

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM13863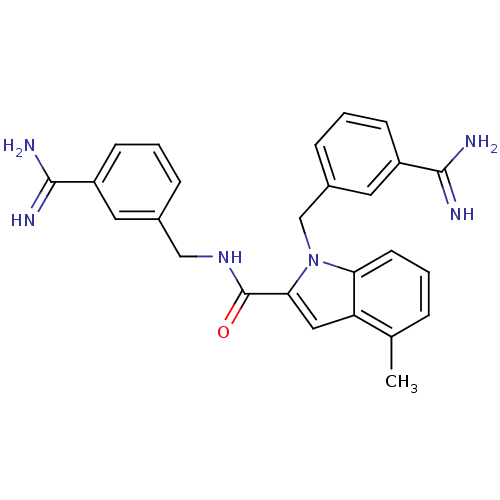 (3-amidinobenzylindole carboxamide 47 | N,1-bis[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13866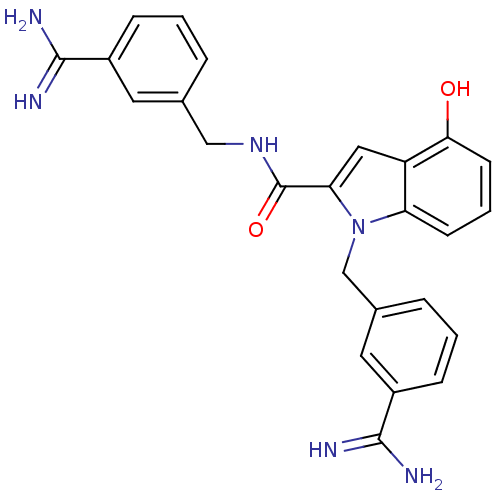 (3-amidinobenzylindole carboxamide 50 | CHEMBL30744...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13861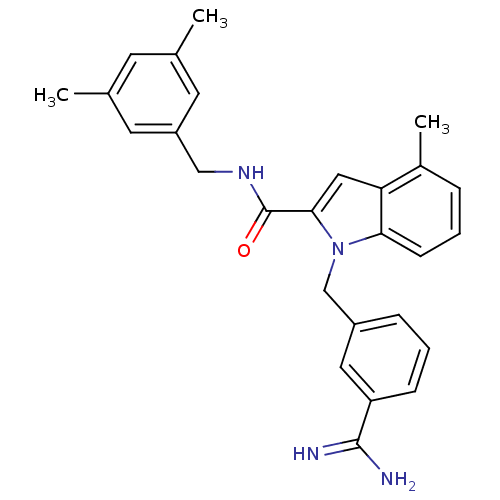 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13825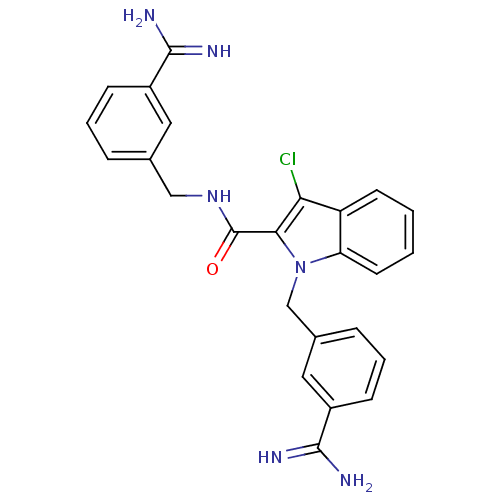 (3-amidinobenzylindole carboxamide 9 | N,1-bis[(3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13862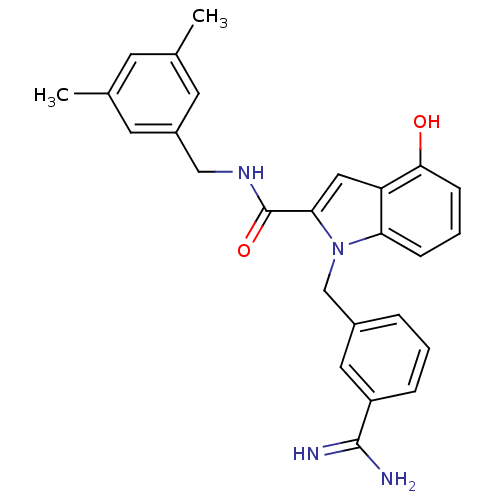 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13880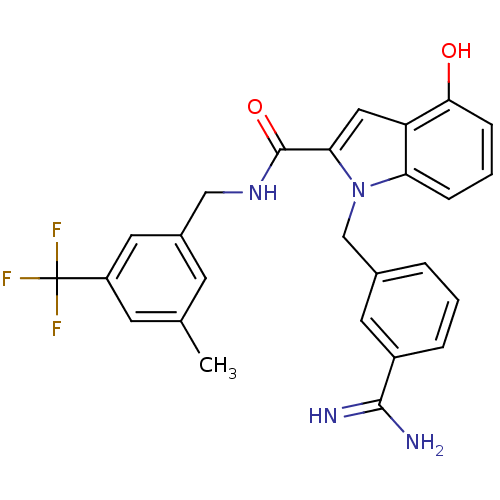 (1-[(3-carbamimidoylphenyl)methyl]-4-hydroxy-N-{[3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13820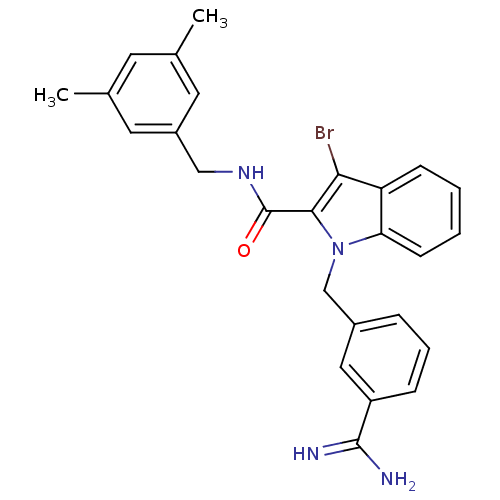 (3-amidinobenzylindole carboxamide 4 | 3-bromo-1-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13898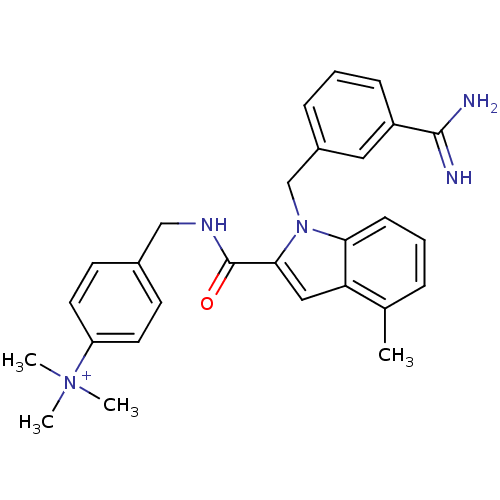 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13869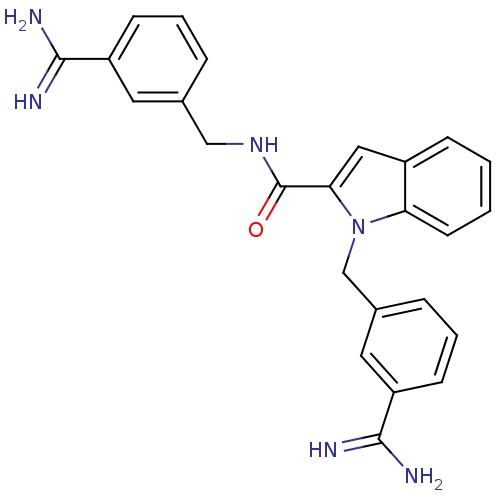 (3-amidinobenzylindole carboxamide 53 | N,1-bis[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13885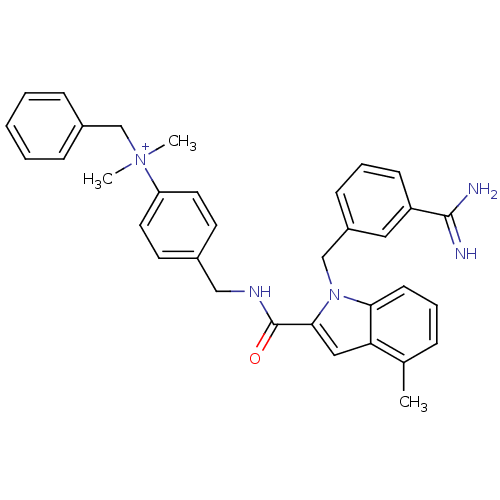 (2,2,2-trifluoroacetate; N-benzyl-4-[({1-[(3-carbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13837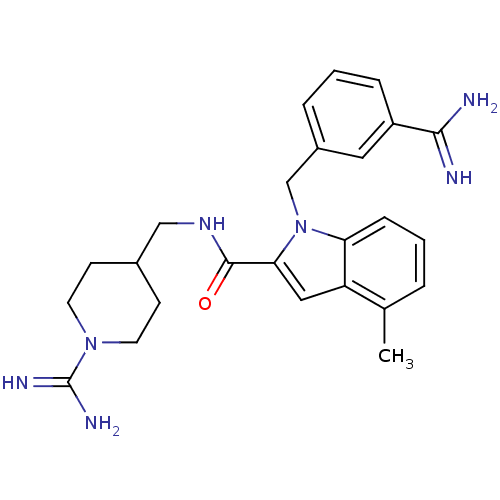 (1-[(3-carbamimidoylphenyl)methyl]-N-[(1-carbamimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12402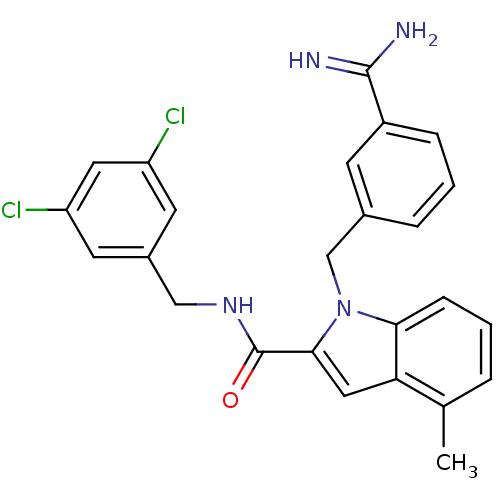 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-dichloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13822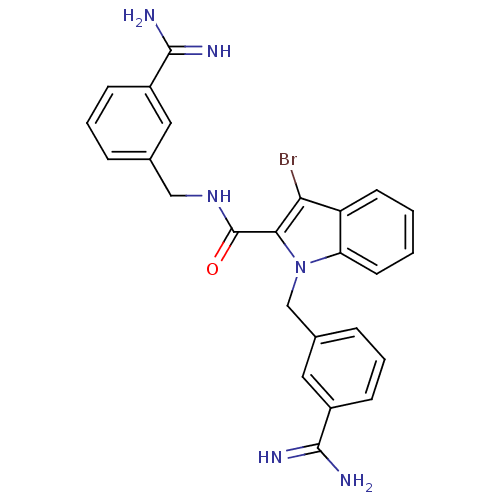 (3-amidinobenzylindole carboxamide 6 | 3-bromo-N,1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13897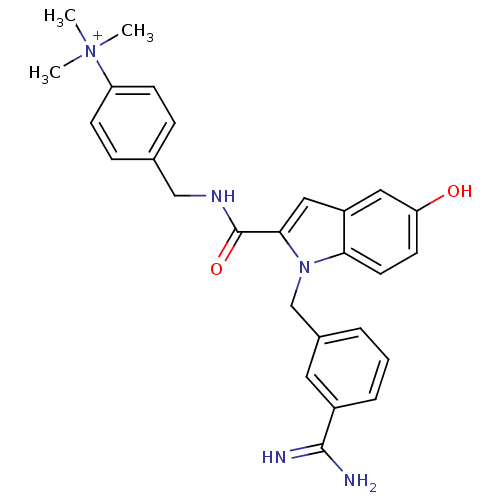 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13874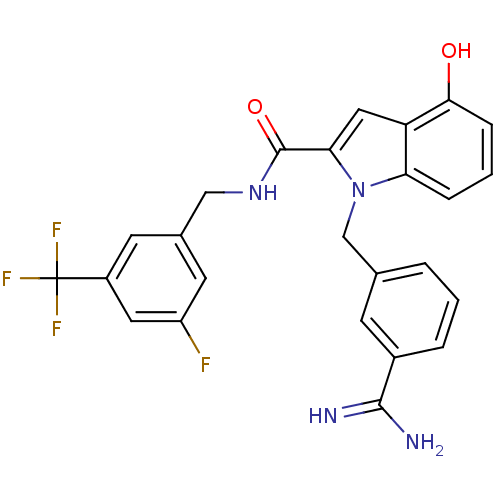 (1-[(3-carbamimidoylphenyl)methyl]-N-{[3-fluoro-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13826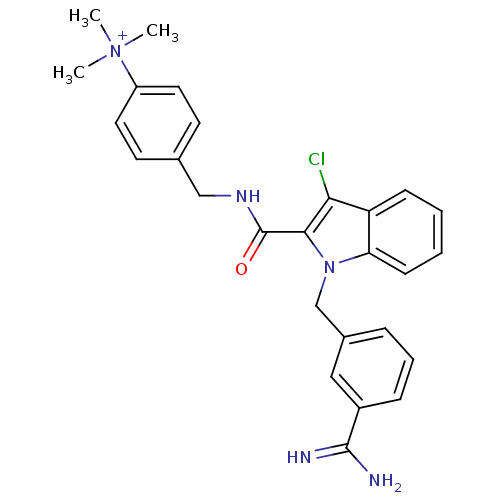 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13877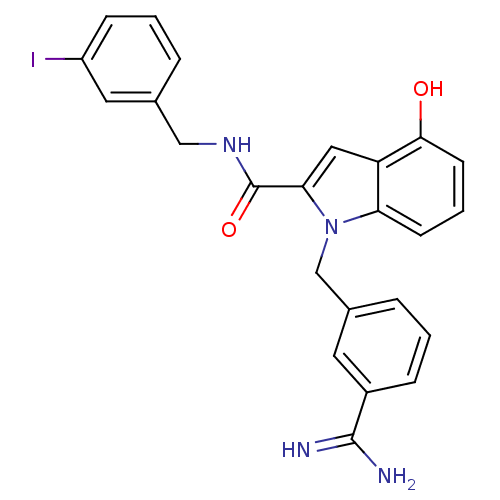 (1-[(3-carbamimidoylphenyl)methyl]-4-hydroxy-N-[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13821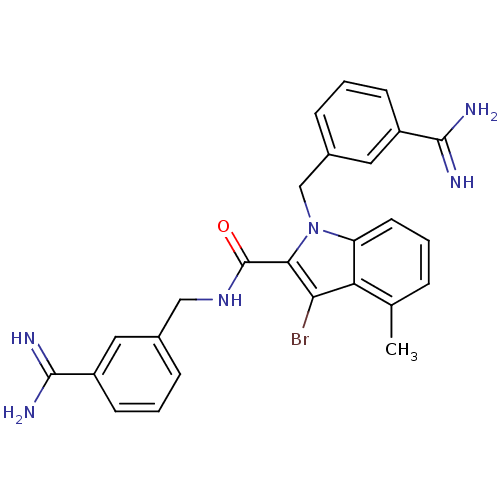 (3-amidinobenzylindole