 Found 45 hits with Last Name = 'delehunt' and Initial = 'j'
Found 45 hits with Last Name = 'delehunt' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009073
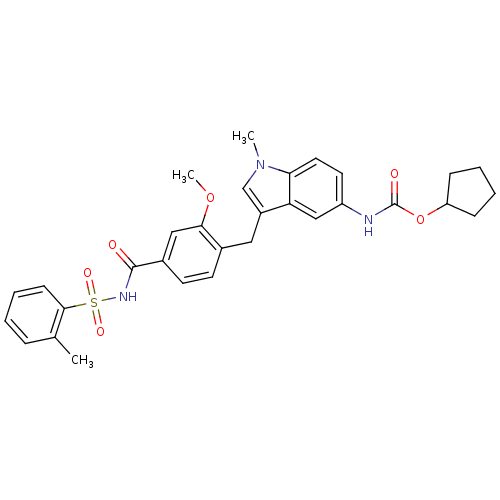
(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-leukotriene D4 (LTD4) from receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50004633
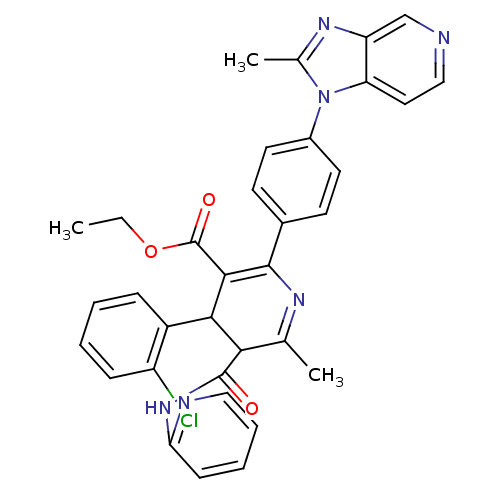
((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...)Show SMILES CCOC(=O)C1=C(N=C(C)C(C1c1ccccc1Cl)C(=O)Nc1ccccn1)c1ccc(cc1)-n1c(C)nc2cnccc12 |t:5,7| Show InChI InChI=1S/C34H29ClN6O3/c1-4-44-34(43)31-30(24-9-5-6-10-25(24)35)29(33(42)40-28-11-7-8-17-37-28)20(2)38-32(31)22-12-14-23(15-13-22)41-21(3)39-26-19-36-18-16-27(26)41/h5-19,29-30H,4H2,1-3H3,(H,37,40,42) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ligand from platelet activating factor (PAF) receptor in rabbit platelets |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 from receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ligand from platelet activating factor (PAF) receptor in rabbit platelets |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50284691
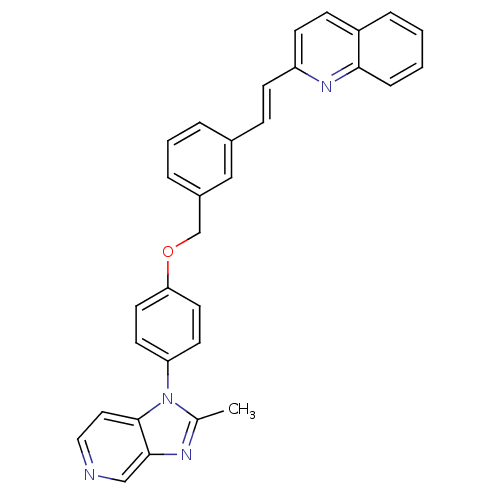
(2-(2-{3-[4-(2-Methyl-imidazo[4,5-c]pyridin-1-yl)-p...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccccc4n3)c2)cc1 Show InChI InChI=1S/C31H24N4O/c1-22-33-30-20-32-18-17-31(30)35(22)27-13-15-28(16-14-27)36-21-24-6-4-5-23(19-24)9-11-26-12-10-25-7-2-3-8-29(25)34-26/h2-20H,21H2,1H3/b11-9+ | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ligand from platelet activating factor (PAF) receptor in rabbit platelets |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-leukotriene D4 (LTD4) from receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ligand from platelet activating factor (PAF) receptor in rabbit platelets |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50284691

(2-(2-{3-[4-(2-Methyl-imidazo[4,5-c]pyridin-1-yl)-p...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccccc4n3)c2)cc1 Show InChI InChI=1S/C31H24N4O/c1-22-33-30-20-32-18-17-31(30)35(22)27-13-15-28(16-14-27)36-21-24-6-4-5-23(19-24)9-11-26-12-10-25-7-2-3-8-29(25)34-26/h2-20H,21H2,1H3/b11-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-leukotriene D4 (LTD4) from receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50284680
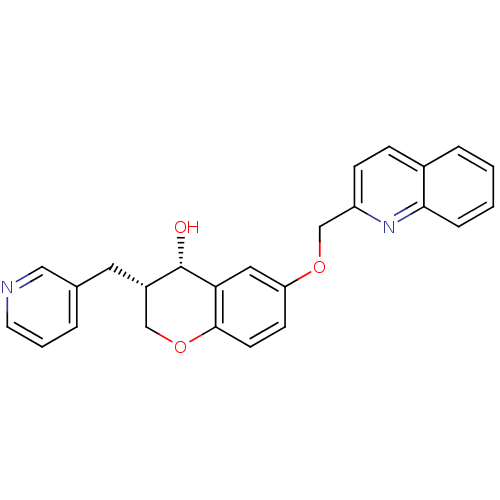
