

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412340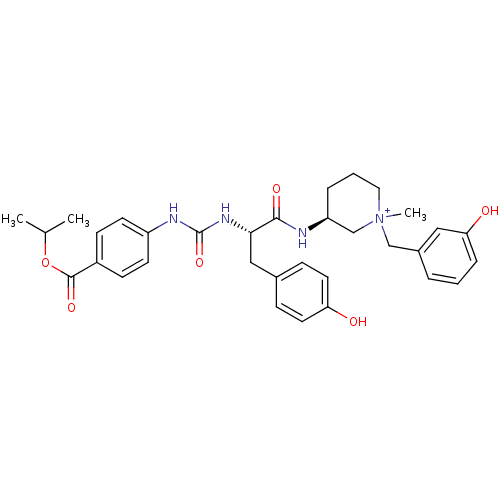 (CHEMBL540359) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M1 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 by scintillatio... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412344 (CHEMBL526009) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412342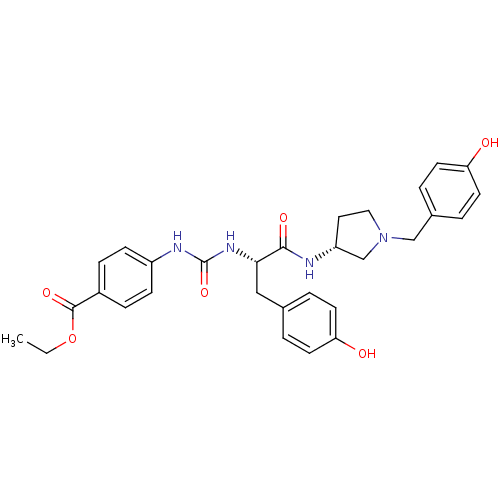 (CHEMBL502000) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50412344 (CHEMBL526009) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 assessed as inhibition of acetylcholin... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50412342 (CHEMBL502000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 assessed as inhibition of acetylcholin... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412344 (CHEMBL526009) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412342 (CHEMBL502000) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50412342 (CHEMBL502000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 assessed as inhibition of acetylcholin... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412342 (CHEMBL502000) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412341 (CHEMBL505453) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412342 (CHEMBL502000) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50412341 (CHEMBL505453) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 assessed as inhibition of acetylcholin... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP1A2 | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412341 (CHEMBL505453) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50412343 (CHEMBL501727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 assessed as inhibition of acetylcholin... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412343 (CHEMBL501727) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412343 (CHEMBL501727) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||