 Found 695 hits with Last Name = 'degraffenreid' and Initial = 'm'
Found 695 hits with Last Name = 'degraffenreid' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable cofactor NADPH concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable substrate cortisol concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001577
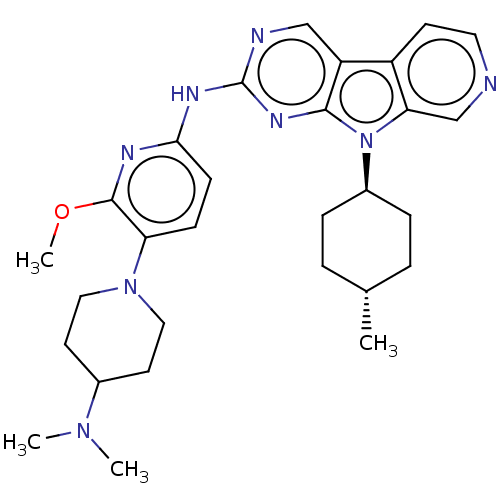
(CHEMBL3237712)Show SMILES COc1nc(Nc2ncc3c4ccncc4n([C@H]4CC[C@H](C)CC4)c3n2)ccc1N1CCC(CC1)N(C)C |r,wU:17.17,wD:20.21,(.78,-65.37,;2.1,-66.15,;3.44,-65.38,;3.44,-63.83,;4.77,-63.06,;4.76,-61.52,;6.09,-60.75,;6.08,-59.22,;7.4,-58.44,;8.75,-59.21,;10.22,-58.73,;10.84,-57.33,;12.37,-57.17,;13.28,-58.42,;12.65,-59.83,;11.12,-59.98,;10.21,-61.23,;10.69,-62.7,;12.2,-63.02,;12.67,-64.49,;11.63,-65.63,;12.1,-67.1,;10.13,-65.3,;9.66,-63.84,;8.74,-60.75,;7.42,-61.52,;6.1,-63.83,;6.11,-65.38,;4.77,-66.15,;4.77,-67.69,;3.43,-68.46,;3.43,-69.99,;4.76,-70.76,;6.09,-70,;6.1,-68.45,;4.75,-72.3,;3.41,-73.07,;6.08,-73.08,)| Show InChI InChI=1S/C29H38N8O/c1-19-5-7-21(8-6-19)37-25-18-30-14-11-22(25)23-17-31-29(34-27(23)37)33-26-10-9-24(28(32-26)38-4)36-15-12-20(13-16-36)35(2)3/h9-11,14,17-21H,5-8,12-13,15-16H2,1-4H3,(H,31,32,33,34)/t19-,21- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM28365
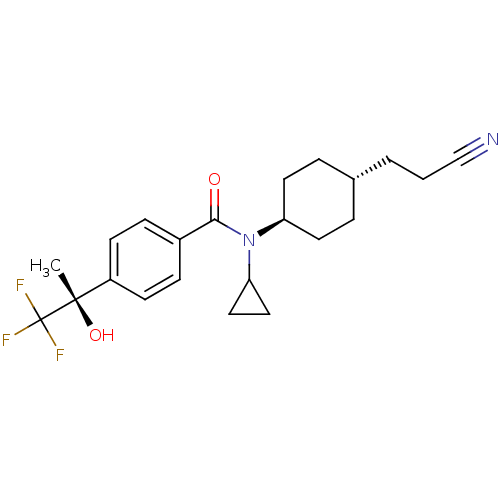
(CHEMBL512355 | N-[4-(2-cyanoethyl)cyclohexyl]-N-cy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@H](CCC#N)CC1)C(F)(F)F |r,wU:15.16,1.0,wD:18.20,1.1,(-12.83,5.36,;-11.49,6.13,;-12.98,6.52,;-10.16,5.36,;-10.16,3.81,;-8.83,3.04,;-7.49,3.81,;-7.49,5.36,;-8.83,6.13,;-6.16,3.05,;-6.16,1.51,;-4.83,3.82,;-4.83,5.36,;-5.57,6.7,;-4.03,6.67,;-3.49,3.05,;-2.16,3.82,;-.82,3.05,;-.82,1.5,;.51,.73,;1.84,1.51,;3.18,.74,;4.51,-.03,;-2.16,.73,;-3.49,1.5,;-11.49,7.67,;-10.16,8.44,;-12.83,8.44,;-11.49,9.21,)| Show InChI InChI=1S/C22H27F3N2O2/c1-21(29,22(23,24)25)17-8-6-16(7-9-17)20(28)27(19-12-13-19)18-10-4-15(5-11-18)3-2-14-26/h6-9,15,18-19,29H,2-5,10-13H2,1H3/t15-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human adipocytes |
J Med Chem 51: 3953-60 (2008)
Article DOI: 10.1021/jm800310g
BindingDB Entry DOI: 10.7270/Q2P84CSZ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001541
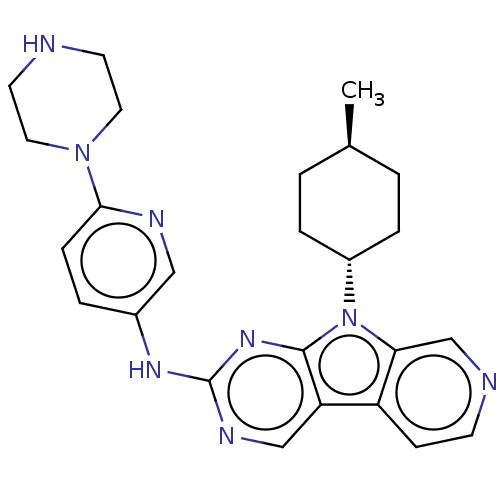
(CHEMBL3237706)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(nc3)N3CCNCC3)nc12 |r,wU:4.7,wD:1.0,(34.74,-35.4,;34.27,-33.94,;35.31,-32.8,;34.84,-31.33,;33.33,-31.01,;32.3,-32.15,;32.77,-33.61,;32.86,-29.55,;33.77,-28.3,;35.3,-28.14,;35.93,-26.73,;35.02,-25.48,;33.49,-25.65,;32.87,-27.05,;31.4,-27.52,;30.05,-26.75,;28.73,-27.53,;28.74,-29.06,;27.41,-29.84,;27.41,-31.38,;28.74,-32.13,;28.75,-33.66,;27.42,-34.45,;26.08,-33.68,;26.08,-32.14,;27.42,-35.99,;26.09,-36.76,;26.1,-38.29,;27.43,-39.06,;28.76,-38.29,;28.76,-36.74,;30.07,-29.83,;31.39,-29.07,)| Show InChI InChI=1S/C25H30N8/c1-17-2-5-19(6-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-18-4-7-23(28-14-18)32-12-10-26-11-13-32/h4,7-9,14-17,19,26H,2-3,5-6,10-13H2,1H3,(H,29,30,31)/t17-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248471
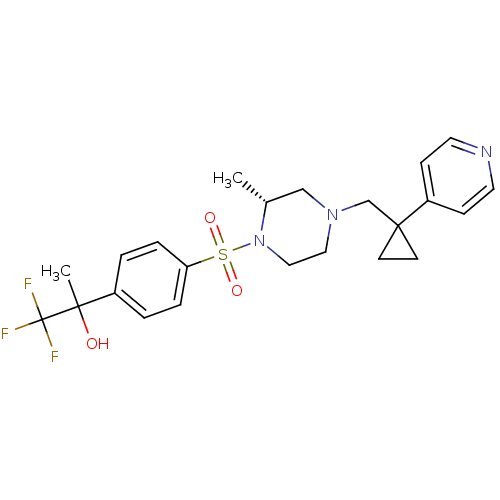
(1,1,1-trifluoro-2-(4-((R)-2-methyl-4-((1-(pyridin-...)Show SMILES C[C@@H]1CN(CC2(CC2)c2ccncc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C23H28F3N3O3S/c1-17-15-28(16-22(9-10-22)19-7-11-27-12-8-19)13-14-29(17)33(31,32)20-5-3-18(4-6-20)21(2,30)23(24,25)26/h3-8,11-12,17,30H,9-10,13-16H2,1-2H3/t17-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248519

(1,1,1-trifluoro-2-(4-((R)-2-methyl-4-((1-(pyridin-...)Show SMILES C[C@@H]1CN(CC2(CCC2)c2cccnc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C24H30F3N3O3S/c1-18-16-29(17-23(10-4-11-23)20-5-3-12-28-15-20)13-14-30(18)34(32,33)21-8-6-19(7-9-21)22(2,31)24(25,26)27/h3,5-9,12,15,18,31H,4,10-11,13-14,16-17H2,1-2H3/t18-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248472
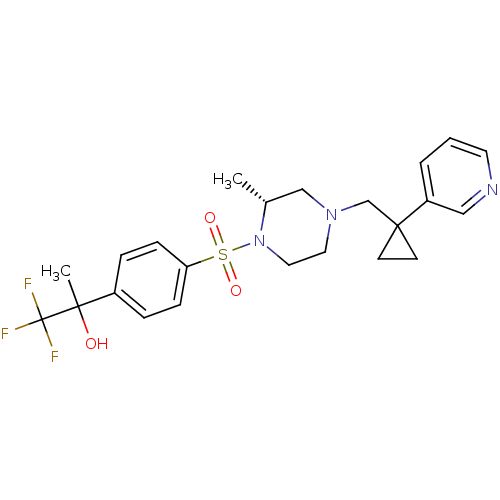
(1,1,1-trifluoro-2-(4-((R)-2-methyl-4-((1-(pyridin-...)Show SMILES C[C@@H]1CN(CC2(CC2)c2cccnc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C23H28F3N3O3S/c1-17-15-28(16-22(9-10-22)19-4-3-11-27-14-19)12-13-29(17)33(31,32)20-7-5-18(6-8-20)21(2,30)23(24,25)26/h3-8,11,14,17,30H,9-10,12-13,15-16H2,1-2H3/t17-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334681

(((1R)-4-(N-cyclopropyl-4-((S)-1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](COC(N)=O)(CC1)c1ccc(F)cc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(.46,-29.77,;1.79,-28.99,;.3,-28.6,;3.13,-29.75,;4.46,-28.98,;5.8,-29.75,;5.79,-31.29,;4.47,-32.06,;3.14,-31.3,;7.13,-32.05,;7.14,-33.59,;8.46,-31.28,;8.46,-29.74,;9.23,-28.41,;7.69,-28.41,;9.8,-32.04,;11.13,-31.27,;12.46,-32.04,;12.46,-33.59,;13.89,-33.03,;15.1,-33.99,;16.53,-33.42,;17.74,-34.39,;16.76,-31.9,;11.13,-34.35,;9.8,-33.59,;13.22,-34.92,;12.44,-36.24,;13.2,-37.57,;14.73,-37.58,;15.49,-38.92,;15.5,-36.25,;14.74,-34.92,;1.78,-27.45,;3.11,-26.67,;.44,-26.68,;1.38,-25.96,)| Show InChI InChI=1S/C27H30F4N2O4/c1-25(36,27(29,30)31)18-4-2-17(3-5-18)23(34)33(21-10-11-21)22-12-14-26(15-13-22,16-37-24(32)35)19-6-8-20(28)9-7-19/h2-9,21-22,36H,10-16H2,1H3,(H2,32,35)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331363

((S)-3'-cyclopropyl-4'-(4-(1,1,1-trifluoro-2-hydrox...)Show SMILES C[C@](O)(c1ccc(cc1)S(=O)(=O)c1ccc(cc1C1CC1)-c1ccc(cc1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C25H20F3NO3S/c1-24(30,25(26,27)28)20-9-11-21(12-10-20)33(31,32)23-13-8-19(14-22(23)18-6-7-18)17-4-2-16(15-29)3-5-17/h2-5,8-14,18,30H,6-7H2,1H3/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7071-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.097
BindingDB Entry DOI: 10.7270/Q21G0MHX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334680
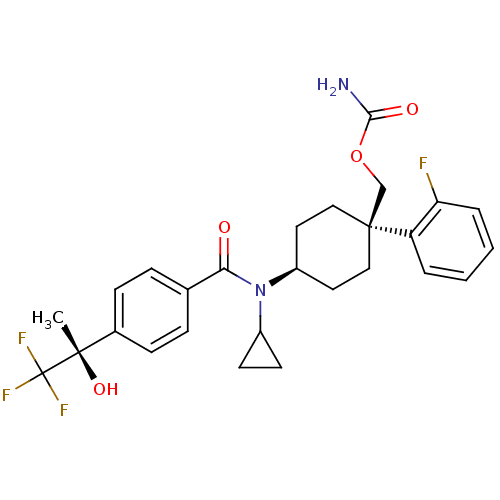
(((1R)-4-(N-cyclopropyl-4-((S)-1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](COC(N)=O)(CC1)c1ccccc1F)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(10.24,-34.27,;11.57,-33.49,;10.08,-33.1,;12.91,-34.25,;14.24,-33.48,;15.58,-34.25,;15.57,-35.79,;14.25,-36.56,;12.92,-35.8,;16.91,-36.55,;16.91,-38.09,;18.24,-35.78,;18.24,-34.24,;19.01,-32.91,;17.46,-32.91,;19.58,-36.54,;20.91,-35.77,;22.24,-36.54,;22.24,-38.09,;23.66,-37.51,;24.88,-38.45,;26.31,-37.87,;27.53,-38.82,;26.52,-36.34,;20.91,-38.85,;19.58,-38.09,;23,-39.42,;24.52,-39.42,;25.28,-40.75,;24.51,-42.08,;22.97,-42.07,;22.22,-40.74,;20.68,-40.73,;11.56,-31.95,;12.89,-31.17,;10.22,-31.18,;11.16,-30.46,)| Show InChI InChI=1S/C27H30F4N2O4/c1-25(36,27(29,30)31)18-8-6-17(7-9-18)23(34)33(19-10-11-19)20-12-14-26(15-13-20,16-37-24(32)35)21-4-2-3-5-22(21)28/h2-9,19-20,36H,10-16H2,1H3,(H2,32,35)/t20-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248521

(1,1,1-trifluoro-2-(4-((R)-2-methyl-4-((1-(pyridin-...)Show SMILES C[C@@H]1CN(CC2(CCCC2)c2cccnc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C25H32F3N3O3S/c1-19-17-30(18-24(11-3-4-12-24)21-6-5-13-29-16-21)14-15-31(19)35(33,34)22-9-7-20(8-10-22)23(2,32)25(26,27)28/h5-10,13,16,19,32H,3-4,11-12,14-15,17-18H2,1-2H3/t19-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001583
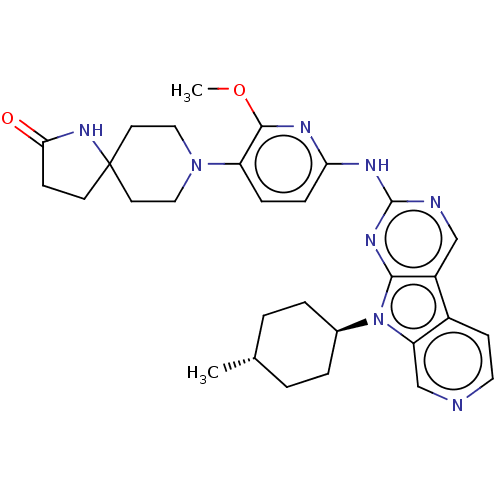
(CHEMBL3237718)Show SMILES COc1nc(Nc2ncc3c4ccncc4n([C@H]4CC[C@H](C)CC4)c3n2)ccc1N1CCC2(CCC(=O)N2)CC1 |r,wU:17.17,wD:20.21,(36.2,-55.73,;37.53,-56.52,;38.87,-55.76,;38.89,-54.23,;40.23,-53.48,;40.24,-51.95,;41.57,-51.18,;41.56,-49.64,;42.89,-48.87,;44.23,-49.63,;45.7,-49.16,;46.33,-47.76,;47.86,-47.59,;48.77,-48.84,;48.14,-50.25,;46.6,-50.41,;45.7,-51.66,;46.17,-53.12,;47.67,-53.44,;48.14,-54.91,;47.11,-56.05,;47.58,-57.52,;45.6,-55.72,;45.13,-54.26,;44.23,-51.18,;42.9,-51.95,;41.54,-54.25,;41.53,-55.78,;40.19,-56.54,;40.18,-58.07,;38.84,-58.83,;38.82,-60.36,;40.14,-61.15,;38.9,-62.06,;39.37,-63.52,;40.91,-63.52,;41.82,-64.77,;41.39,-62.06,;41.49,-60.4,;41.51,-58.85,)| Show InChI InChI=1S/C30H36N8O2/c1-19-3-5-20(6-4-19)38-24-18-31-14-10-21(24)22-17-32-29(35-27(22)38)34-25-8-7-23(28(33-25)40-2)37-15-12-30(13-16-37)11-9-26(39)36-30/h7-8,10,14,17-20H,3-6,9,11-13,15-16H2,1-2H3,(H,36,39)(H,32,33,34,35)/t19-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001576
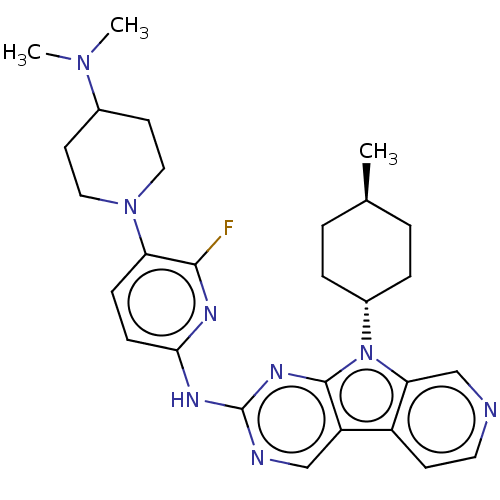
(CHEMBL3237711)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(N4CCC(CC4)N(C)C)c(F)n3)nc12 |r,wU:4.7,wD:1.0,(48.6,-50.76,;48.13,-49.29,;49.17,-48.15,;48.7,-46.68,;47.19,-46.36,;46.16,-47.5,;46.63,-48.96,;46.72,-44.9,;47.63,-43.65,;49.16,-43.49,;49.79,-42.08,;48.88,-40.83,;47.35,-40.99,;46.73,-42.4,;45.26,-42.87,;43.91,-42.1,;42.58,-42.88,;42.6,-44.41,;41.27,-45.19,;41.25,-46.72,;42.56,-47.49,;42.55,-49.02,;41.22,-49.78,;41.2,-51.31,;39.86,-52.07,;39.84,-53.6,;41.17,-54.39,;42.51,-53.63,;42.53,-52.09,;41.15,-55.93,;39.8,-56.68,;42.47,-56.72,;39.9,-49,;38.55,-49.76,;39.91,-47.47,;43.93,-45.18,;45.25,-44.42,)| Show InChI InChI=1S/C28H35FN8/c1-18-4-6-20(7-5-18)37-24-17-30-13-10-21(24)22-16-31-28(34-27(22)37)33-25-9-8-23(26(29)32-25)36-14-11-19(12-15-36)35(2)3/h8-10,13,16-20H,4-7,11-12,14-15H2,1-3H3,(H,31,32,33,34)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248518
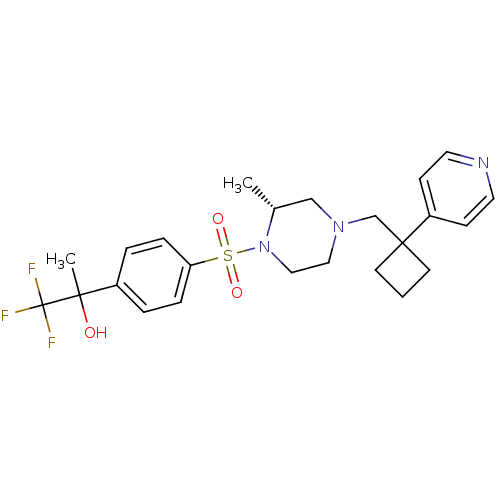
(1,1,1-trifluoro-2-(4-((R)-2-methyl-4-((1-(pyridin-...)Show SMILES C[C@@H]1CN(CC2(CCC2)c2ccncc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C24H30F3N3O3S/c1-18-16-29(17-23(10-3-11-23)20-8-12-28-13-9-20)14-15-30(18)34(32,33)21-6-4-19(5-7-21)22(2,31)24(25,26)27/h4-9,12-13,18,31H,3,10-11,14-17H2,1-2H3/t18-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM28365

(CHEMBL512355 | N-[4-(2-cyanoethyl)cyclohexyl]-N-cy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@H](CCC#N)CC1)C(F)(F)F |r,wU:15.16,1.0,wD:18.20,1.1,(-12.83,5.36,;-11.49,6.13,;-12.98,6.52,;-10.16,5.36,;-10.16,3.81,;-8.83,3.04,;-7.49,3.81,;-7.49,5.36,;-8.83,6.13,;-6.16,3.05,;-6.16,1.51,;-4.83,3.82,;-4.83,5.36,;-5.57,6.7,;-4.03,6.67,;-3.49,3.05,;-2.16,3.82,;-.82,3.05,;-.82,1.5,;.51,.73,;1.84,1.51,;3.18,.74,;4.51,-.03,;-2.16,.73,;-3.49,1.5,;-11.49,7.67,;-10.16,8.44,;-12.83,8.44,;-11.49,9.21,)| Show InChI InChI=1S/C22H27F3N2O2/c1-21(29,22(23,24)25)17-8-6-16(7-9-17)20(28)27(19-12-13-19)18-10-4-15(5-11-18)3-2-14-26/h6-9,15,18-19,29H,2-5,10-13H2,1H3/t15-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cloned 11beta-HSD1 by scintillation proximity assay |
J Med Chem 51: 3953-60 (2008)
Article DOI: 10.1021/jm800310g
BindingDB Entry DOI: 10.7270/Q2P84CSZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50243635

((S)-N-[trans-4-(4-Cyanophenyl)cyclohexyl]-4-[1,1,1...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@H](CC1)c1ccc(cc1)C#N)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.23,(-8.78,-33.82,;-8.02,-32.49,;-9.36,-31.72,;-6.68,-33.26,;-6.69,-34.8,;-5.35,-35.57,;-4.01,-34.8,;-4.02,-33.25,;-5.35,-32.49,;-2.68,-35.57,;-2.68,-37.11,;-1.35,-34.8,;-1.35,-33.26,;-.59,-31.92,;-2.13,-31.92,;-.01,-35.57,;1.31,-34.79,;2.65,-35.57,;2.65,-37.11,;1.31,-37.88,;-.02,-37.11,;3.97,-37.88,;3.97,-39.42,;5.3,-40.19,;6.63,-39.43,;6.63,-37.88,;5.3,-37.11,;7.97,-40.2,;9.29,-40.97,;-7.25,-31.15,;-6.49,-29.81,;-5.91,-31.92,;-8.59,-30.39,)| Show InChI InChI=1S/C26H27F3N2O2/c1-25(33,26(27,28)29)21-10-6-20(7-11-21)24(32)31(23-14-15-23)22-12-8-19(9-13-22)18-4-2-17(16-30)3-5-18/h2-7,10-11,19,22-23,33H,8-9,12-15H2,1H3/t19-,22-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cloned 11beta-HSD1 by scintillation proximity assay |
J Med Chem 51: 3953-60 (2008)
Article DOI: 10.1021/jm800310g
BindingDB Entry DOI: 10.7270/Q2P84CSZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50243635

((S)-N-[trans-4-(4-Cyanophenyl)cyclohexyl]-4-[1,1,1...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@H](CC1)c1ccc(cc1)C#N)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.23,(-8.78,-33.82,;-8.02,-32.49,;-9.36,-31.72,;-6.68,-33.26,;-6.69,-34.8,;-5.35,-35.57,;-4.01,-34.8,;-4.02,-33.25,;-5.35,-32.49,;-2.68,-35.57,;-2.68,-37.11,;-1.35,-34.8,;-1.35,-33.26,;-.59,-31.92,;-2.13,-31.92,;-.01,-35.57,;1.31,-34.79,;2.65,-35.57,;2.65,-37.11,;1.31,-37.88,;-.02,-37.11,;3.97,-37.88,;3.97,-39.42,;5.3,-40.19,;6.63,-39.43,;6.63,-37.88,;5.3,-37.11,;7.97,-40.2,;9.29,-40.97,;-7.25,-31.15,;-6.49,-29.81,;-5.91,-31.92,;-8.59,-30.39,)| Show InChI InChI=1S/C26H27F3N2O2/c1-25(33,26(27,28)29)21-10-6-20(7-11-21)24(32)31(23-14-15-23)22-12-8-19(9-13-22)18-4-2-17(16-30)3-5-18/h2-7,10-11,19,22-23,33H,8-9,12-15H2,1H3/t19-,22-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50243632

((S)-N-Cyclohexyl-4-(1,1,1-trifluoro-2-hydroxypropa...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)C1CCCCC1)C(F)(F)F |r| Show InChI InChI=1S/C19H24F3NO2/c1-18(25,19(20,21)22)14-9-7-13(8-10-14)17(24)23(16-11-12-16)15-5-3-2-4-6-15/h7-10,15-16,25H,2-6,11-12H2,1H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cloned 11beta-HSD1 by scintillation proximity assay |
J Med Chem 51: 3953-60 (2008)
Article DOI: 10.1021/jm800310g
BindingDB Entry DOI: 10.7270/Q2P84CSZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334673

(CHEMBL1642593 | N-((4S)-4-(3-amino-3-oxopropyl)-4-...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CCC(N)=O)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(8.32,-10.39,;9.65,-9.61,;8.16,-9.22,;10.99,-10.38,;12.32,-9.6,;13.66,-10.37,;13.66,-11.91,;12.34,-12.68,;11,-11.93,;14.99,-12.67,;15,-14.22,;16.33,-11.9,;16.32,-10.36,;17.09,-9.03,;15.55,-9.03,;17.66,-12.67,;18.99,-11.89,;20.32,-12.67,;20.32,-14.21,;21.65,-13.43,;22.99,-14.19,;24.32,-13.41,;25.66,-14.17,;24.31,-11.87,;18.99,-14.97,;17.66,-14.21,;21.08,-15.54,;20.3,-16.86,;21.06,-18.19,;22.59,-18.2,;23.36,-16.87,;22.6,-15.55,;9.65,-8.07,;10.98,-7.29,;8.31,-7.3,;9.24,-6.58,)| Show InChI InChI=1S/C28H33F3N2O3/c1-26(36,28(29,30)31)20-9-7-19(8-10-20)25(35)33(22-11-12-22)23-13-16-27(17-14-23,18-15-24(32)34)21-5-3-2-4-6-21/h2-10,22-23,36H,11-18H2,1H3,(H2,32,34)/t23-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248520

(1,1,1-trifluoro-2-(4-((R)-2-methyl-4-((1-(pyridin-...)Show SMILES C[C@@H]1CN(CC2(CCCC2)c2ccncc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C25H32F3N3O3S/c1-19-17-30(18-24(11-3-4-12-24)21-9-13-29-14-10-21)15-16-31(19)35(33,34)22-7-5-20(6-8-22)23(2,32)25(26,27)28/h5-10,13-14,19,32H,3-4,11-12,15-18H2,1-2H3/t19-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7071-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.097
BindingDB Entry DOI: 10.7270/Q21G0MHX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331361
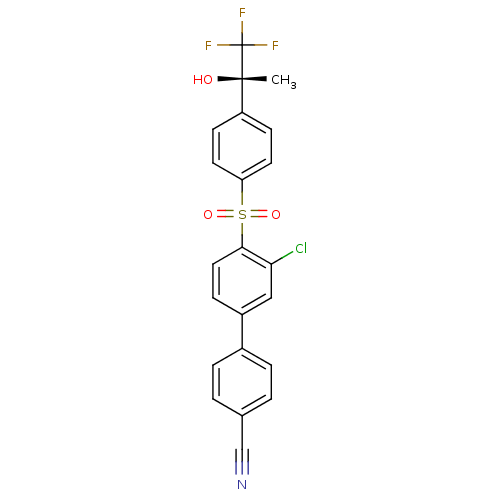
((S)-3'-chloro-4'-(4-(1,1,1-trifluoro-2-hydroxyprop...)Show SMILES C[C@](O)(c1ccc(cc1)S(=O)(=O)c1ccc(cc1Cl)-c1ccc(cc1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C22H15ClF3NO3S/c1-21(28,22(24,25)26)17-7-9-18(10-8-17)31(29,30)20-11-6-16(12-19(20)23)15-4-2-14(13-27)3-5-15/h2-12,28H,1H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7071-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.097
BindingDB Entry DOI: 10.7270/Q21G0MHX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248469
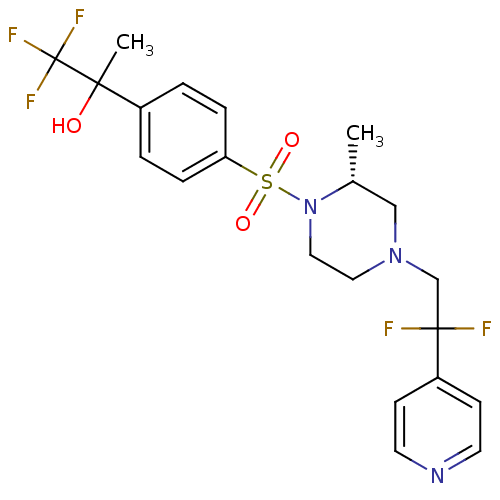
(2-(4-((R)-4-(2,2-difluoro-2-(pyridin-4-yl)ethyl)-2...)Show SMILES C[C@@H]1CN(CC(F)(F)c2ccncc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C21H24F5N3O3S/c1-15-13-28(14-20(22,23)17-7-9-27-10-8-17)11-12-29(15)33(31,32)18-5-3-16(4-6-18)19(2,30)21(24,25)26/h3-10,15,30H,11-14H2,1-2H3/t15-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334675
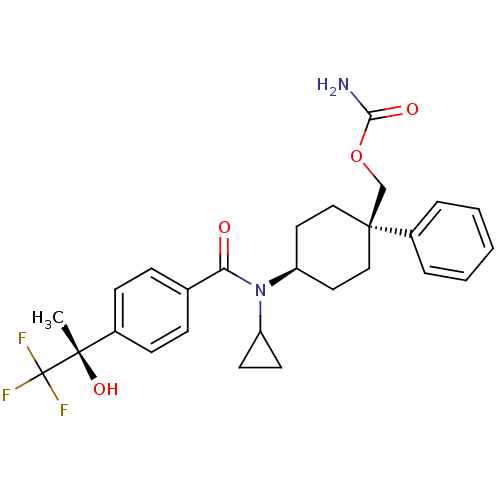
(((1R)-4-(N-cyclopropyl-4-((S)-1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](COC(N)=O)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(-5.93,-6.52,;-4.6,-5.74,;-6.09,-5.35,;-3.26,-6.51,;-1.92,-5.73,;-.59,-6.5,;-.59,-8.04,;-1.91,-8.82,;-3.25,-8.06,;.75,-8.81,;.75,-10.35,;2.08,-8.03,;2.08,-6.49,;2.84,-5.16,;1.3,-5.16,;3.42,-8.8,;4.75,-8.02,;6.08,-8.8,;6.08,-10.34,;7.56,-9.94,;7.95,-8.44,;9.44,-8.04,;9.83,-6.55,;10.53,-9.12,;4.75,-11.1,;3.42,-10.34,;6.84,-11.67,;6.06,-12.99,;6.81,-14.32,;8.35,-14.33,;9.12,-13,;8.36,-11.68,;-4.6,-4.2,;-3.27,-3.42,;-5.94,-3.44,;-5.01,-2.71,)| Show InChI InChI=1S/C27H31F3N2O4/c1-25(35,27(28,29)30)19-9-7-18(8-10-19)23(33)32(21-11-12-21)22-13-15-26(16-14-22,17-36-24(31)34)20-5-3-2-4-6-20/h2-10,21-22,35H,11-17H2,1H3,(H2,31,34)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001576

(CHEMBL3237711)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(N4CCC(CC4)N(C)C)c(F)n3)nc12 |r,wU:4.7,wD:1.0,(48.6,-50.76,;48.13,-49.29,;49.17,-48.15,;48.7,-46.68,;47.19,-46.36,;46.16,-47.5,;46.63,-48.96,;46.72,-44.9,;47.63,-43.65,;49.16,-43.49,;49.79,-42.08,;48.88,-40.83,;47.35,-40.99,;46.73,-42.4,;45.26,-42.87,;43.91,-42.1,;42.58,-42.88,;42.6,-44.41,;41.27,-45.19,;41.25,-46.72,;42.56,-47.49,;42.55,-49.02,;41.22,-49.78,;41.2,-51.31,;39.86,-52.07,;39.84,-53.6,;41.17,-54.39,;42.51,-53.63,;42.53,-52.09,;41.15,-55.93,;39.8,-56.68,;42.47,-56.72,;39.9,-49,;38.55,-49.76,;39.91,-47.47,;43.93,-45.18,;45.25,-44.42,)| Show InChI InChI=1S/C28H35FN8/c1-18-4-6-20(7-5-18)37-24-17-30-13-10-21(24)22-16-31-28(34-27(22)37)33-25-9-8-23(26(29)32-25)36-14-11-19(12-15-36)35(2)3/h8-10,13,16-20H,4-7,11-12,14-15H2,1-3H3,(H,31,32,33,34)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001539
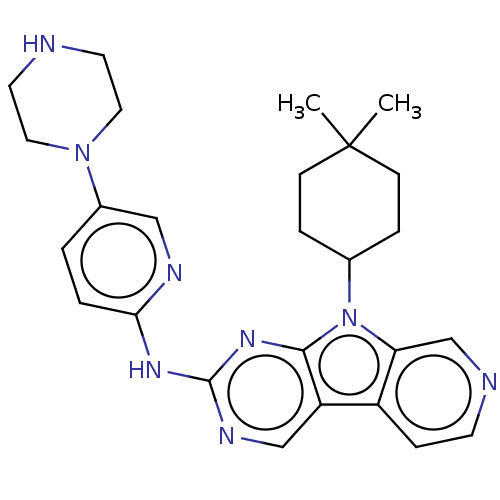
(CHEMBL3237704 | US8841312, 204)Show SMILES CC1(C)CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 Show InChI InChI=1S/C26H32N8/c1-26(2)8-5-18(6-9-26)34-22-17-28-10-7-20(22)21-16-30-25(32-24(21)34)31-23-4-3-19(15-29-23)33-13-11-27-12-14-33/h3-4,7,10,15-18,27H,5-6,8-9,11-14H2,1-2H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248310
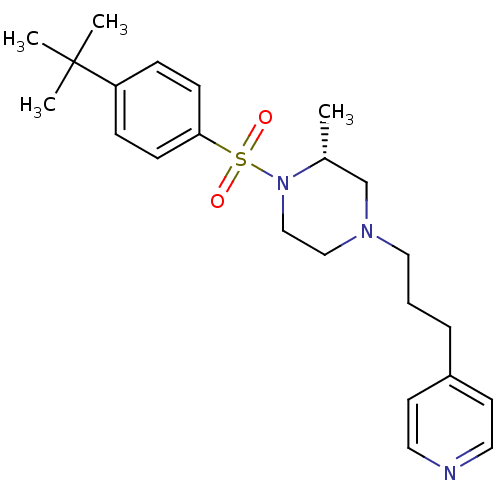
((R)-1-(4-tert-butylphenylsulfonyl)-2-methyl-4-(3-(...)Show SMILES C[C@@H]1CN(CCCc2ccncc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C23H33N3O2S/c1-19-18-25(15-5-6-20-11-13-24-14-12-20)16-17-26(19)29(27,28)22-9-7-21(8-10-22)23(2,3)4/h7-14,19H,5-6,15-18H2,1-4H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001537
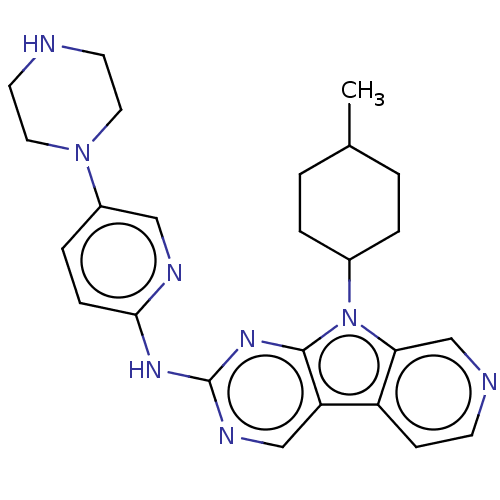
(CHEMBL3237702 | US8841312, 55)Show SMILES CC1CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 |(35.98,-56.71,;35.51,-55.25,;34.01,-54.92,;33.54,-53.46,;34.57,-52.32,;36.08,-52.63,;36.55,-54.1,;34.1,-50.85,;35.01,-49.6,;36.54,-49.45,;37.17,-48.04,;36.26,-46.79,;34.73,-46.95,;34.11,-48.35,;32.64,-48.83,;31.29,-48.06,;29.97,-48.84,;29.98,-50.37,;28.65,-51.14,;28.65,-52.68,;29.99,-53.45,;29.99,-55,;28.66,-55.77,;27.32,-55,;27.33,-53.45,;28.66,-57.31,;27.32,-58.08,;27.31,-59.61,;28.64,-60.38,;29.98,-59.62,;29.99,-58.07,;31.31,-51.14,;32.63,-50.37,)| Show InChI InChI=1S/C25H30N8/c1-17-2-4-18(5-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-23-7-6-19(14-28-23)32-12-10-26-11-13-32/h6-9,14-18,26H,2-5,10-13H2,1H3,(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112464
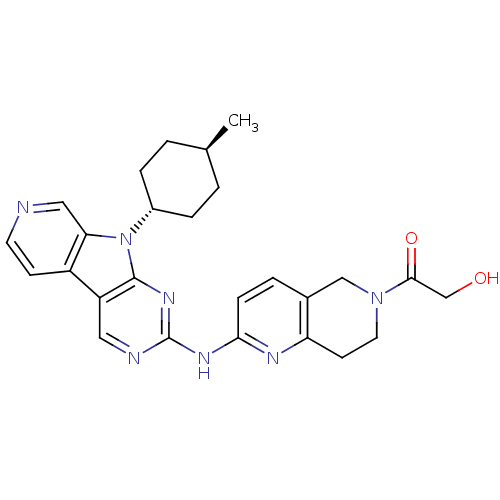
(US8623885, 5)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CN(CCc4n3)C(=O)CO)nc12 |r,wU:4.7,wD:1.0,(4.13,-3.21,;3.73,-1.73,;4.82,-.64,;4.42,.85,;2.93,1.25,;1.84,.16,;2.24,-1.33,;2.53,2.74,;3.44,3.98,;4.97,4.14,;5.6,5.55,;4.69,6.8,;3.16,6.64,;2.53,5.23,;1.07,4.75,;-.26,5.52,;-1.6,4.75,;-1.6,3.21,;-2.93,2.44,;-2.93,.9,;-1.6,.13,;-1.6,-1.41,;-2.93,-2.18,;-2.93,-3.72,;-4.26,-4.49,;-5.6,-3.72,;-5.6,-2.18,;-4.26,-1.41,;-4.26,.13,;-4.26,-6.03,;-5.6,-6.8,;-2.93,-6.8,;-1.6,-6.03,;-.26,2.44,;1.07,3.21,)| Show InChI InChI=1S/C26H29N7O2/c1-16-2-5-18(6-3-16)33-22-13-27-10-8-19(22)20-12-28-26(31-25(20)33)30-23-7-4-17-14-32(24(35)15-34)11-9-21(17)29-23/h4,7-8,10,12-13,16,18,34H,2-3,5-6,9,11,14-15H2,1H3,(H,28,29,30,31)/t16-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001543