((3S,4S)-3-Pyridin-3-ylmethyl-6-(quinolin-2-ylmetho...)Show SMILES O[C@H]1[C@@H](Cc2cccnc2)COc2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C25H22N2O3/c28-25-19(12-17-4-3-11-26-14-17)15-30-24-10-9-21(13-22(24)25)29-16-20-8-7-18-5-1-2-6-23(18)27-20/h1-11,13-14,19,25,28H,12,15-16H2/t19-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTD4 binding to LTD4 receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1365-1370 (1995)
Article DOI: 10.1016/0960-894X(95)00225-I
BindingDB Entry DOI: 10.7270/Q2VH5NSM |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50284682
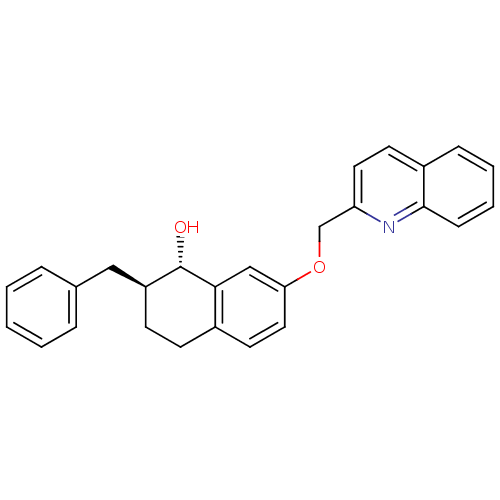
(2-Benzyl-7-(quinolin-2-ylmethoxy)-1,2,3,4-tetrahyd...)Show SMILES O[C@H]1[C@H](Cc2ccccc2)CCc2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C27H25NO2/c29-27-22(16-19-6-2-1-3-7-19)11-10-20-13-15-24(17-25(20)27)30-18-23-14-12-21-8-4-5-9-26(21)28-23/h1-9,12-15,17,22,27,29H,10-11,16,18H2/t22-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTD4 binding to LTD4 receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1365-1370 (1995)
Article DOI: 10.1016/0960-894X(95)00225-I
BindingDB Entry DOI: 10.7270/Q2VH5NSM |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50284681
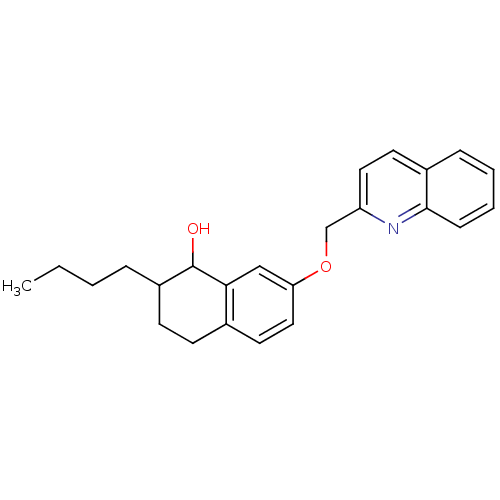
(2-Butyl-7-(quinolin-2-ylmethoxy)-1,2,3,4-tetrahydr...)Show InChI InChI=1S/C24H27NO2/c1-2-3-6-19-10-9-17-12-14-21(15-22(17)24(19)26)27-16-20-13-11-18-7-4-5-8-23(18)25-20/h4-5,7-8,11-15,19,24,26H,2-3,6,9-10,16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTD4 binding to LTD4 receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1365-1370 (1995)
Article DOI: 10.1016/0960-894X(95)00225-I
BindingDB Entry DOI: 10.7270/Q2VH5NSM |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50012434
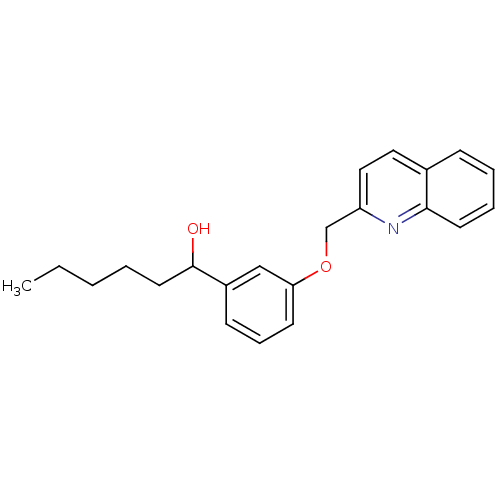
((REV-5,901)1-[3-(Quinolin-2-ylmethoxy)-phenyl]-hex...)Show InChI InChI=1S/C22H25NO2/c1-2-3-4-12-22(24)18-9-7-10-20(15-18)25-16-19-14-13-17-8-5-6-11-21(17)23-19/h5-11,13-15,22,24H,2-4,12,16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTD4 binding to LTD4 receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1365-1370 (1995)
Article DOI: 10.1016/0960-894X(95)00225-I
BindingDB Entry DOI: 10.7270/Q2VH5NSM |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50070921
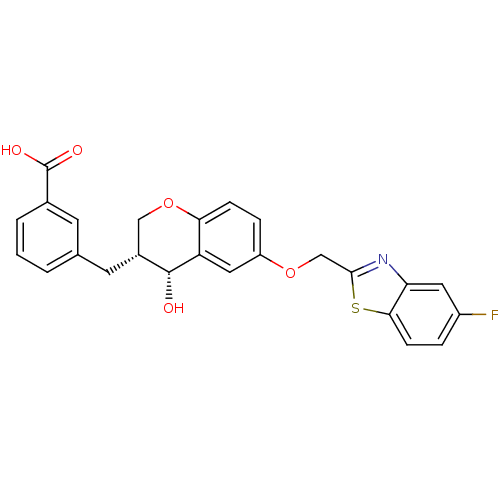
(3-[(3R,4R)-6-(5-Fluoro-benzothiazol-2-ylmethoxy)-4...)Show SMILES O[C@@H]1[C@H](Cc2cccc(c2)C(O)=O)COc2ccc(OCc3nc4cc(F)ccc4s3)cc12 Show InChI InChI=1S/C25H20FNO5S/c26-17-4-7-22-20(10-17)27-23(33-22)13-31-18-5-6-21-19(11-18)24(28)16(12-32-21)9-14-2-1-3-15(8-14)25(29)30/h1-8,10-11,16,24,28H,9,12-13H2,(H,29,30)/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTD4 binding to LTD4 receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1365-1370 (1995)
Article DOI: 10.1016/0960-894X(95)00225-I
BindingDB Entry DOI: 10.7270/Q2VH5NSM |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50004633

((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...)Show SMILES CCOC(=O)C1=C(N=C(C)C(C1c1ccccc1Cl)C(=O)Nc1ccccn1)c1ccc(cc1)-n1c(C)nc2cnccc12 |t:5,7| Show InChI InChI=1S/C34H29ClN6O3/c1-4-44-34(43)31-30(24-9-5-6-10-25(24)35)29(33(42)40-28-11-7-8-17-37-28)20(2)38-32(31)22-12-14-23(15-13-22)41-21(3)39-26-19-36-18-16-27(26)41/h5-19,29-30H,4H2,1-3H3,(H,37,40,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-leukotriene D4 (LTD4) from receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ligand from platelet activating factor (PAF) receptor in rabbit platelets |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50012434

((REV-5,901)1-[3-(Quinolin-2-ylmethoxy)-phenyl]-hex...)Show InChI InChI=1S/C22H25NO2/c1-2-3-4-12-22(24)18-9-7-10-20(15-18)25-16-19-14-13-17-8-5-6-11-21(17)23-19/h5-11,13-15,22,24H,2-4,12,16H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of 5-Lipoxygenase (5-LO) in rat basophilic leukemic cells |
Bioorg Med Chem Lett 5: 1365-1370 (1995)
Article DOI: 10.1016/0960-894X(95)00225-I
BindingDB Entry DOI: 10.7270/Q2VH5NSM |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50284680

((3S,4S)-3-Pyridin-3-ylmethyl-6-(quinolin-2-ylmetho...)Show SMILES O[C@H]1[C@@H](Cc2cccnc2)COc2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C25H22N2O3/c28-25-19(12-17-4-3-11-26-14-17)15-30-24-10-9-21(13-22(24)25)29-16-20-8-7-18-5-1-2-6-23(18)27-20/h1-11,13-14,19,25,28H,12,15-16H2/t19-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of 5-Lipoxygenase (5-LO) in rat basophilic leukemia cells |
Bioorg Med Chem Lett 5: 1365-1370 (1995)
Article DOI: 10.1016/0960-894X(95)00225-I
BindingDB Entry DOI: 10.7270/Q2VH5NSM |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50070921

(3-[(3R,4R)-6-(5-Fluoro-benzothiazol-2-ylmethoxy)-4...)Show SMILES O[C@@H]1[C@H](Cc2cccc(c2)C(O)=O)COc2ccc(OCc3nc4cc(F)ccc4s3)cc12 Show InChI InChI=1S/C25H20FNO5S/c26-17-4-7-22-20(10-17)27-23(33-22)13-31-18-5-6-21-19(11-18)24(28)16(12-32-21)9-14-2-1-3-15(8-14)25(29)30/h1-8,10-11,16,24,28H,9,12-13H2,(H,29,30)/t16-,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of 5-Lipoxygenase (5-LO) in rat basophilic leukemia cells |
Bioorg Med Chem Lett 5: 1365-1370 (1995)
Article DOI: 10.1016/0960-894X(95)00225-I
BindingDB Entry DOI: 10.7270/Q2VH5NSM |
More data for this
Ligand-Target Pair | |
Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Alpha-2 adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Beta adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Beta adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against muscarinic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A/2B/2C
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-hydroxytryptamine 2 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Alpha-2 adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Alpha-1 adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against muscarinic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Alpha-2 adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Alpha-1 adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A/2B/2C
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-hydroxytryptamine 2 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against muscarinic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Beta adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A/2B/2C
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-hydroxytryptamine 2 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Opioid receptor mu 1 |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against H1 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Opioid receptor mu 1 |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Adenosine A1 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Dopamine receptor D2 |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Adenosine A1 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against H1 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Alpha-1 adrenergic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Opioid receptor mu 1 |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Dopamine receptor D2 |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against H1 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Dopamine receptor D2 |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Adenosine A1 receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data