 Found 287 hits with Last Name = 'delker' and Initial = 'sl'
Found 287 hits with Last Name = 'delker' and Initial = 'sl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330882
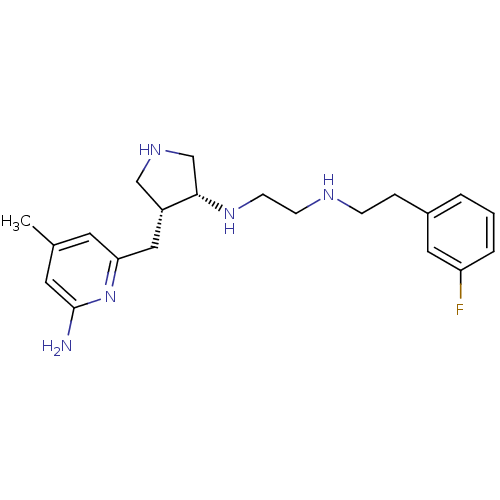
(CHEMBL1277951 | CHEMBL594682 | N1-((3R,4R)-4-((6-a...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50330882

(CHEMBL1277951 | CHEMBL594682 | N1-((3R,4R)-4-((6-a...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of nNOS |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50364378
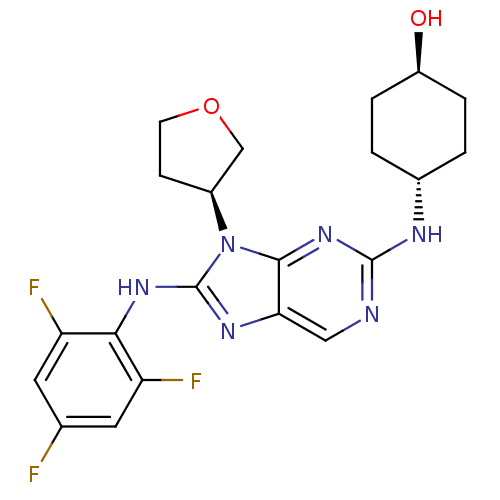
(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK2 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50328814
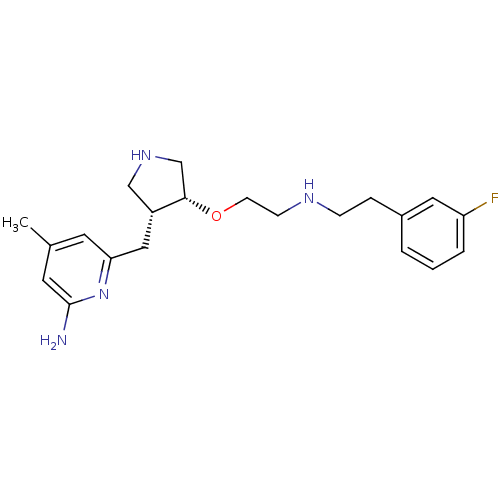
((+/-)-6-((4-(2-(3-fluorophenethylamino)ethoxy)pyrr...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H29FN4O/c1-15-9-19(26-21(23)10-15)12-17-13-25-14-20(17)27-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-25H,5-8,12-14H2,1H3,(H2,23,26)/t17-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide by hemoglobin capture assay |
Bioorg Med Chem 21: 1333-43 (2013)
Article DOI: 10.1016/j.bmc.2012.12.019
BindingDB Entry DOI: 10.7270/Q20G3MG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50328814