(CHEMBL3237708)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCC(N)CC3)nc12 |r,wU:4.7,wD:1.0,(11.29,-49.99,;10.82,-48.52,;11.85,-47.38,;11.38,-45.91,;9.88,-45.59,;8.84,-46.74,;9.31,-48.2,;9.41,-44.13,;10.31,-42.88,;11.84,-42.73,;12.48,-41.32,;11.56,-40.07,;10.04,-40.23,;9.41,-41.63,;7.94,-42.1,;6.6,-41.34,;5.27,-42.12,;5.28,-43.65,;3.95,-44.42,;3.96,-45.96,;5.29,-46.72,;5.3,-48.25,;3.96,-49.03,;2.63,-48.27,;2.62,-46.73,;3.97,-50.58,;2.64,-51.35,;2.64,-52.89,;3.98,-53.66,;3.98,-55.2,;5.31,-52.88,;5.31,-51.34,;6.62,-44.42,;7.94,-43.65,)| Show InChI InChI=1S/C26H32N8/c1-17-2-4-19(5-3-17)34-23-16-28-11-8-21(23)22-15-30-26(32-25(22)34)31-24-7-6-20(14-29-24)33-12-9-18(27)10-13-33/h6-8,11,14-19H,2-5,9-10,12-13,27H2,1H3,(H,29,30,31,32)/t17-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248470
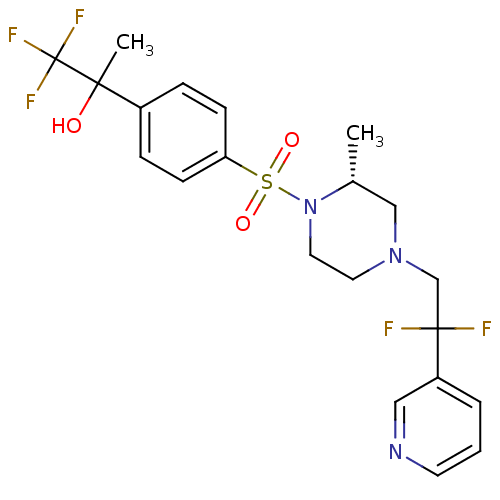
(2-(4-((R)-4-(2,2-difluoro-2-(pyridin-3-yl)ethyl)-2...)Show SMILES C[C@@H]1CN(CC(F)(F)c2cccnc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C21H24F5N3O3S/c1-15-13-28(14-20(22,23)17-4-3-9-27-12-17)10-11-29(15)33(31,32)18-7-5-16(6-8-18)19(2,30)21(24,25)26/h3-9,12,15,30H,10-11,13-14H2,1-2H3/t15-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG tagged human recombinant 11beta-HSD1 reductase activity expressed in Trichoplusia ni Hi5 cells |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331365
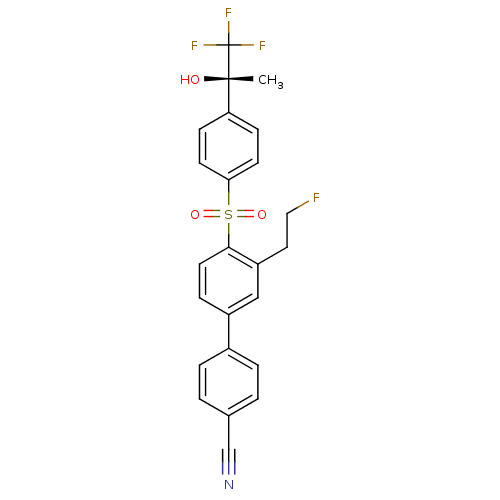
((S)-3'-(2-fluoroethyl)-4'-(4-(1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)S(=O)(=O)c1ccc(cc1CCF)-c1ccc(cc1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C24H19F4NO3S/c1-23(30,24(26,27)28)20-7-9-21(10-8-20)33(31,32)22-11-6-18(14-19(22)12-13-25)17-4-2-16(15-29)3-5-17/h2-11,14,30H,12-13H2,1H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7071-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.097
BindingDB Entry DOI: 10.7270/Q21G0MHX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331343
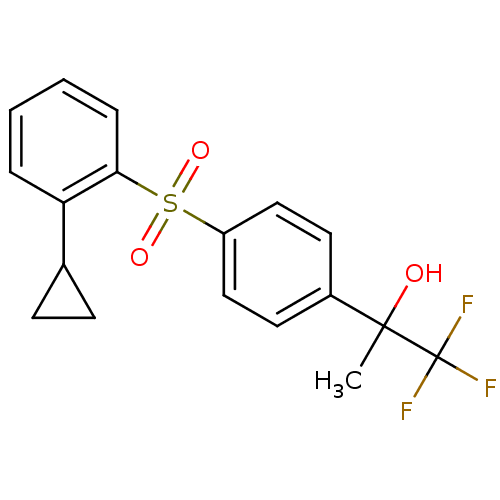
(CHEMBL1290164 | rac-2-(4-(2-cyclopropylphenylsulfo...)Show SMILES CC(O)(c1ccc(cc1)S(=O)(=O)c1ccccc1C1CC1)C(F)(F)F Show InChI InChI=1S/C18H17F3O3S/c1-17(22,18(19,20)21)13-8-10-14(11-9-13)25(23,24)16-5-3-2-4-15(16)12-6-7-12/h2-5,8-12,22H,6-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7071-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.097
BindingDB Entry DOI: 10.7270/Q21G0MHX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331341
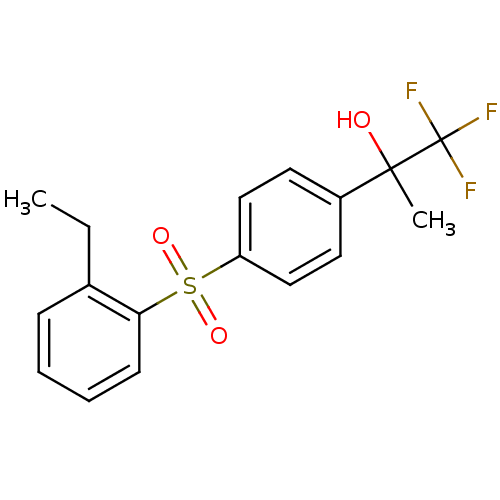
(CHEMBL1290057 | rac-2-(4-(2-ethylphenylsulfonyl)ph...)Show SMILES CCc1ccccc1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F Show InChI InChI=1S/C17H17F3O3S/c1-3-12-6-4-5-7-15(12)24(22,23)14-10-8-13(9-11-14)16(2,21)17(18,19)20/h4-11,21H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7071-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.097
BindingDB Entry DOI: 10.7270/Q21G0MHX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
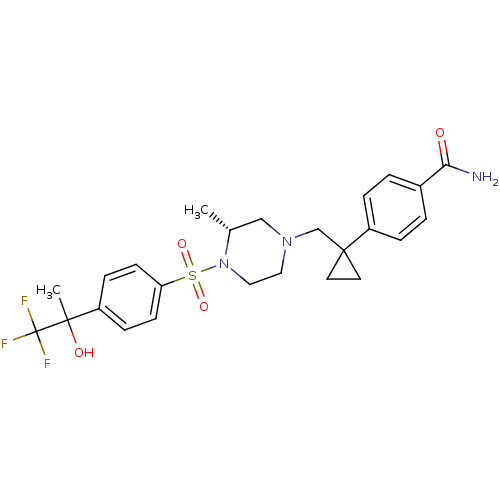
(Homo sapiens (Human)) | BDBM50248568
(4-(1-(((3R)-3-methyl-4-(4-(1,1,1-trifluoro-2-hydro...)Show SMILES C[C@@H]1CN(CC2(CC2)c2ccc(cc2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C25H30F3N3O4S/c1-17-15-30(16-24(11-12-24)20-5-3-18(4-6-20)22(29)32)13-14-31(17)36(34,35)21-9-7-19(8-10-21)23(2,33)25(26,27)28/h3-10,17,33H,11-16H2,1-2H3,(H2,29,32)/t17-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248427

(1,1,1-trifluoro-2-(4-((R)-2-methyl-4-(2-(pyridin-3...)Show SMILES C[C@@H]1CN(CCc2cccnc2)CCN1S(=O)(=O)c1ccc(cc1)C(C)(O)C(F)(F)F |r| Show InChI InChI=1S/C21H26F3N3O3S/c1-16-15-26(11-9-17-4-3-10-25-14-17)12-13-27(16)31(29,30)19-7-5-18(6-8-19)20(2,28)21(22,23)24/h3-8,10,14,16,28H,9,11-13,15H2,1-2H3/t16-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... |
Bioorg Med Chem Lett 19: 1522-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.114
BindingDB Entry DOI: 10.7270/Q2QC03BN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM28365

(CHEMBL512355 | N-[4-(2-cyanoethyl)cyclohexyl]-N-cy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@H](CCC#N)CC1)C(F)(F)F |r,wU:15.16,1.0,wD:18.20,1.1,(-12.83,5.36,;-11.49,6.13,;-12.98,6.52,;-10.16,5.36,;-10.16,3.81,;-8.83,3.04,;-7.49,3.81,;-7.49,5.36,;-8.83,6.13,;-6.16,3.05,;-6.16,1.51,;-4.83,3.82,;-4.83,5.36,;-5.57,6.7,;-4.03,6.67,;-3.49,3.05,;-2.16,3.82,;-.82,3.05,;-.82,1.5,;.51,.73,;1.84,1.51,;3.18,.74,;4.51,-.03,;-2.16,.73,;-3.49,1.5,;-11.49,7.67,;-10.16,8.44,;-12.83,8.44,;-11.49,9.21,)| Show InChI InChI=1S/C22H27F3N2O2/c1-21(29,22(23,24)25)17-8-6-16(7-9-17)20(28)27(19-12-13-19)18-10-4-15(5-11-18)3-2-14-26/h6-9,15,18-19,29H,2-5,10-13H2,1H3/t15-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cells |
J Med Chem 51: 3953-60 (2008)
Article DOI: 10.1021/jm800310g
BindingDB Entry DOI: 10.7270/Q2P84CSZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
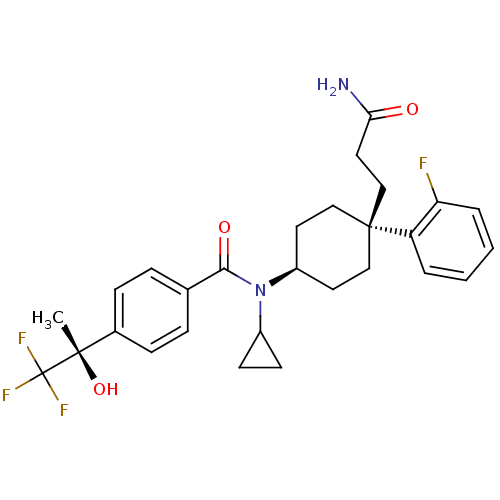
(Homo sapiens (Human)) | BDBM50334677
(CHEMBL1642597 | N-((4S)-4-(3-amino-3-oxopropyl)-4-...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CCC(N)=O)(CC1)c1ccccc1F)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(24.79,-23.04,;26.12,-22.26,;24.63,-21.87,;27.46,-23.03,;28.79,-22.25,;30.13,-23.02,;30.12,-24.56,;28.8,-25.34,;27.47,-24.58,;31.46,-25.33,;31.47,-26.87,;32.79,-24.55,;32.79,-23.01,;33.56,-21.68,;32.02,-21.68,;34.13,-25.32,;35.46,-24.54,;36.79,-25.32,;36.79,-26.86,;38.12,-26.08,;39.46,-26.84,;40.79,-26.06,;42.13,-26.82,;40.78,-24.52,;35.46,-27.62,;34.13,-26.86,;37.55,-28.19,;39.07,-28.2,;39.83,-29.52,;39.06,-30.85,;37.53,-30.84,;36.77,-29.51,;35.23,-29.5,;26.11,-20.72,;27.44,-19.94,;24.77,-19.96,;25.71,-19.23,)| Show InChI InChI=1S/C28H32F4N2O3/c1-26(37,28(30,31)32)19-8-6-18(7-9-19)25(36)34(20-10-11-20)21-12-15-27(16-13-21,17-14-24(33)35)22-4-2-3-5-23(22)29/h2-9,20-21,37H,10-17H2,1H3,(H2,33,35)/t21-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50243633

((S)-N-(trans-4-Phenyl)cyclohexyl-4-(1,1,1-trifluor...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@H](CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.23,(-6.04,-22.98,;-5.28,-21.65,;-6.62,-20.88,;-3.95,-22.42,;-3.95,-23.96,;-2.61,-24.73,;-1.28,-23.96,;-1.28,-22.41,;-2.62,-21.64,;.06,-24.73,;.06,-26.27,;1.39,-23.95,;1.39,-22.41,;2.15,-21.08,;.61,-21.08,;2.73,-24.72,;4.05,-23.95,;5.39,-24.73,;5.38,-26.27,;4.05,-27.04,;2.72,-26.27,;6.71,-27.04,;6.7,-28.58,;8.03,-29.35,;9.37,-28.58,;9.37,-27.04,;8.04,-26.27,;-4.51,-20.31,;-3.75,-18.97,;-3.17,-21.08,;-5.85,-19.55,)| Show InChI InChI=1S/C25H28F3NO2/c1-24(31,25(26,27)28)20-11-7-19(8-12-20)23(30)29(22-15-16-22)21-13-9-18(10-14-21)17-5-3-2-4-6-17/h2-8,11-12,18,21-22,31H,9-10,13-16H2,1H3/t18-,21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cloned 11beta-HSD1 by scintillation proximity assay |
J Med Chem 51: 3953-60 (2008)
Article DOI: 10.1021/jm800310g
BindingDB Entry DOI: 10.7270/Q2P84CSZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
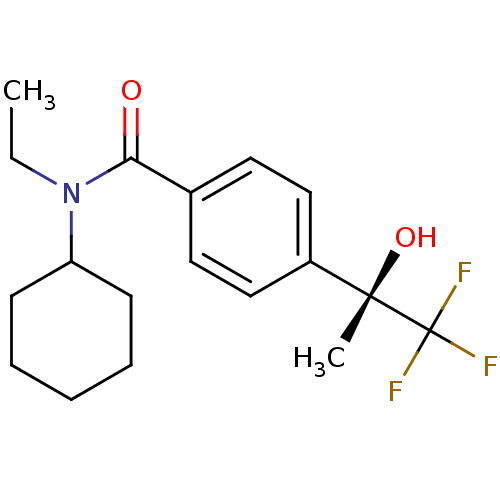
(Homo sapiens (Human)) | BDBM50243595
((S)-N-Cyclohexyl-4-(1,1,1-trifluoro-2-hydroxypropa...)Show SMILES CCN(C1CCCCC1)C(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C18H24F3NO2/c1-3-22(15-7-5-4-6-8-15)16(23)13-9-11-14(12-10-13)17(2,24)18(19,20)21/h9-12,15,24H,3-8H2,1-2H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cells |
J Med Chem 51: 3953-60 (2008)
Article DOI: 10.1021/jm800310g
BindingDB Entry DOI: 10.7270/Q2P84CSZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50243633

((S)-N-(trans-4-Phenyl)cyclohexyl-4-(1,1,1-trifluor...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@H](CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.23,(-6.04,-22.98,;-5.28,-21.65,;-6.62,-20.88,;-3.95,-22.42,;-3.95,-23.96,;-2.61,-24.73,;-1.28,-23.96,;-1.28,-22.41,;-2.62,-21.64,;.06,-24.73,;.06,-26.27,;1.39,-23.95,;1.39,-22.41,;2.15,-21.08,;.61,-21.08,;2.73,-24.72,;4.05,-23.95,;5.39,-24.73,;5.38,-26.27,;4.05,-27.04,;2.72,-26.27,;6.71,-27.04,;6.7,-28.58,;8.03,-29.35,;9.37,-28.58,;9.37,-27.04,;8.04,-26.27,;-4.51,-20.31,;-3.75,-18.97,;-3.17,-21.08,;-5.85,-19.55,)| Show InChI InChI=1S/C25H28F3NO2/c1-24(31,25(26,27)28)20-11-7-19(8-12-20)23(30)29(22-15-16-22)21-13-9-18(10-14-21)17-5-3-2-4-6-17/h2-8,11-12,18,21-22,31H,9-10,13-16H2,1H3/t18-,21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM28361

(N-cyclopropyl-N-[4-(pyridin-3-yl)cyclohexyl]-4-[(2...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@H](CC1)c1cccnc1)C(F)(F)F |r,wU:15.16,1.0,wD:18.23,1.1,(-12.83,5.36,;-11.49,6.13,;-12.98,6.52,;-10.16,5.36,;-10.16,3.81,;-8.83,3.04,;-7.49,3.81,;-7.49,5.36,;-8.83,6.13,;-6.16,3.05,;-6.16,1.51,;-4.83,3.82,;-4.83,5.36,;-5.57,6.7,;-4.03,6.67,;-3.49,3.05,;-2.16,3.82,;-.82,3.05,;-.82,1.5,;-2.16,.73,;-3.49,1.5,;.51,.73,;.51,-.81,;1.84,-1.58,;3.18,-.81,;3.18,.73,;1.84,1.51,;-11.49,7.67,;-10.16,8.44,;-12.83,8.44,;-11.49,9.21,)| Show InChI InChI=1S/C24H27F3N2O2/c1-23(31,24(25,26)27)19-8-4-17(5-9-19)22(30)29(21-12-13-21)20-10-6-16(7-11-20)18-3-2-14-28-15-18/h2-5,8-9,14-16,20-21,31H,6-7,10-13H2,1H3/t16-,20-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cloned 11beta-HSD1 by scintillation proximity assay |
J Med Chem 51: 3953-60 (2008)
Article DOI: 10.1021/jm800310g
BindingDB Entry DOI: 10.7270/Q2P84CSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334686

