 Found 1841 hits with Last Name = 'deaton' and Initial = 'dn'
Found 1841 hits with Last Name = 'deaton' and Initial = 'dn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50167296
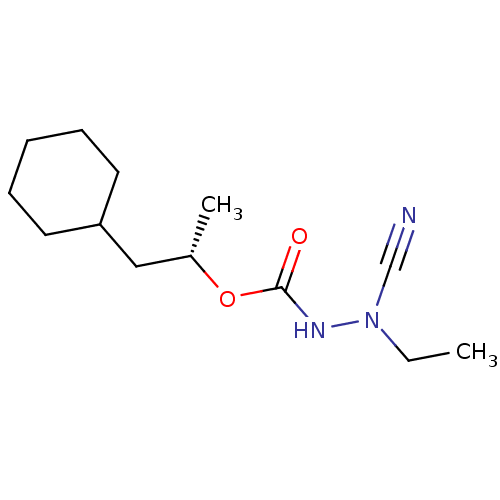
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-ethylhyd...)Show InChI InChI=1S/C13H23N3O2/c1-3-16(10-14)15-13(17)18-11(2)9-12-7-5-4-6-8-12/h11-12H,3-9H2,1-2H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167295
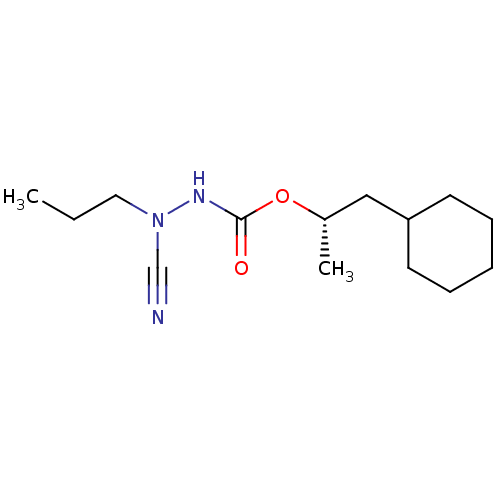
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-propylhy...)Show InChI InChI=1S/C14H25N3O2/c1-3-9-17(11-15)16-14(18)19-12(2)10-13-7-5-4-6-8-13/h12-13H,3-10H2,1-2H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167302

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isobutyl...)Show InChI InChI=1S/C15H27N3O2/c1-12(2)10-18(11-16)17-15(19)20-13(3)9-14-7-5-4-6-8-14/h12-14H,4-10H2,1-3H3,(H,17,19)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167298
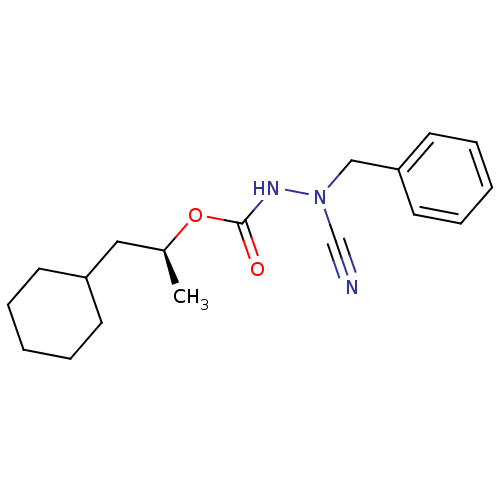
((1S)-2-cyclohexyl-1-methylethyl 2-benzyl-2-cyanohy...)Show InChI InChI=1S/C18H25N3O2/c1-15(12-16-8-4-2-5-9-16)23-18(22)20-21(14-19)13-17-10-6-3-7-11-17/h3,6-7,10-11,15-16H,2,4-5,8-9,12-13H2,1H3,(H,20,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167303
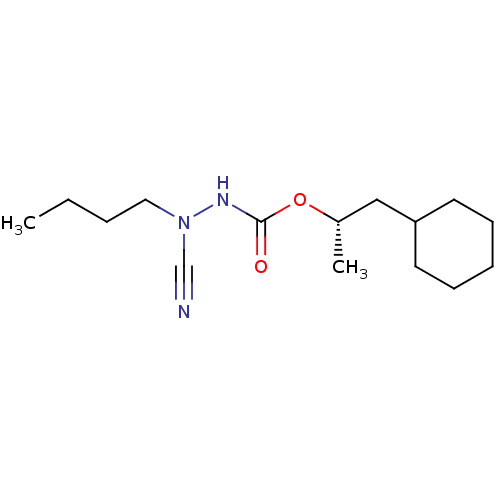
((1S)-2-cyclohexyl-1-methylethyl 2-butyl-2-cyanohyd...)Show InChI InChI=1S/C15H27N3O2/c1-3-4-10-18(12-16)17-15(19)20-13(2)11-14-8-6-5-7-9-14/h13-14H,3-11H2,1-2H3,(H,17,19)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167289
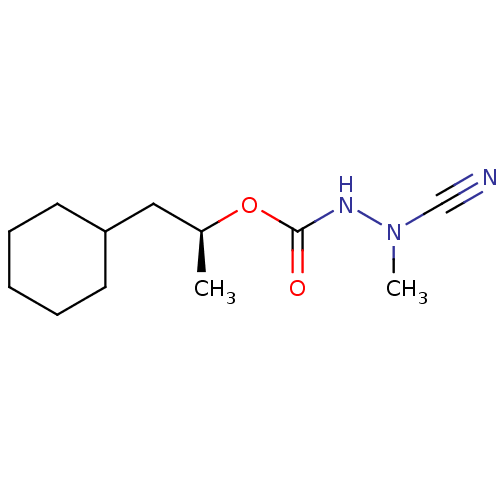
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167290
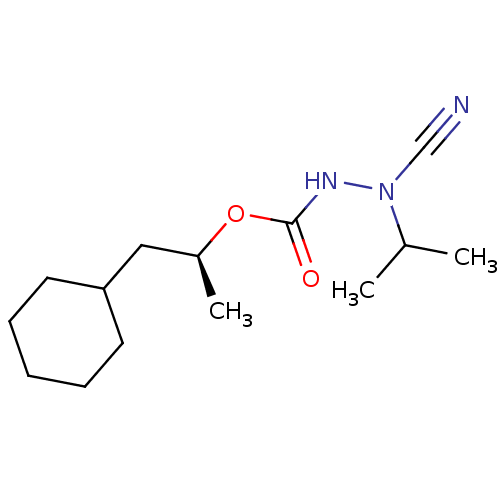
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)17(10-15)16-14(18)19-12(3)9-13-7-5-4-6-8-13/h11-13H,4-9H2,1-3H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50167289