carboxamide 5 | 3-bromo-N,1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13838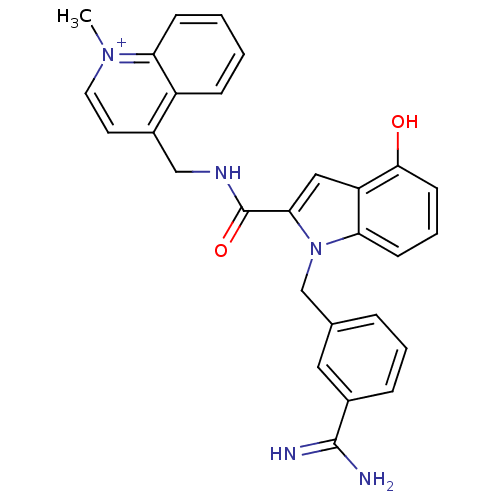 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13865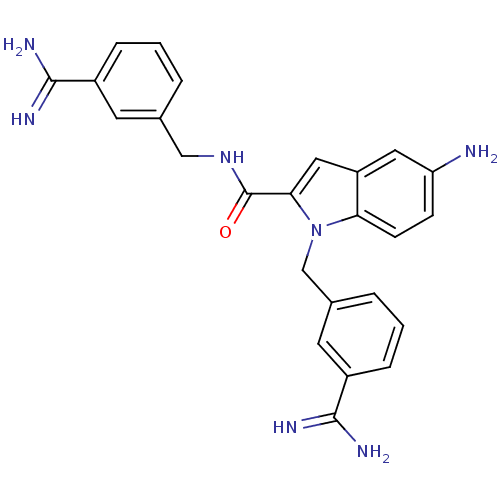 (3-amidinobenzylindole carboxamide 49 | 5-amino-N,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13864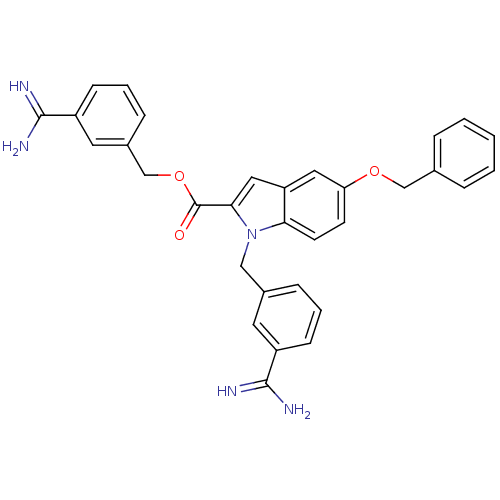 ((3-carbamimidoylphenyl)methyl 5-(benzyloxy)-1-[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13893 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13895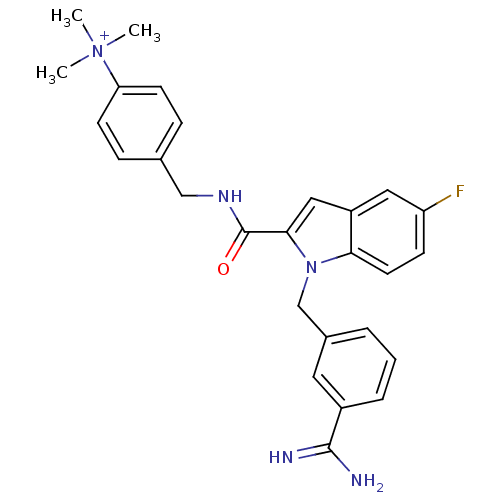 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13840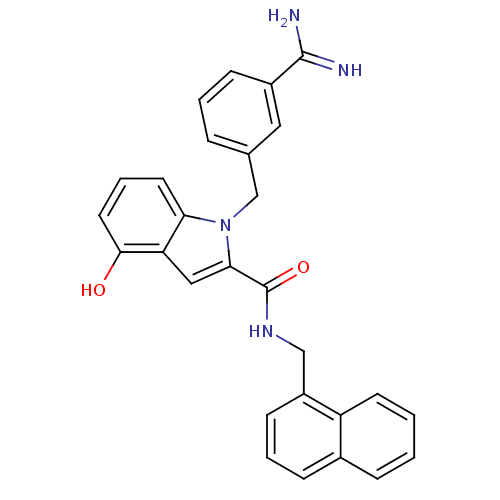 (1-[(3-carbamimidoylphenyl)methyl]-4-hydroxy-N-(nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13890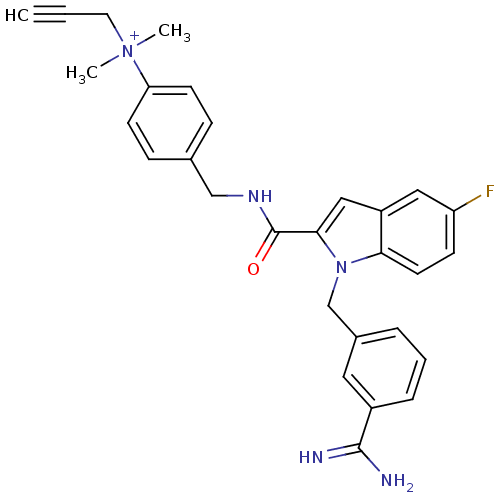 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13867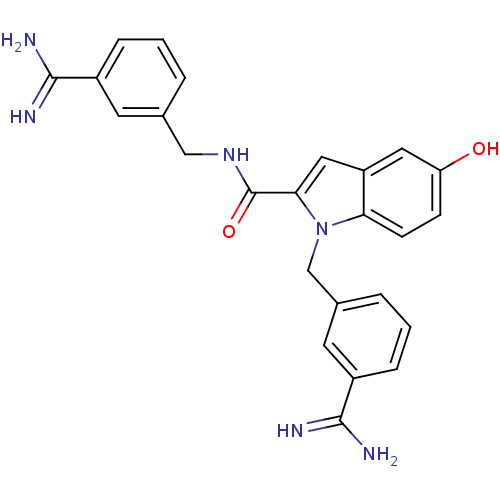 (3-amidinobenzylindole carboxamide 51 | N,1-bis[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13894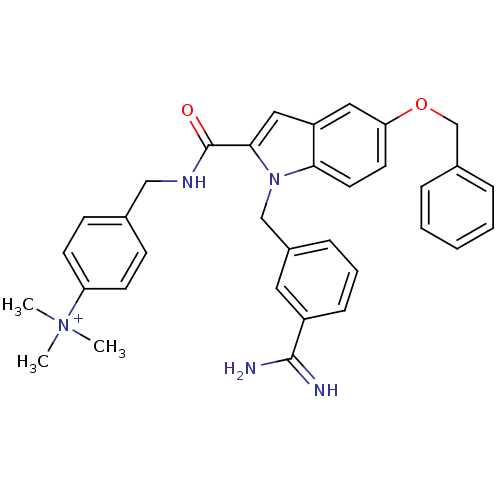 (2,2,2-trifluoroacetate; 4-({[5-(benzyloxy)-1-[(3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13819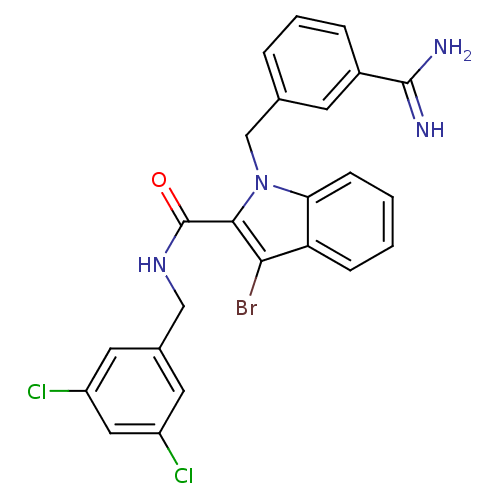 (3-amidinobenzylindole carboxamide 3 | 3-bromo-1-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13891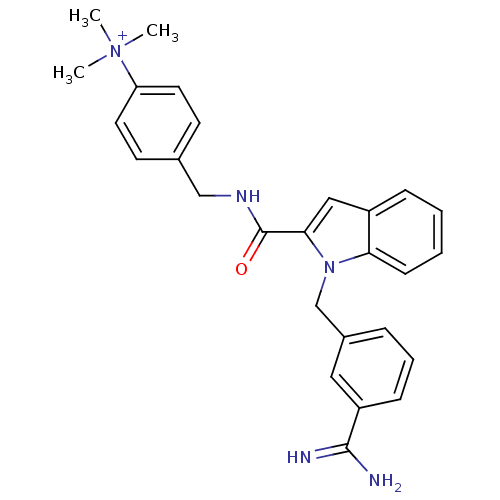 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13834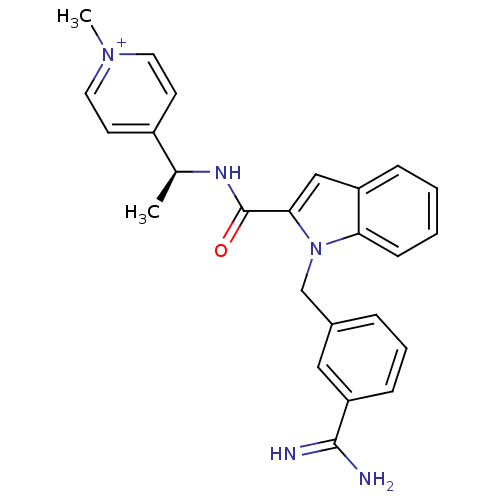 (2,2,2-trifluoroacetate; 4-[(1S)-1-({1-[(3-carbamim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13839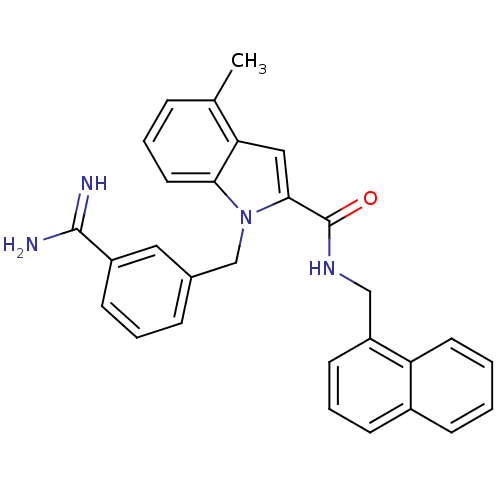 (1-[(3-carbamimidoylphenyl)methyl]-4-methyl-N-(naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13850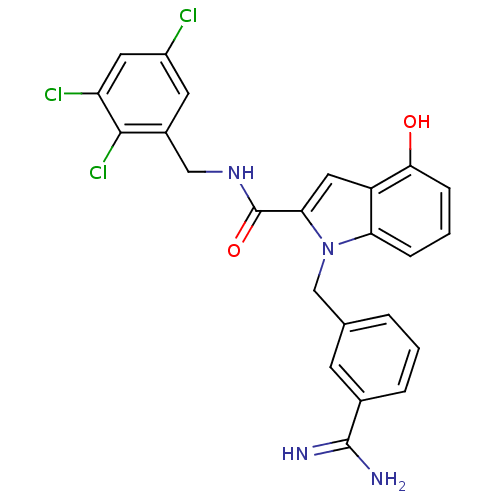 (1-[(3-carbamimidoylphenyl)methyl]-4-hydroxy-N-[(2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13856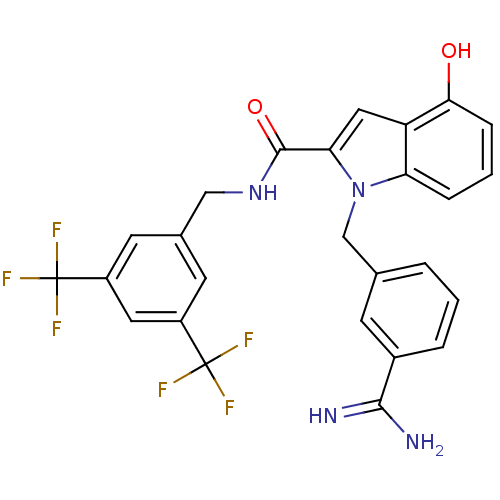 (3-amidinobenzylindole carboxamide 40 | N-{[3,5-bis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13832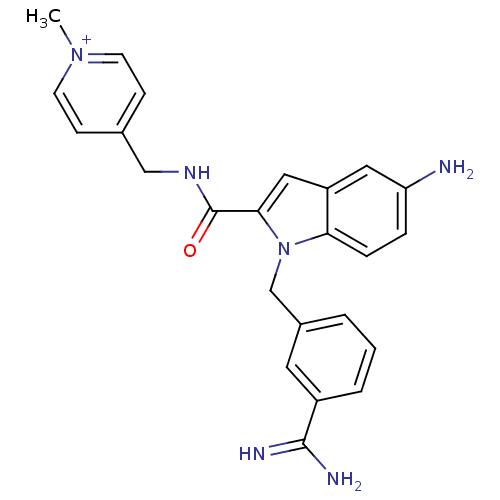 (2,2,2-trifluoroacetate; 4-[({5-amino-1-[(3-carbami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13888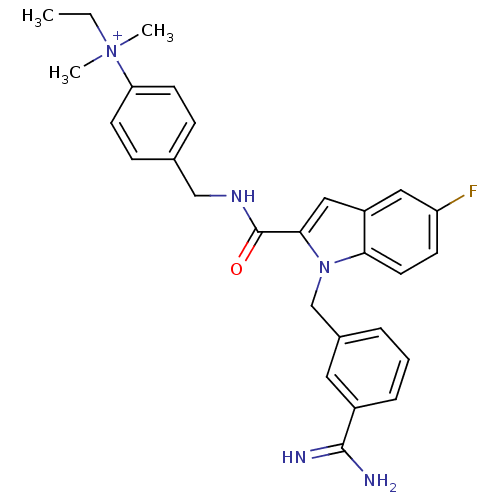 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13833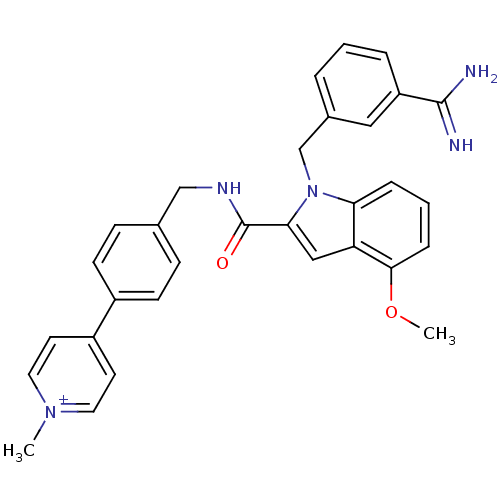 (2,2,2-trifluoroacetate; 4-{4-[({1-[(3-carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13870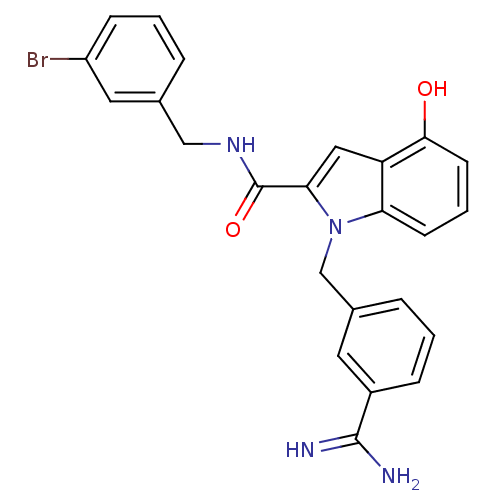 (3-amidinobenzylindole carboxamide 55 | N-[(3-bromo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13881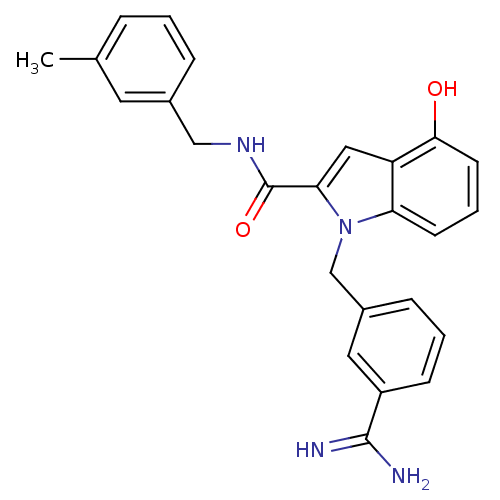 (1-[(3-carbamimidoylphenyl)methyl]-4-hydroxy-N-[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13868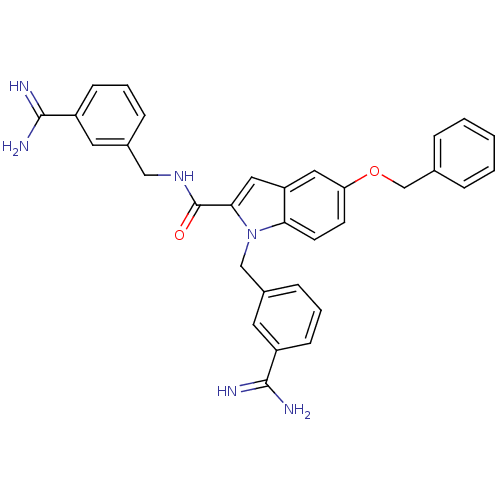 (3-amidinobenzylindole carboxamide 52 | 5-(benzylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13896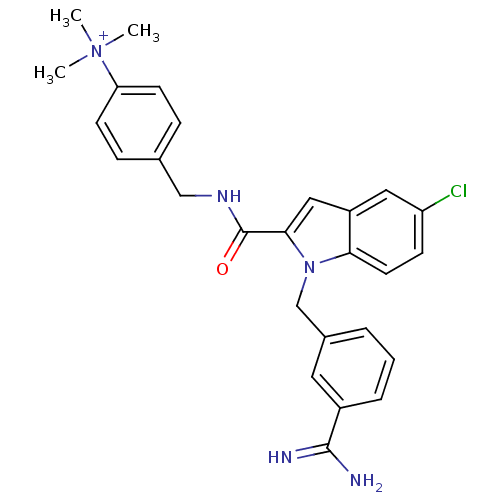 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13889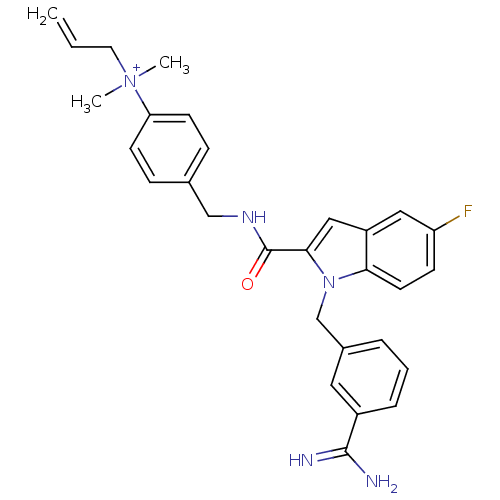 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13899 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13831 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13828 (2,2,2-trifluoroacetate; 4-[1-({1-[(3-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13817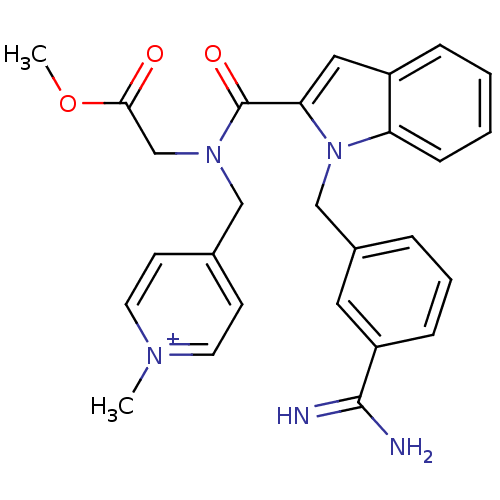 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13823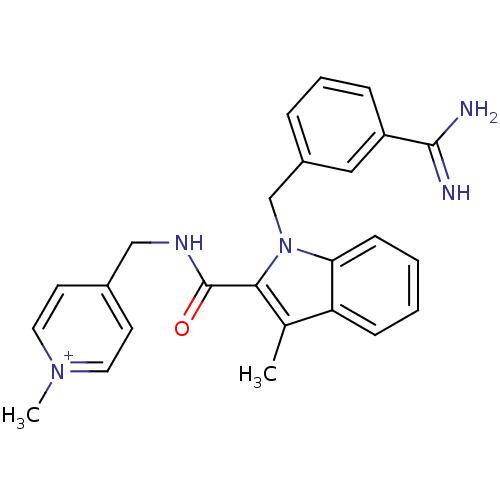 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13871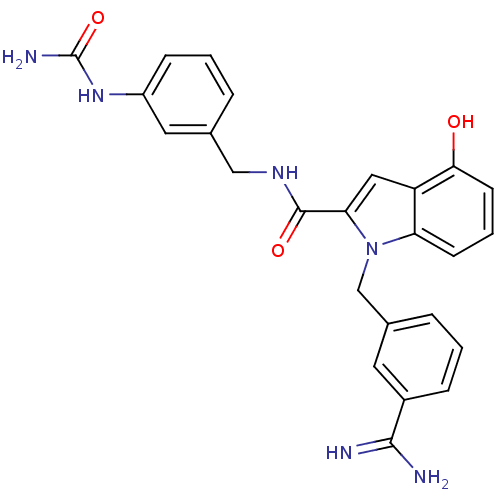 (1-[(3-carbamimidoylphenyl)methyl]-N-{[3-(carbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13859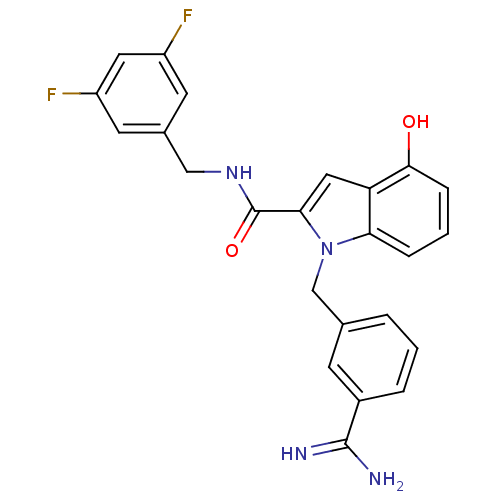 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13860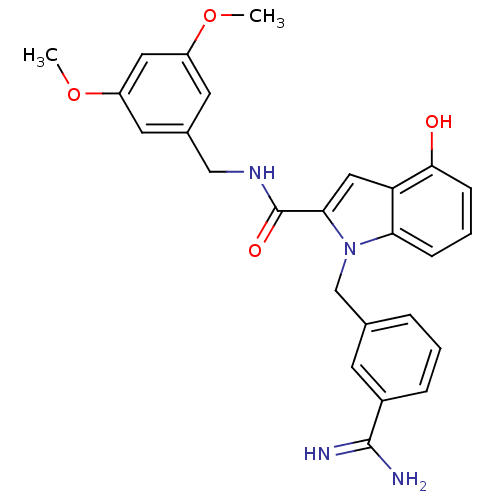 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13872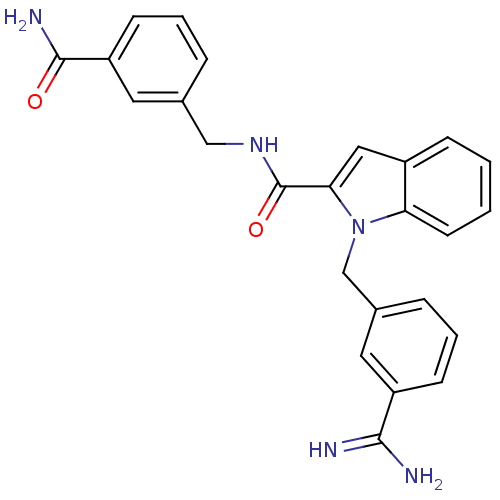 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3-carbamoylp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 201 total ) | Next | Last >> |