(CHEMBL1642590 | N-((4S)-4-(cyanomethyl)-4-phenylcy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CC#N)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.26,(24.32,2.92,;25.66,3.69,;24.17,4.09,;27,2.93,;28.33,3.7,;29.67,2.94,;29.66,1.4,;28.34,.62,;27,1.38,;31,.63,;31,-.91,;32.33,1.41,;32.33,2.95,;33.1,4.28,;31.56,4.27,;33.67,.64,;35,1.42,;36.33,.64,;36.33,-.9,;37.66,-.12,;39,-.89,;40.34,-1.64,;35,-1.67,;33.67,-.9,;37.09,-2.23,;36.31,-3.56,;37.07,-4.89,;38.6,-4.9,;39.37,-3.57,;38.61,-2.24,;25.65,5.24,;26.98,6.01,;24.31,6,;25.24,6.72,)| Show InChI InChI=1S/C27H29F3N2O2/c1-25(34,27(28,29)30)20-9-7-19(8-10-20)24(33)32(22-11-12-22)23-13-15-26(16-14-23,17-18-31)21-5-3-2-4-6-21/h2-10,22-23,34H,11-17H2,1H3/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
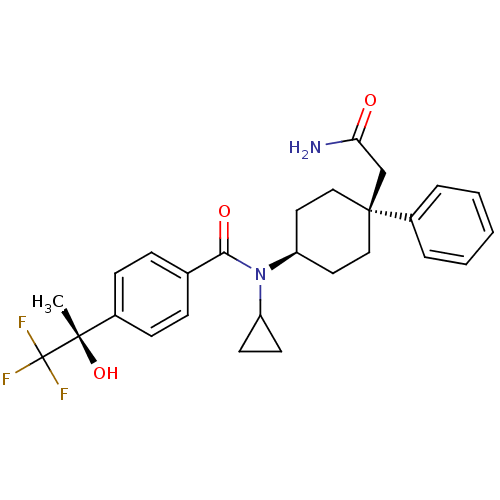
(Homo sapiens (Human)) | BDBM50334688
(CHEMBL1642592 | N-((4S)-4-(2-amino-2-oxoethyl)-4-p...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CC(N)=O)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.27,(-9.59,-9.92,;-8.25,-9.14,;-9.74,-8.75,;-6.92,-9.91,;-5.58,-9.13,;-4.25,-9.9,;-4.25,-11.44,;-5.57,-12.22,;-6.91,-11.46,;-2.91,-12.21,;-2.91,-13.75,;-1.58,-11.43,;-1.58,-9.89,;-.82,-8.56,;-2.36,-8.57,;-.24,-12.2,;1.09,-11.42,;2.42,-12.2,;2.42,-13.74,;3.9,-13.34,;4.29,-11.84,;5.78,-11.44,;3.2,-10.76,;1.09,-14.5,;-.24,-13.74,;3.18,-15.07,;2.4,-16.39,;3.15,-17.72,;4.68,-17.73,;5.46,-16.4,;4.7,-15.08,;-8.26,-7.6,;-6.93,-6.83,;-9.6,-6.84,;-8.67,-6.12,)| Show InChI InChI=1S/C27H31F3N2O3/c1-25(35,27(28,29)30)19-9-7-18(8-10-19)24(34)32(21-11-12-21)22-13-15-26(16-14-22,17-23(31)33)20-5-3-2-4-6-20/h2-10,21-22,35H,11-17H2,1H3,(H2,31,33)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50385037

(CHEMBL2035600)Show SMILES CC(C)N(c1ccc(cc1)[C@](C)(O)C(F)(F)F)S(=O)(=O)c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C18H18Cl2F3NO3S/c1-11(2)24(28(26,27)16-10-13(19)6-9-15(16)20)14-7-4-12(5-8-14)17(3,25)18(21,22)23/h4-11,25H,1-3H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using [3H]cortisone as substrate assessed as production of [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3786-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.005
BindingDB Entry DOI: 10.7270/Q21V5G07 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248133
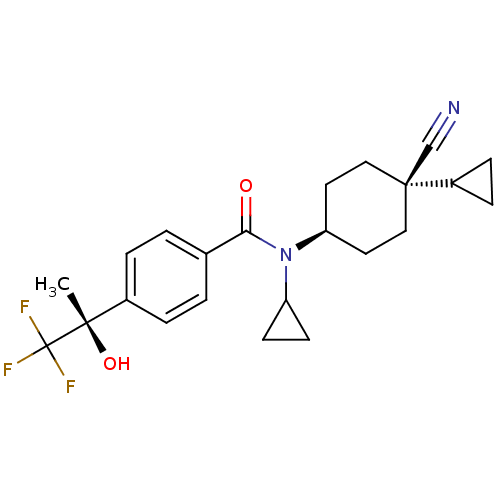
(CHEMBL518644 | N-((1s,4R)-4-cyano-4-cyclopropylcyc...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@](CC1)(C#N)C1CC1)C(F)(F)F |r,wU:15.16,18.23,1.2,wD:1.1,(24.17,-43.66,;24.94,-42.33,;25.71,-40.99,;26.27,-43.1,;26.27,-44.64,;27.6,-45.41,;28.94,-44.64,;28.93,-43.09,;27.6,-42.33,;30.28,-45.41,;30.28,-46.95,;31.61,-44.64,;31.61,-43.1,;32.37,-41.77,;30.83,-41.77,;32.94,-45.4,;34.27,-44.63,;35.6,-45.41,;35.6,-46.95,;34.26,-47.72,;32.94,-46.95,;36.93,-46.17,;38.26,-45.39,;36.93,-47.72,;37.7,-49.05,;38.48,-47.72,;23.6,-41.56,;22.26,-40.79,;22.83,-42.89,;24.36,-40.22,)| Show InChI InChI=1S/C23H27F3N2O2/c1-21(30,23(24,25)26)16-4-2-15(3-5-16)20(29)28(18-8-9-18)19-10-12-22(14-27,13-11-19)17-6-7-17/h2-5,17-19,30H,6-13H2,1H3/t19-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248020
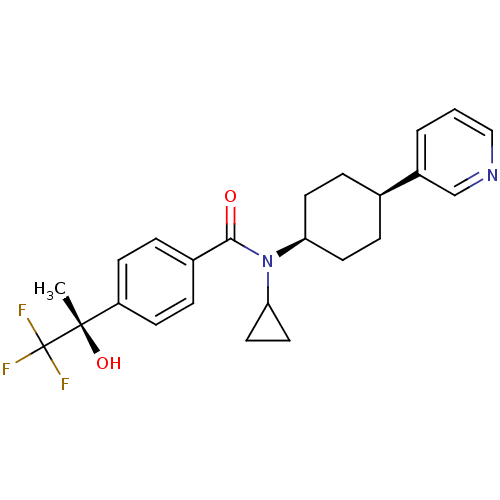
(CHEMBL455443 | N-cyclopropyl-N-((1s,4R)-4-(pyridin...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@H](CC1)c1cccnc1)C(F)(F)F |r,wU:15.16,18.23,1.2,wD:1.1,(23.78,1.56,;24.55,2.9,;25.32,4.24,;25.88,2.13,;25.88,.59,;27.21,-.18,;28.55,.59,;28.55,2.14,;27.21,2.9,;29.89,-.18,;29.89,-1.72,;31.22,.59,;31.22,2.13,;31.98,3.46,;30.44,3.46,;32.55,-.18,;33.88,.59,;35.21,-.18,;35.21,-1.72,;33.88,-2.49,;32.55,-1.72,;36.54,-2.5,;36.53,-4.04,;37.86,-4.81,;39.2,-4.05,;39.2,-2.51,;37.87,-1.73,;23.21,3.67,;21.87,4.44,;22.44,2.34,;23.98,5.01,)| Show InChI InChI=1S/C24H27F3N2O2/c1-23(31,24(25,26)27)19-8-4-17(5-9-19)22(30)29(21-12-13-21)20-10-6-16(7-11-20)18-3-2-14-28-15-18/h2-5,8-9,14-16,20-21,31H,6-7,10-13H2,1H3/t16-,20+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data