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Rattus norvegicus) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against rat cathepsin K |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50167289

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411736
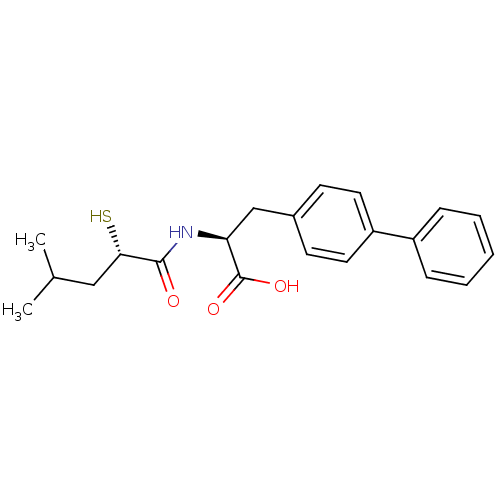
(CHEMBL271225)Show SMILES CC(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-14(2)12-19(26)20(23)22-18(21(24)25)13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,26H,12-13H2,1-2H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086433
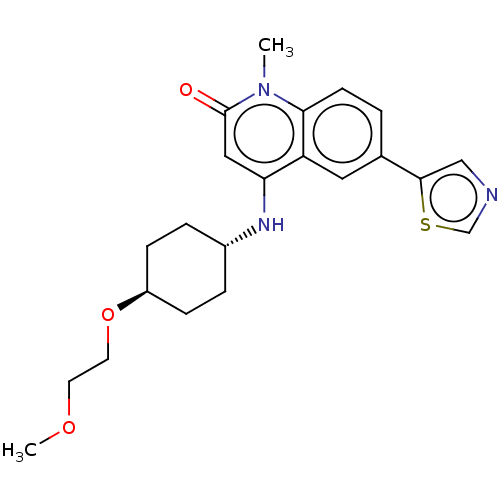
(CHEMBL3426034)Show SMILES COCCO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2ccc(cc12)-c1cncs1 |r,wU:5.4,wD:8.11,(9.09,10.61,;8.02,10,;8.02,8.46,;6.68,7.69,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.01,6.15,;1.33,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.4,.91,;-6.43,2.07,;-5.65,3.39,;-4.14,3.06,)| Show InChI InChI=1S/C22H27N3O3S/c1-25-20-8-3-15(21-13-23-14-29-21)11-18(20)19(12-22(25)26)24-16-4-6-17(7-5-16)28-10-9-27-2/h3,8,11-14,16-17,24H,4-7,9-10H2,1-2H3/t16-,17- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50275902

(CHEMBL525577 | D-Phe-Gln-Trp-Ala-Val-b-Ala-His-Phe...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34+,40-,42+,43+,44+,45+,46+,48+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRS-3 receptor (unknown origin) |
Bioorg Med Chem Lett 18: 5451-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.033
BindingDB Entry DOI: 10.7270/Q2H9952R |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086438

(CHEMBL3426039)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2c(C)cc(cc12)-c1cncs1 |r,wU:2.1,wD:5.8,(6.69,7.38,;6.68,6.15,;5.35,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.02,6.16,;1.34,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.39,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.02,1.54,;-5.41,.92,;-6.43,2.07,;-5.65,3.4,;-4.15,3.07,)| Show InChI InChI=1S/C21H25N3O2S/c1-13-8-14(19-11-22-12-27-19)9-17-18(10-20(25)24(2)21(13)17)23-15-4-6-16(26-3)7-5-15/h8-12,15-16,23H,4-7H2,1-3H3/t15-,16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
Pro-cathepsin H
(Homo sapiens (Human)) | BDBM50167290

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)17(10-15)16-14(18)19-12(3)9-13-7-5-4-6-8-13/h11-13H,4-9H2,1-3H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin H using L-Arg-b-naphthalamide |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086434
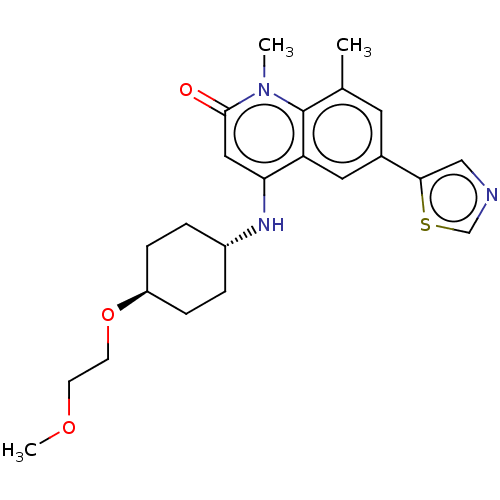
(CHEMBL3426035)Show SMILES COCCO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2c(C)cc(cc12)-c1cncs1 |r,wU:5.4,wD:8.11,(9.09,10.61,;8.02,10,;8.02,8.46,;6.68,7.69,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.01,6.15,;1.33,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.4,.91,;-6.43,2.07,;-5.65,3.39,;-4.14,3.06,)| Show InChI InChI=1S/C23H29N3O3S/c1-15-10-16(21-13-24-14-30-21)11-19-20(12-22(27)26(2)23(15)19)25-17-4-6-18(7-5-17)29-9-8-28-3/h10-14,17-18,25H,4-9H2,1-3H3/t17-,18- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50167290

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)17(10-15)16-14(18)19-12(3)9-13-7-5-4-6-8-13/h11-13H,4-9H2,1-3H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411731
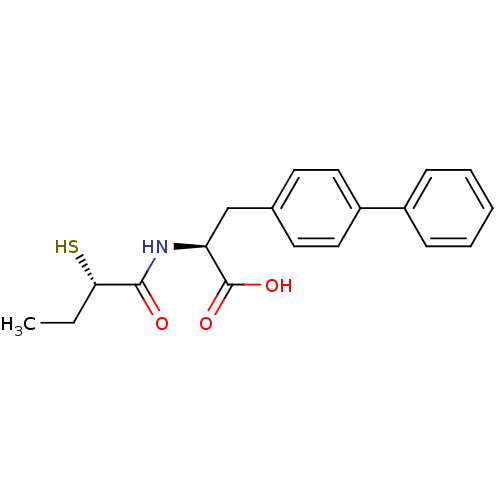
(CHEMBL257726)Show SMILES CC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C19H21NO3S/c1-2-17(24)18(21)20-16(19(22)23)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11,16-17,24H,2,12H2,1H3,(H,20,21)(H,22,23)/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50275902

(CHEMBL525577 | D-Phe-Gln-Trp-Ala-Val-b-Ala-His-Phe...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34+,40-,42+,43+,44+,45+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to gastrin releasing peptide receptor (unknown origin) |
Bioorg Med Chem Lett 18: 5451-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.033
BindingDB Entry DOI: 10.7270/Q2H9952R |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50286715
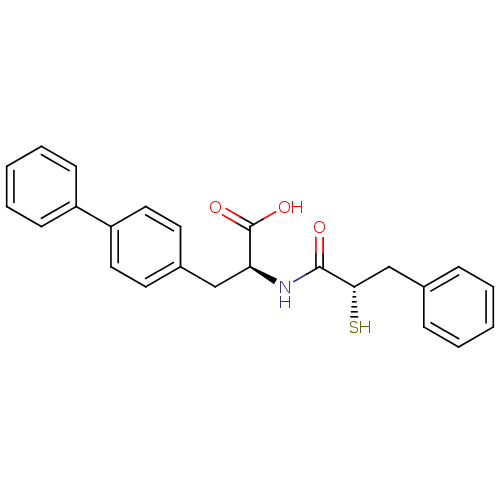
((S)-3-biphenyl-4-yl-2-((S)-2-mercapto-3-phenyl-pro...)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C24H23NO3S/c26-23(22(29)16-17-7-3-1-4-8-17)25-21(24(27)28)15-18-11-13-20(14-12-18)19-9-5-2-6-10-19/h1-14,21-22,29H,15-16H2,(H,25,26)(H,27,28)/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411730
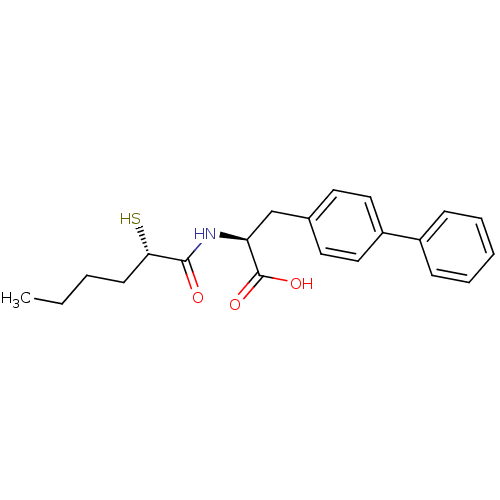
(CHEMBL257270)Show SMILES CCCC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-2-3-9-19(26)20(23)22-18(21(24)25)14-15-10-12-17(13-11-15)16-7-5-4-6-8-16/h4-8,10-13,18-19,26H,2-3,9,14H2,1H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50286724
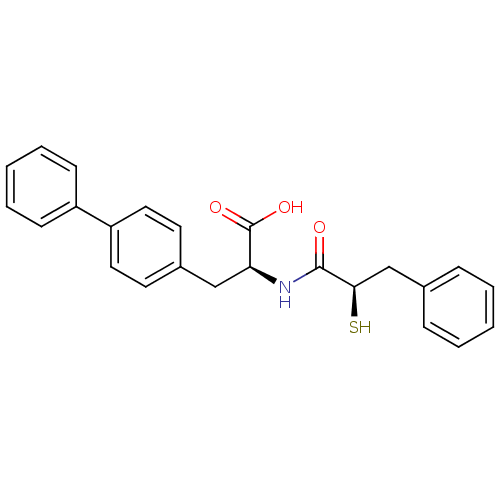
((S)-3-Biphenyl-4-yl-2-((R)-2-mercapto-3-phenyl-pro...)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](S)Cc1ccccc1 Show InChI InChI=1S/C24H23NO3S/c26-23(22(29)16-17-7-3-1-4-8-17)25-21(24(27)28)15-18-11-13-20(14-12-18)19-9-5-2-6-10-19/h1-14,21-22,29H,15-16H2,(H,25,26)(H,27,28)/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50167290

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)17(10-15)16-14(18)19-12(3)9-13-7-5-4-6-8-13/h11-13H,4-9H2,1-3H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Pro-cathepsin H
(Homo sapiens (Human)) | BDBM50167289

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin H using L-Arg-b-naphthalamide |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411731

(CHEMBL257726)Show SMILES CC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C19H21NO3S/c1-2-17(24)18(21)20-16(19(22)23)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11,16-17,24H,2,12H2,1H3,(H,20,21)(H,22,23)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411736

(CHEMBL271225)Show SMILES CC(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-14(2)12-19(26)20(23)22-18(21(24)25)13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,26H,12-13H2,1-2H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411733
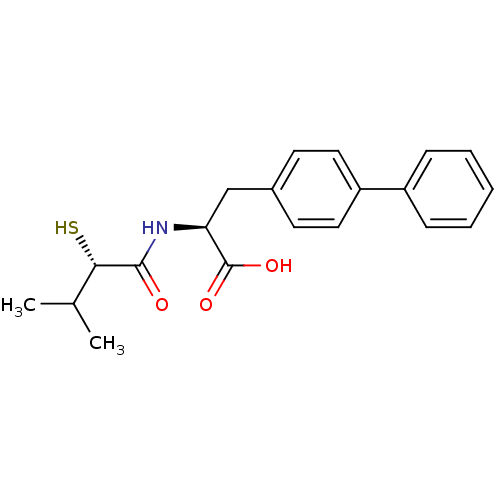
(CHEMBL269997)Show SMILES CC(C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C20H23NO3S/c1-13(2)18(25)19(22)21-17(20(23)24)12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,13,17-18,25H,12H2,1-2H3,(H,21,22)(H,23,24)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411605
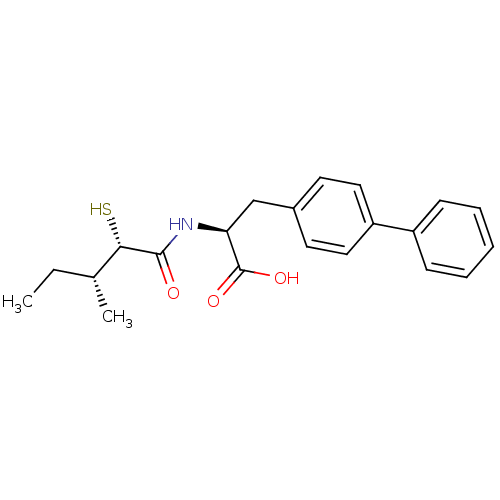
(CHEMBL252391)Show SMILES CC[C@@H](C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-3-14(2)19(26)20(23)22-18(21(24)25)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,14,18-19,26H,3,13H2,1-2H3,(H,22,23)(H,24,25)/t14-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411729
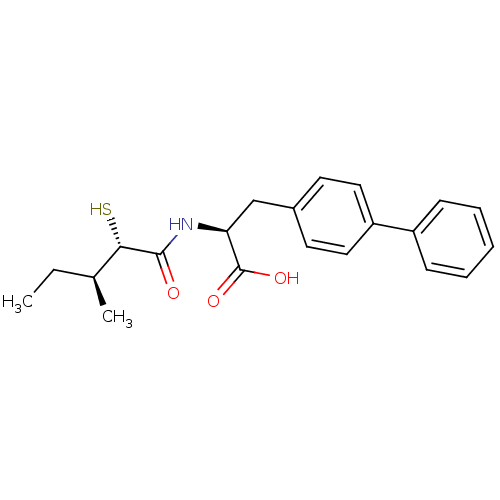
(CHEMBL269996)Show SMILES CC[C@H](C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-3-14(2)19(26)20(23)22-18(21(24)25)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,14,18-19,26H,3,13H2,1-2H3,(H,22,23)(H,24,25)/t14-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411737
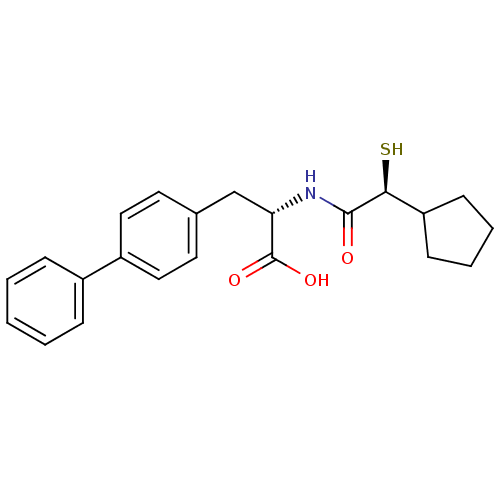
(CHEMBL404117)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)C1CCCC1 Show InChI InChI=1S/C22H25NO3S/c24-21(20(27)18-8-4-5-9-18)23-19(22(25)26)14-15-10-12-17(13-11-15)16-6-2-1-3-7-16/h1-3,6-7,10-13,18-20,27H,4-5,8-9,14H2,(H,23,24)(H,25,26)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163832
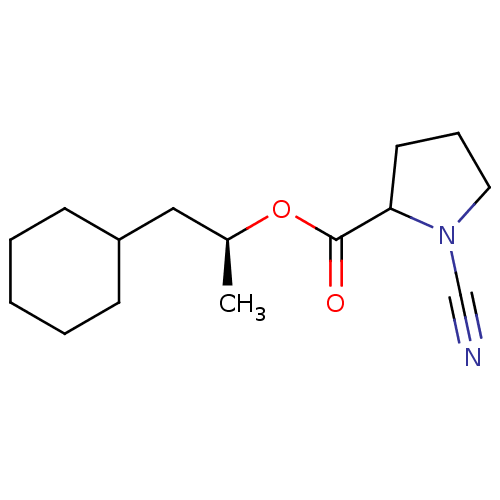
((1S)-2-cyclohexyl-1-methylethyl (2S)-1-cyanopyrrol...)Show InChI InChI=1S/C15H24N2O2/c1-12(10-13-6-3-2-4-7-13)19-15(18)14-8-5-9-17(14)11-16/h12-14H,2-10H2,1H3/t12-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411730

(CHEMBL257270)Show SMILES CCCC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-2-3-9-19(26)20(23)22-18(21(24)25)14-15-10-12-17(13-11-15)16-7-5-4-6-8-16/h4-8,10-13,18-19,26H,2-3,9,14H2,1H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411729