((+/-)-6-((4-(2-(3-fluorophenethylamino)ethoxy)pyrr...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H29FN4O/c1-15-9-19(26-21(23)10-15)12-17-13-25-14-20(17)27-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-25H,5-8,12-14H2,1H3,(H2,23,26)/t17-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50255365
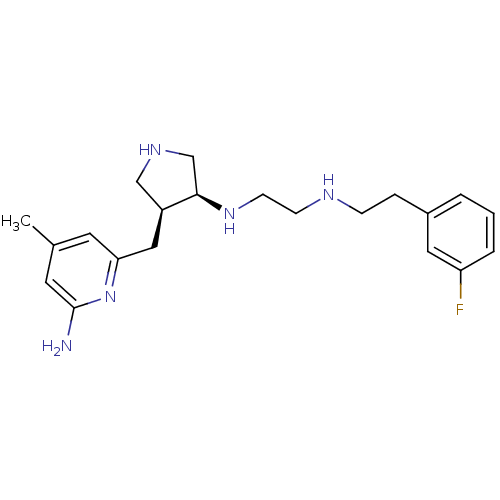
((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50278675

((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2OCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H29FN4O/c1-15-9-19(26-21(23)10-15)12-17-13-25-14-20(17)27-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-25H,5-8,12-14H2,1H3,(H2,23,26)/t17-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330883
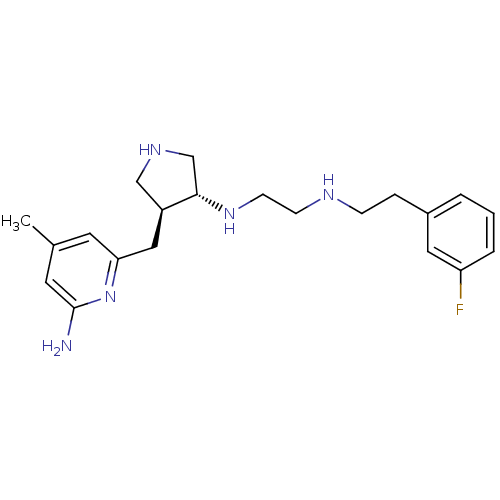
(CHEMBL1233715 | CHEMBL1277952 | N1-((3R,4S)-4-((6-...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50341682
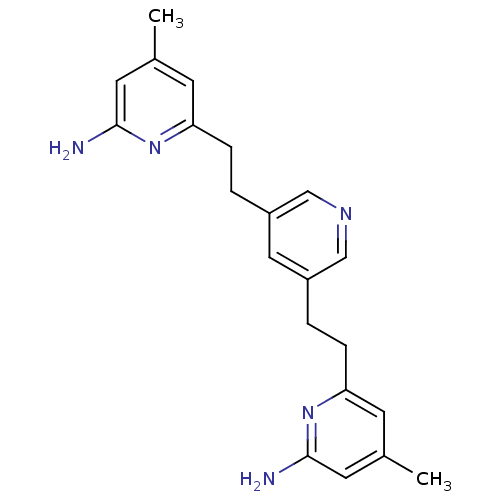
(6,6'-(2,2'-(Pyridine-3,5-diyl)bis(ethane-2,1-diyl)...)Show InChI InChI=1S/C21H25N5/c1-14-7-18(25-20(22)9-14)5-3-16-11-17(13-24-12-16)4-6-19-8-15(2)10-21(23)26-19/h7-13H,3-6H2,1-2H3,(H2,22,25)(H2,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Irvine
| Assay Description
Inhibition assay using nitric oxide synthases. |
Biochemistry 49: 10803-10 (2010)
Article DOI: 10.1021/bi1013479
BindingDB Entry DOI: 10.7270/Q2KS6Q51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341682

(6,6'-(2,2'-(Pyridine-3,5-diyl)bis(ethane-2,1-diyl)...)Show InChI InChI=1S/C21H25N5/c1-14-7-18(25-20(22)9-14)5-3-16-11-17(13-24-12-16)4-6-19-8-15(2)10-21(23)26-19/h7-13H,3-6H2,1-2H3,(H2,22,25)(H2,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50352520
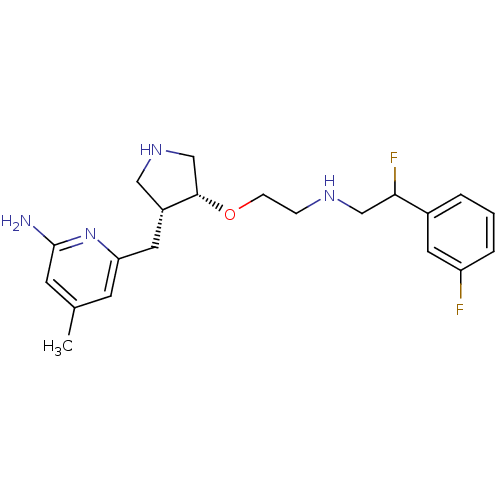
(CHEMBL1824857)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCC(F)c2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H28F2N4O/c1-14-7-18(27-21(24)8-14)10-16-11-26-13-20(16)28-6-5-25-12-19(23)15-3-2-4-17(22)9-15/h2-4,7-9,16,19-20,25-26H,5-6,10-13H2,1H3,(H2,24,27)/t16-,19?,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli assessed as reduction in formation of nitric oxide by hemoglobin capture assay |
J Med Chem 54: 6399-403 (2011)
Article DOI: 10.1021/jm200411j
BindingDB Entry DOI: 10.7270/Q2TX3FRR |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50341684
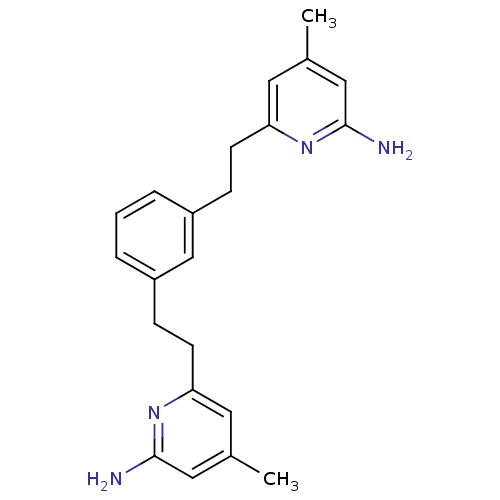
(6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...)Show InChI InChI=1S/C22H26N4/c1-15-10-19(25-21(23)12-15)8-6-17-4-3-5-18(14-17)7-9-20-11-16(2)13-22(24)26-20/h3-5,10-14H,6-9H2,1-2H3,(H2,23,25)(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Irvine
| Assay Description
Inhibition assay using nitric oxide synthases. |
Biochemistry 49: 10803-10 (2010)
Article DOI: 10.1021/bi1013479
BindingDB Entry DOI: 10.7270/Q2KS6Q51 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341683
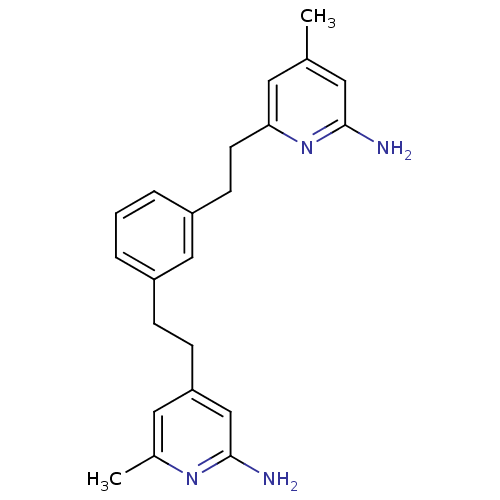
(4-(3-(2-(6-Amino-4-methylpyridin-2-yl)ethyl)phenet...)Show InChI InChI=1S/C22H26N4/c1-15-10-20(26-21(23)11-15)9-8-18-5-3-4-17(13-18)6-7-19-12-16(2)25-22(24)14-19/h3-5,10-14H,6-9H2,1-2H3,(H2,23,26)(H2,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50364378

(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK1 expressed in baculoviral system using GST-tagged c-Jun as substrate preincubated for 15 mins prior ATP addition me... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50429229

(CHEMBL2332793)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCN[C@H]2C[C@@H]2c2cccc(F)c2)c1 |r| