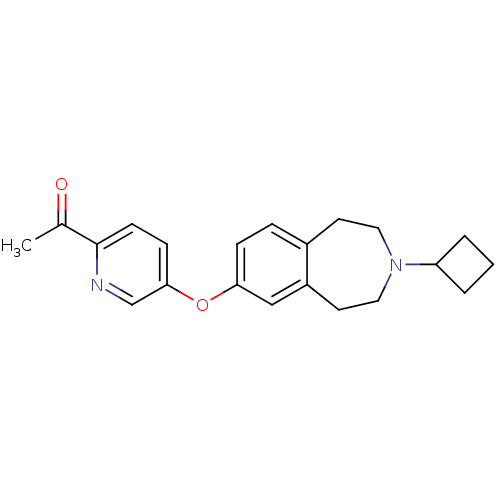 (CHEMBL3092828) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50247054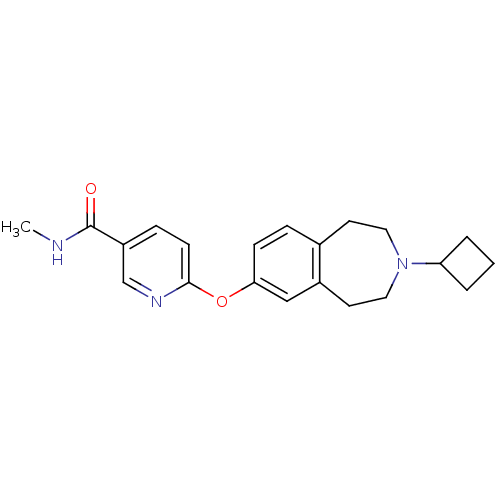 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444489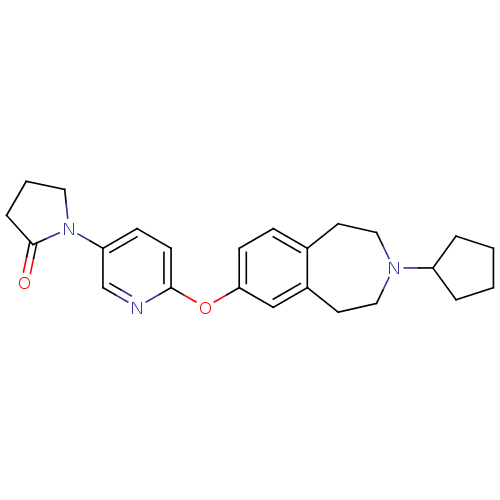 (CHEMBL3092825) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444495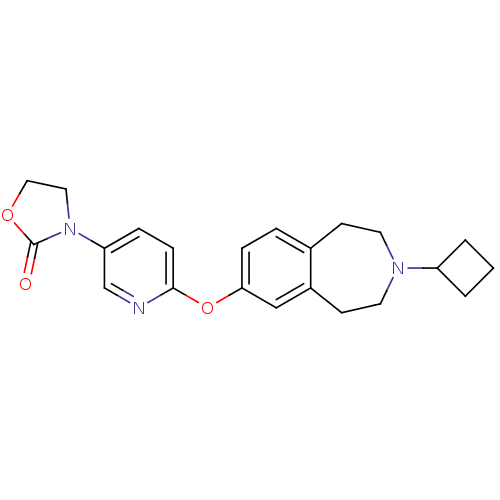 (CHEMBL3092651) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346209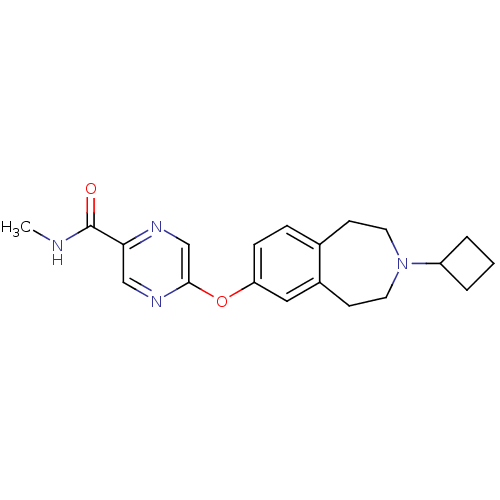 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444494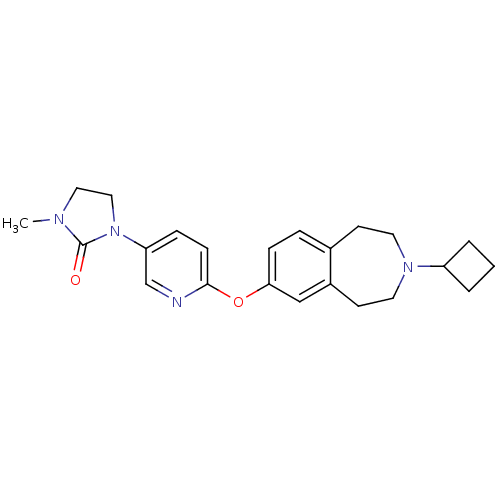 (CHEMBL3092820) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444506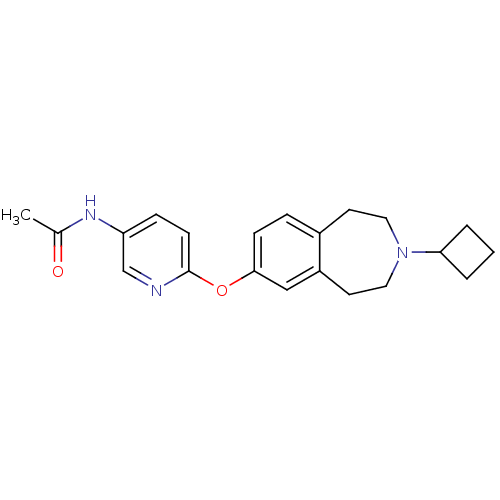 (CHEMBL3092827) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444493 (CHEMBL3092821) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444510 (CHEMBL3092839) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444499 (CHEMBL3092835) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444497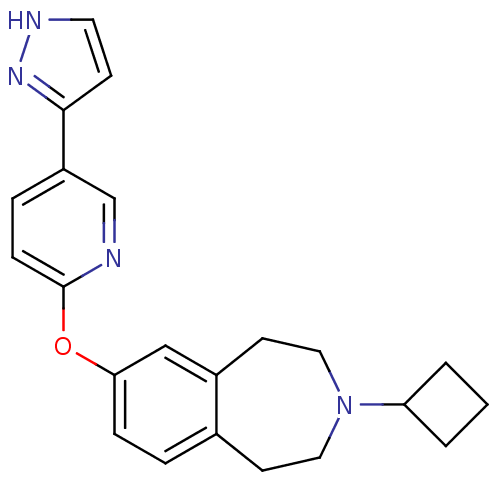 (CHEMBL3092649) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444490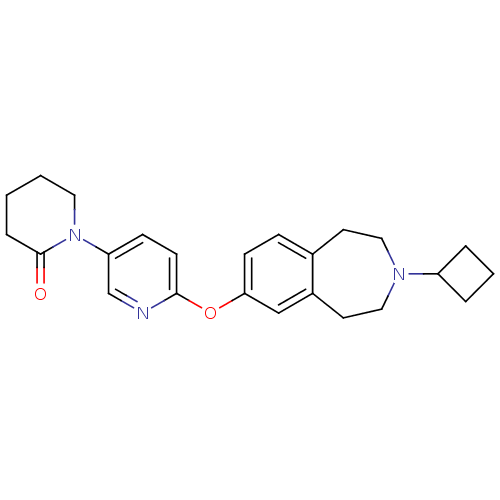 (CHEMBL3092824) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085043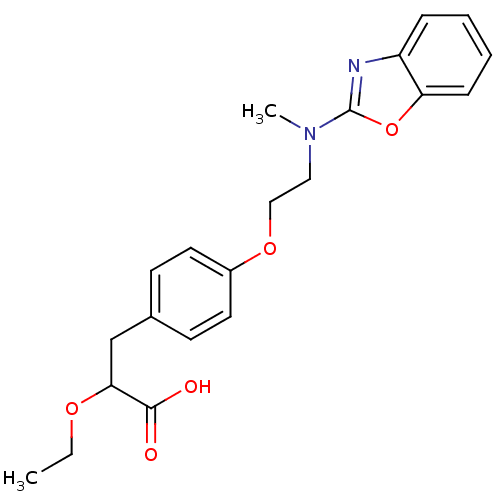 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2127-2130 (1996) Article DOI: 10.1016/0960-894X(96)00382-4 BindingDB Entry DOI: 10.7270/Q2J1034Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085043 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444492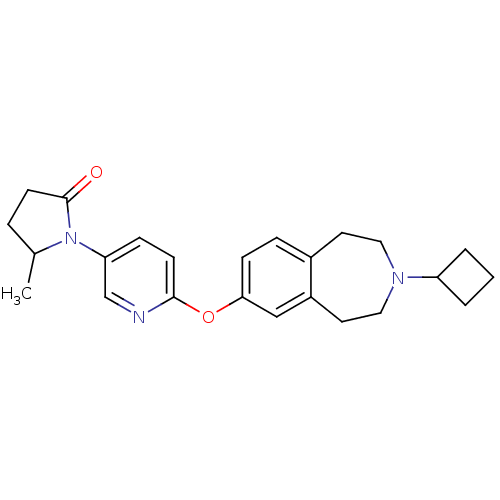 (CHEMBL3092822) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50247054 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444498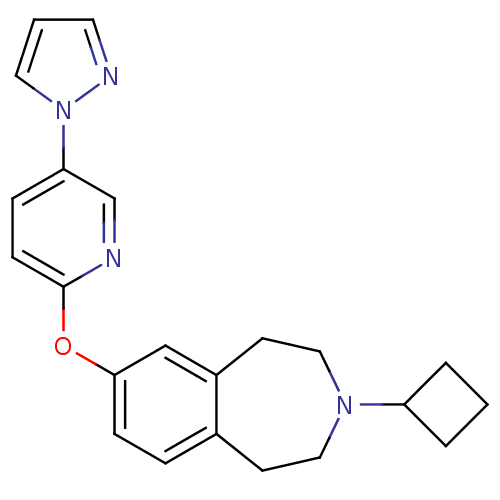 (CHEMBL3092648) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444502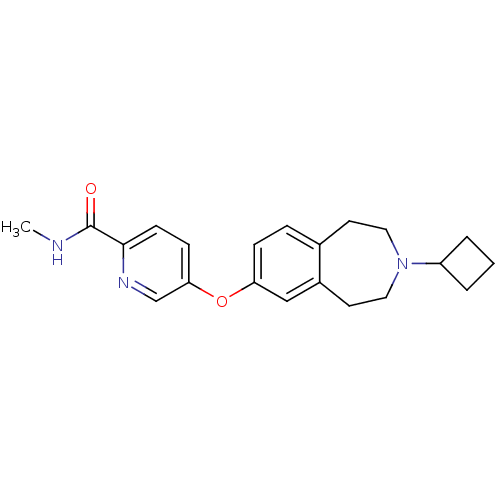 (CHEMBL3092831) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444501 (CHEMBL3092832) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230294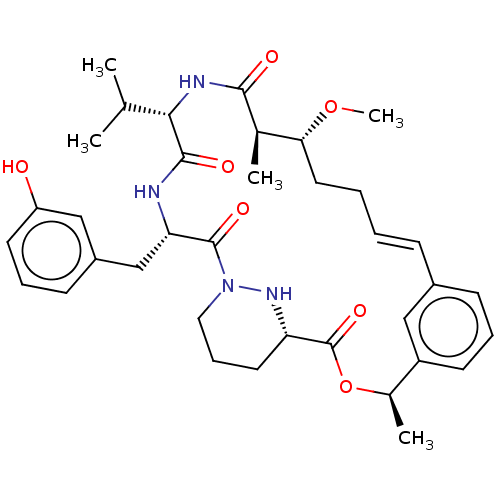 (CHEMBL4071740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444511 (CHEMBL3092838) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230211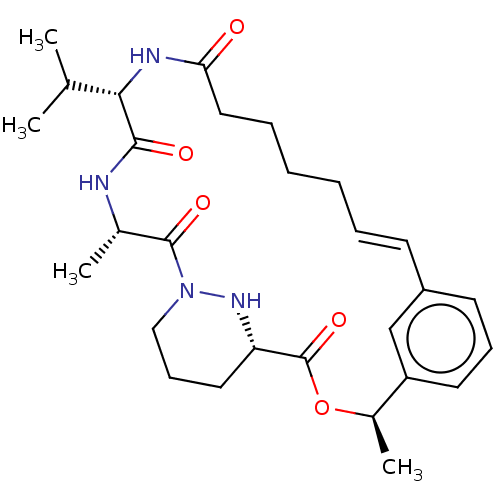 (CHEMBL4063126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444504 (CHEMBL3092829) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815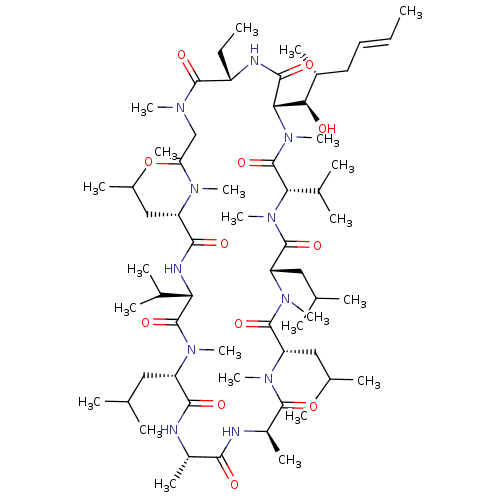 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230212 (CHEMBL4084776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444503 (CHEMBL3092830) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444508 (CHEMBL3091477) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50477760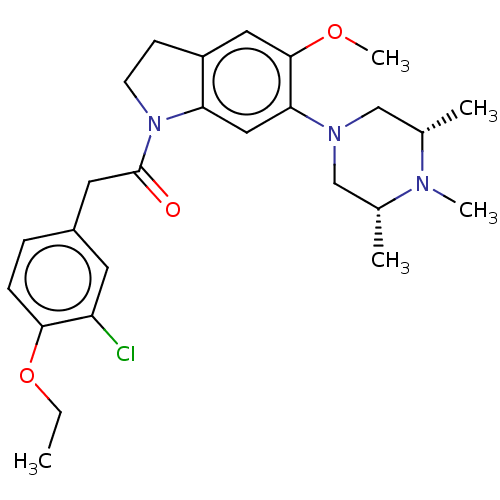 (CHEMBL250973) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity at 5HT1B receptor | Bioorg Med Chem Lett 17: 6584-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.067 BindingDB Entry DOI: 10.7270/Q2MW2KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50443776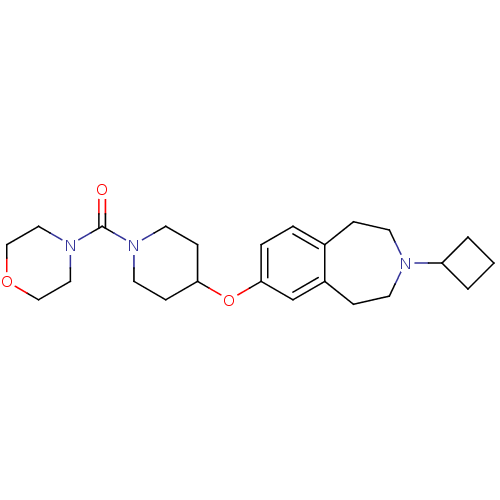 (CHEMBL3092836) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50443776 (CHEMBL3092836) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylhistamine from histamine H3 receptor in rat cerebral cortical membranes after 45 mins by liquid scintillation spect... | Bioorg Med Chem Lett 23: 6897-901 (2013) Article DOI: 10.1016/j.bmcl.2013.09.089 BindingDB Entry DOI: 10.7270/Q2HM59X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230213 (CHEMBL4092526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287732 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230210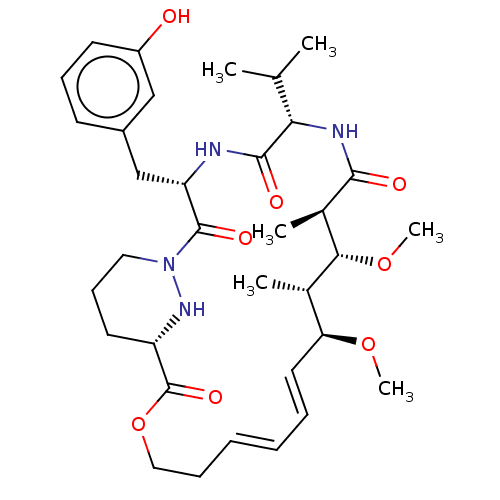 (CHEMBL4090107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50049244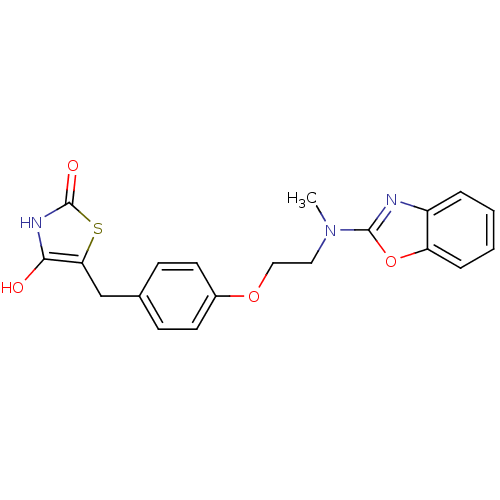 (5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2127-2130 (1996) Article DOI: 10.1016/0960-894X(96)00382-4 BindingDB Entry DOI: 10.7270/Q2J1034Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50049244 (5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287733 (2-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2127-2130 (1996) Article DOI: 10.1016/0960-894X(96)00382-4 BindingDB Entry DOI: 10.7270/Q2J1034Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287727 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287730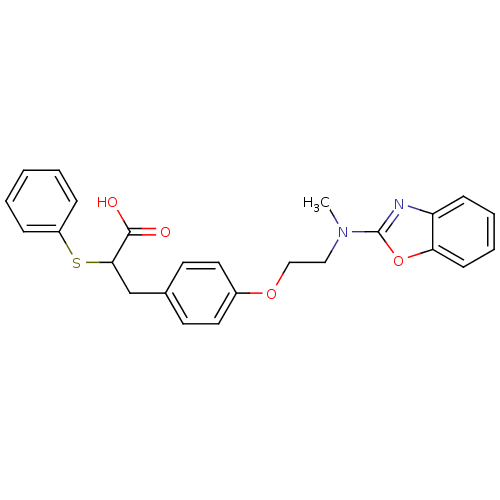 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230209 (CHEMBL4100272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287728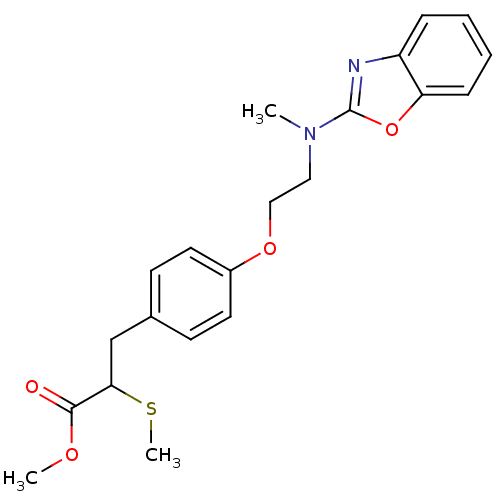 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230293 (CHEMBL4082253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 795 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50443787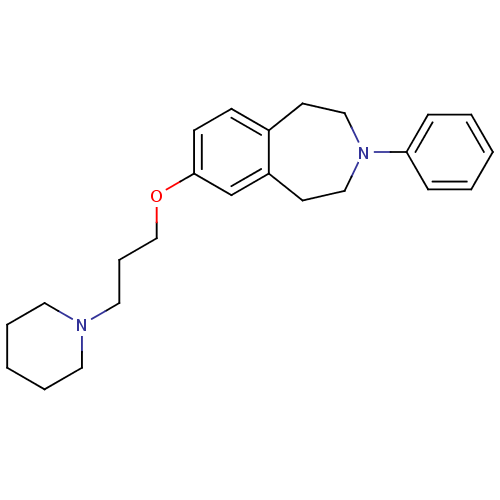 (CHEMBL3094214) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced effect by FLIPR ass... | Bioorg Med Chem Lett 23: 6897-901 (2013) Article DOI: 10.1016/j.bmcl.2013.09.089 BindingDB Entry DOI: 10.7270/Q2HM59X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 386 total ) | Next | Last >> |