(CHEMBL269996)Show SMILES CC[C@H](C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-3-14(2)19(26)20(23)22-18(21(24)25)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,14,18-19,26H,3,13H2,1-2H3,(H,22,23)(H,24,25)/t14-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411728
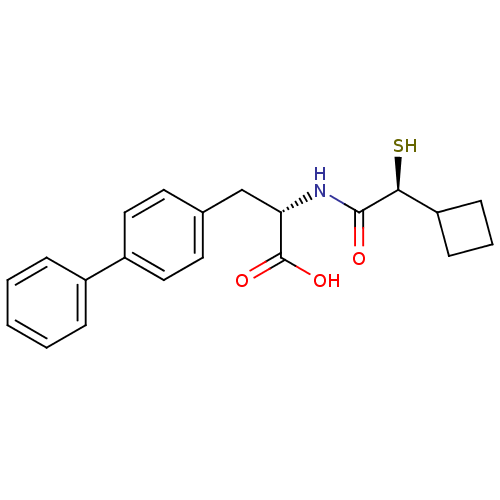
(CHEMBL257229)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)C1CCC1 Show InChI InChI=1S/C21H23NO3S/c23-20(19(26)17-7-4-8-17)22-18(21(24)25)13-14-9-11-16(12-10-14)15-5-2-1-3-6-15/h1-3,5-6,9-12,17-19,26H,4,7-8,13H2,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411735
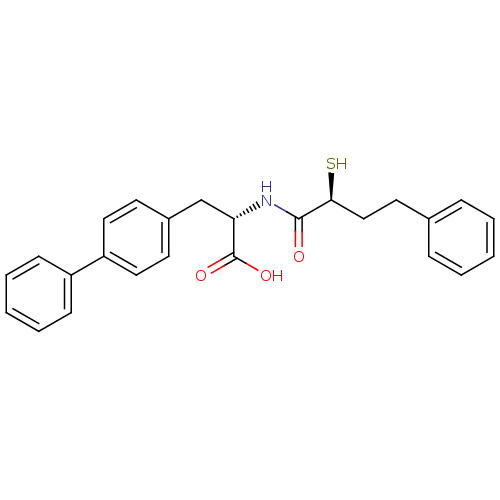
(CHEMBL402987)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)CCc1ccccc1 Show InChI InChI=1S/C25H25NO3S/c27-24(23(30)16-13-18-7-3-1-4-8-18)26-22(25(28)29)17-19-11-14-21(15-12-19)20-9-5-2-6-10-20/h1-12,14-15,22-23,30H,13,16-17H2,(H,26,27)(H,28,29)/t22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411725
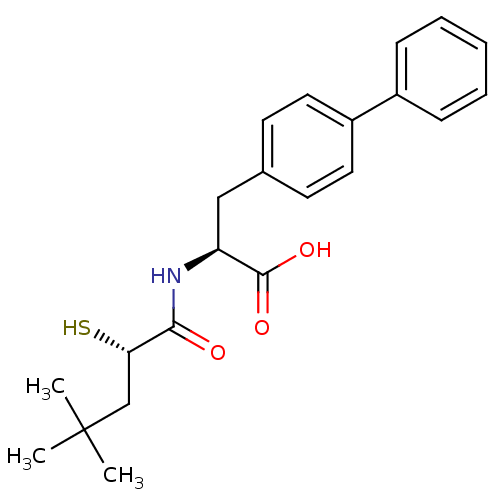
(CHEMBL271223)Show SMILES CC(C)(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C22H27NO3S/c1-22(2,3)14-19(27)20(24)23-18(21(25)26)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,18-19,27H,13-14H2,1-3H3,(H,23,24)(H,25,26)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50411736

(CHEMBL271225)Show SMILES CC(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-14(2)12-19(26)20(23)22-18(21(24)25)13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,26H,12-13H2,1-2H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411728

(CHEMBL257229)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)C1CCC1 Show InChI InChI=1S/C21H23NO3S/c23-20(19(26)17-7-4-8-17)22-18(21(24)25)13-14-9-11-16(12-10-14)15-5-2-1-3-6-15/h1-3,5-6,9-12,17-19,26H,4,7-8,13H2,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411734

(CHEMBL257727)Show SMILES C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C18H19NO3S/c1-12(23)17(20)19-16(18(21)22)11-13-7-9-15(10-8-13)14-5-3-2-4-6-14/h2-10,12,16,23H,11H2,1H3,(H,19,20)(H,21,22)/t12-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411725

(CHEMBL271223)Show SMILES CC(C)(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C22H27NO3S/c1-22(2,3)14-19(27)20(24)23-18(21(25)26)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,18-19,27H,13-14H2,1-3H3,(H,23,24)(H,25,26)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50411731

(CHEMBL257726)Show SMILES CC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C19H21NO3S/c1-2-17(24)18(21)20-16(19(22)23)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11,16-17,24H,2,12H2,1H3,(H,20,21)(H,22,23)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50275902

(CHEMBL525577 | D-Phe-Gln-Trp-Ala-Val-b-Ala-His-Phe...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34+,40-,42+,43+,44+,45+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to neuromedin B receptor (unknown origin) |
Bioorg Med Chem Lett 18: 5451-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.033
BindingDB Entry DOI: 10.7270/Q2H9952R |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50411730

(CHEMBL257270)Show SMILES CCCC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-2-3-9-19(26)20(23)22-18(21(24)25)14-15-10-12-17(13-11-15)16-7-5-4-6-8-16/h4-8,10-13,18-19,26H,2-3,9,14H2,1H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411606
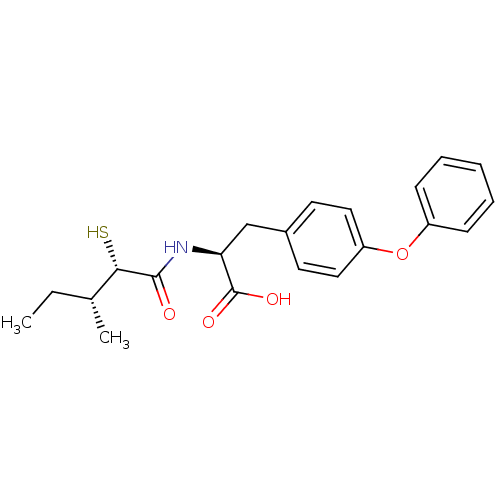
(CHEMBL254282)Show SMILES CC[C@@H](C)[C@H](S)C(=O)N[C@@H](Cc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C21H25NO4S/c1-3-14(2)19(27)20(23)22-18(21(24)25)13-15-9-11-17(12-10-15)26-16-7-5-4-6-8-16/h4-12,14,18-19,27H,3,13H2,1-2H3,(H,22,23)(H,24,25)/t14-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 1681-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.046
BindingDB Entry DOI: 10.7270/Q29K49Z2 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411608

(CHEMBL400527)Show SMILES CC[C@@H](C)[C@H](S)C(=O)N[C@@H](Cc1cccc2ccccc12)C(O)=O Show InChI InChI=1S/C19H23NO3S/c1-3-12(2)17(24)18(21)20-16(19(22)23)11-14-9-6-8-13-7-4-5-10-15(13)14/h4-10,12,16-17,24H,3,11H2,1-2H3,(H,20,21)(H,22,23)/t12-,16+,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 1681-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.046
BindingDB Entry DOI: 10.7270/Q29K49Z2 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411722
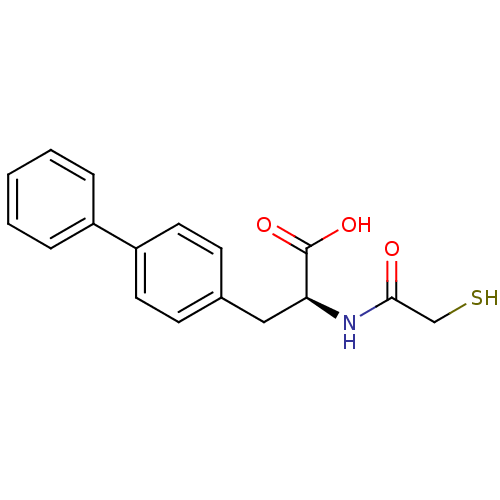
(CHEMBL258333)Show InChI InChI=1S/C17H17NO3S/c19-16(11-22)18-15(17(20)21)10-12-6-8-14(9-7-12)13-4-2-1-3-5-13/h1-9,15,22H,10-11H2,(H,18,19)(H,20,21)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411733

(CHEMBL269997)Show SMILES CC(C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C20H23NO3S/c1-13(2)18(25)19(22)21-17(20(23)24)12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,13,17-18,25H,12H2,1-2H3,(H,21,22)(H,23,24)/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data