

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449917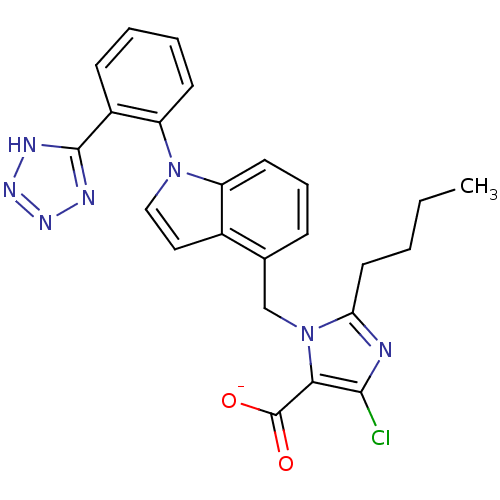 (BMS-180560 | CHEMBL2021417) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004154 (2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004155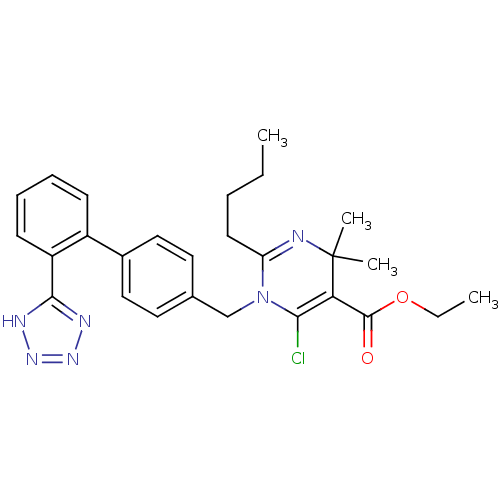 (2-Butyl-6-chloro-4,4-dimethyl-1-[2'-(1H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282324 (2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50049201 (2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282318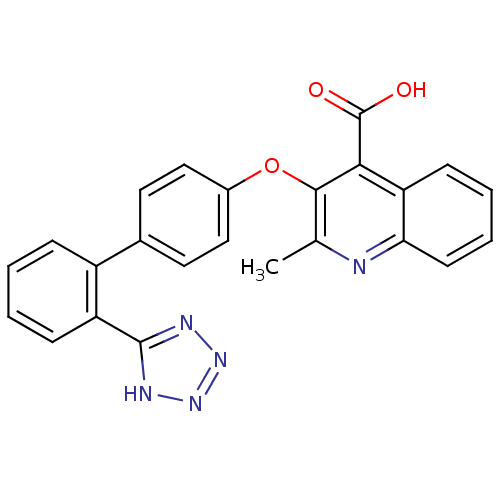 (2-Methyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282322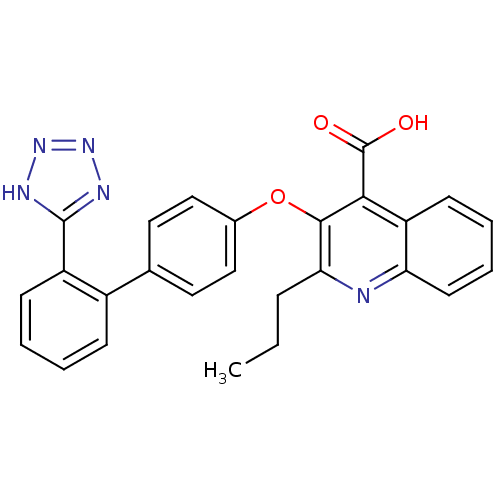 (2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231140 (CHEMBL77029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449914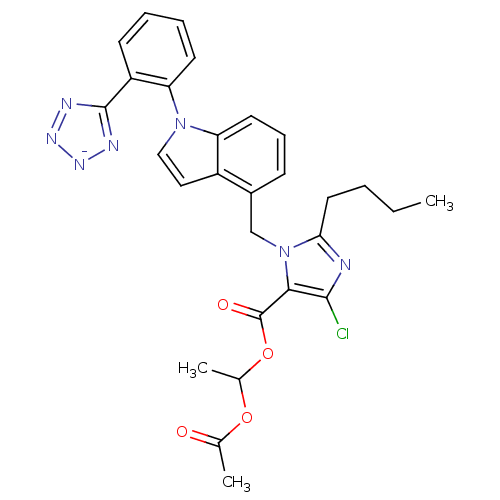 (CHEMBL2079784) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282316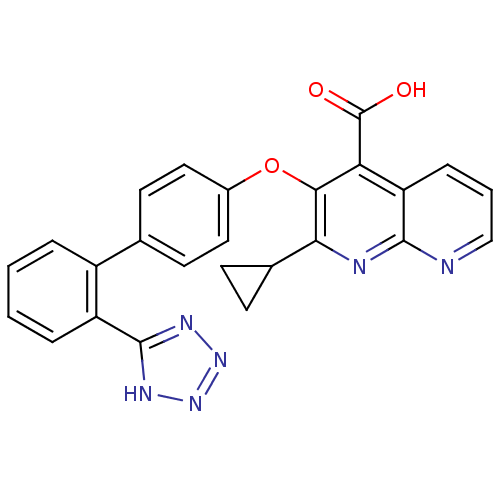 (2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145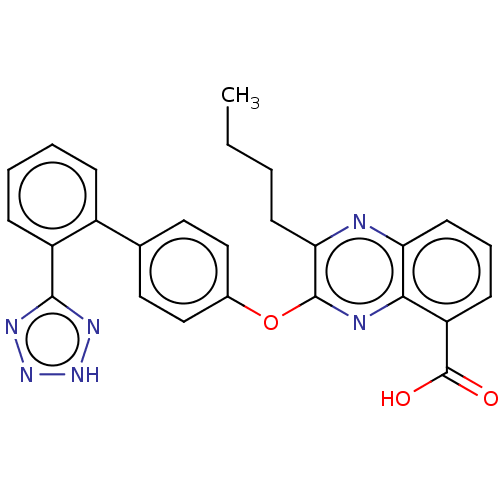 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282315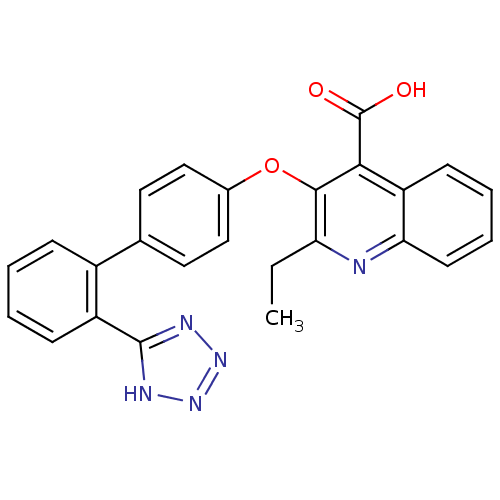 (2-Ethyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449919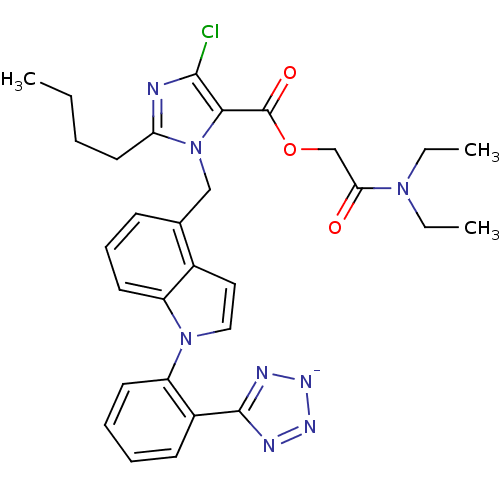 (CHEMBL2021415) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50366331 (CHEMBL1790055) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449910 (CHEMBL2079782) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231150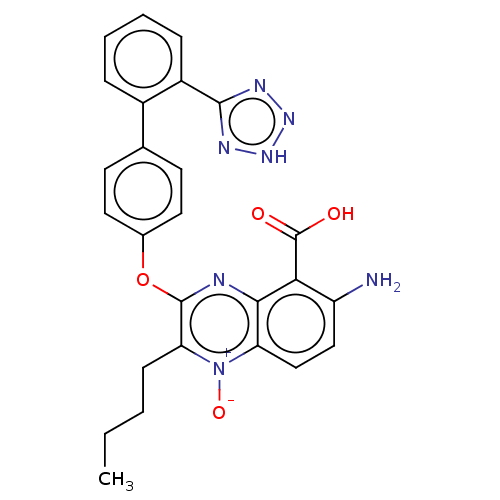 (CHEMBL77935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004164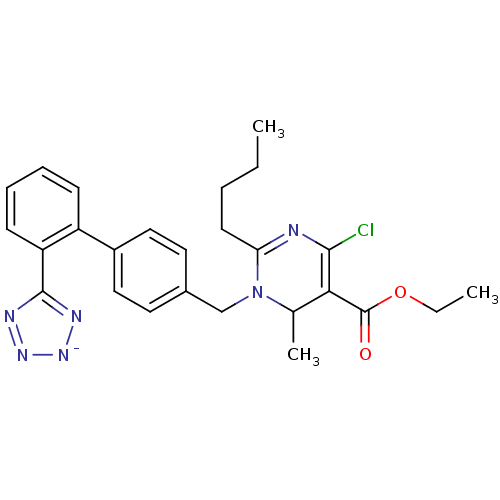 (2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449912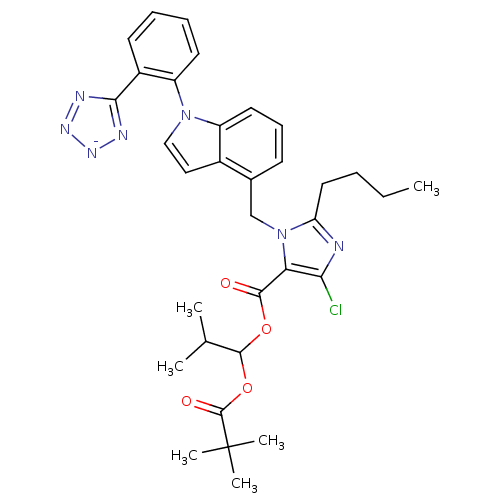 (CHEMBL2079781) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50406795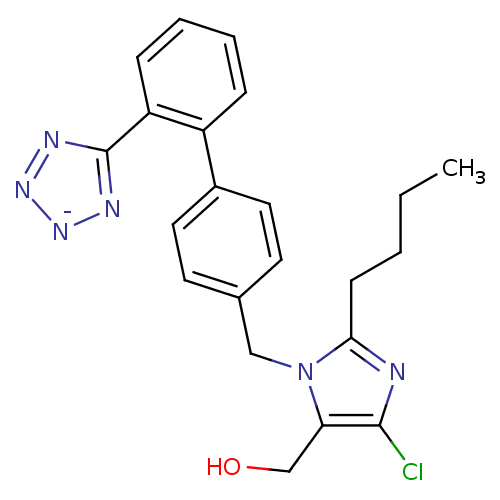 (Cozaar | LOSARTAN POTASSIUM) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231139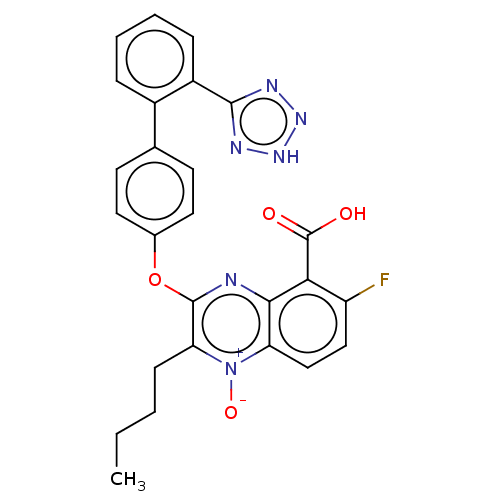 (CHEMBL77827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282317 (2-Cyclopropyl-5-methyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449909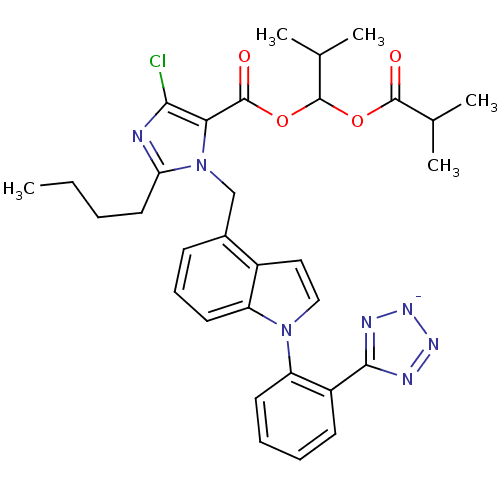 (CHEMBL2079769) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282319 (2-Cyclopropyl-6-fluoro-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231149 (CHEMBL306278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231143 (CHEMBL75053) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231007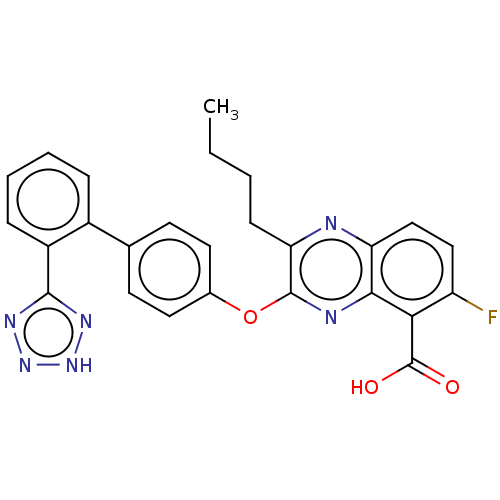 (CHEMBL74445) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231142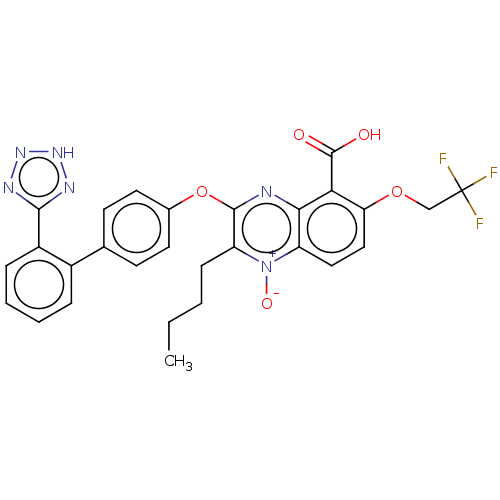 (CHEMBL312068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449918 (BMS-181688 | CHEMBL2021416) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231148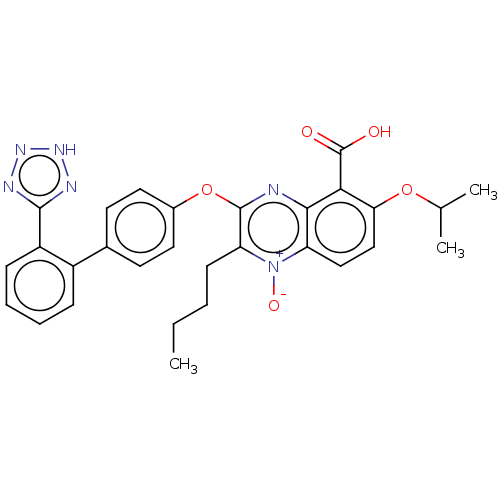 (CHEMBL77376) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449920 (CHEMBL2079768) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282321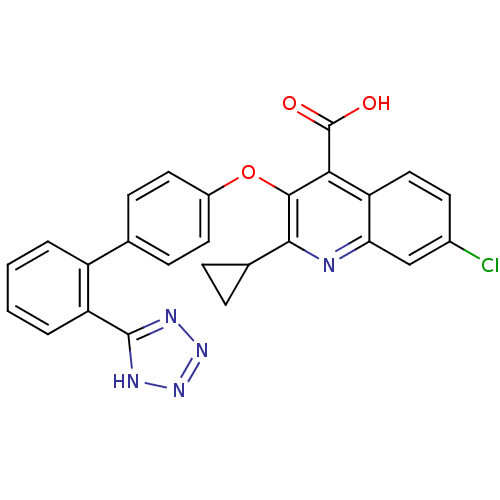 (7-Chloro-2-cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282313 (2-Cyclopropyl-6-methoxy-3-[2'-(2H-tetrazol-5-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449911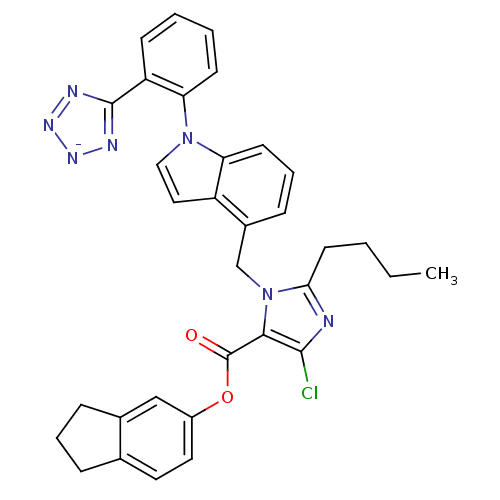 (CHEMBL2079770) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004153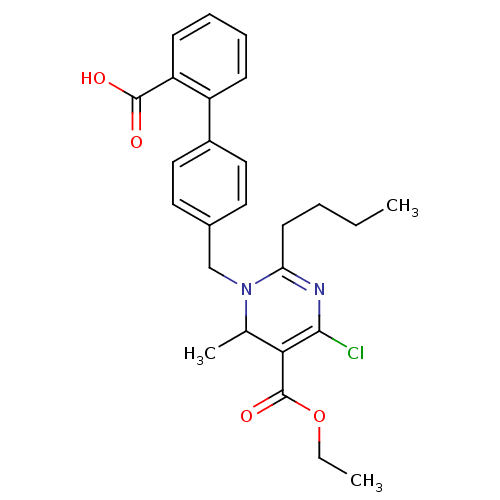 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231011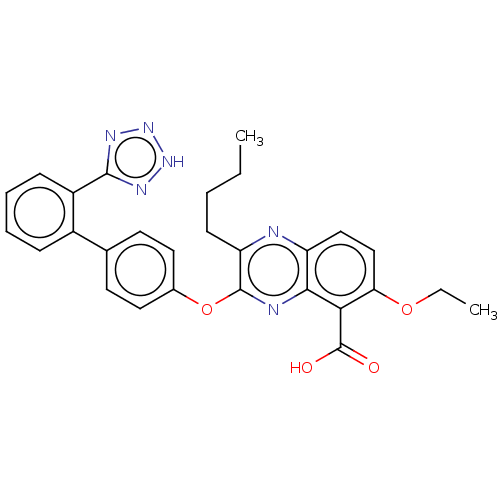 (CHEMBL309489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231009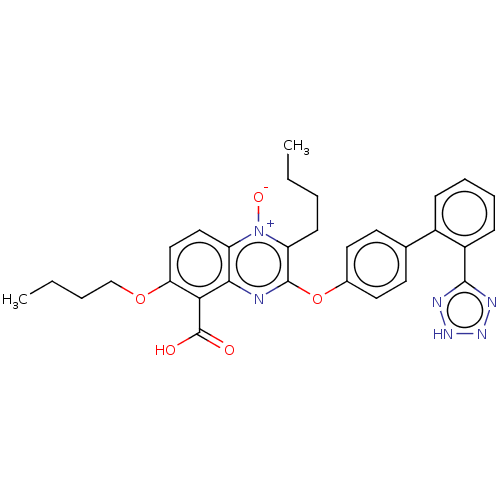 (CHEMBL76765) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004160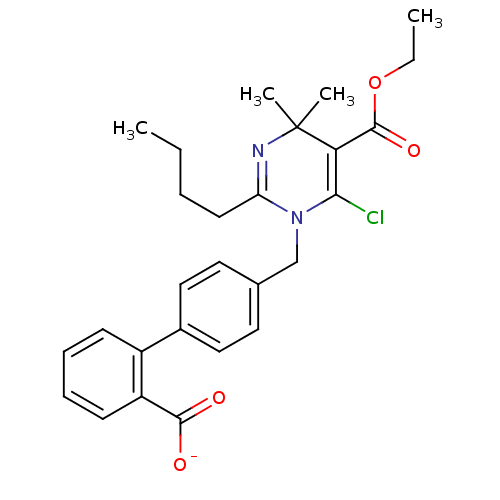 (CHEMBL145036 | Sodium; 4'-(2-butyl-6-chloro-5-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231147 (CHEMBL433310) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231008 (CHEMBL75101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231008 (CHEMBL75101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449915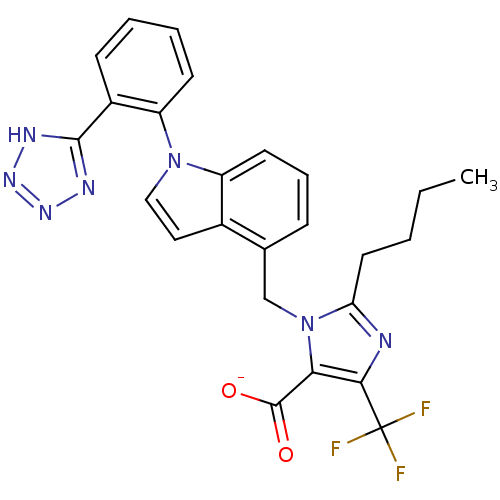 (CHEMBL2021419) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282240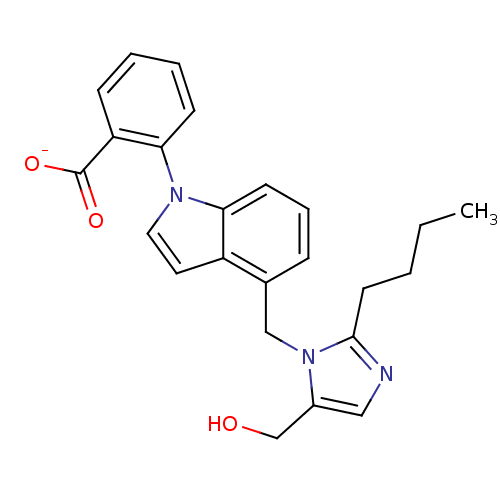 (CHEMBL288691 | Lithium; 2-[4-(2-butyl-5-hydroxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004163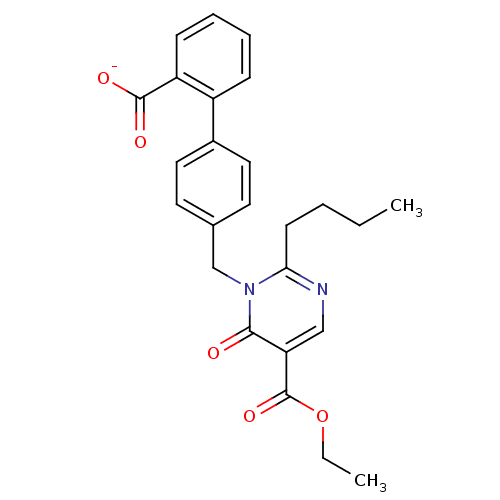 (CHEMBL342287 | Sodium; 4'-(2-butyl-5-ethoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282323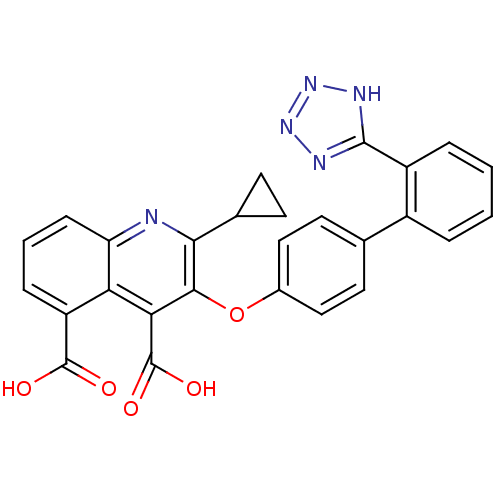 (2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |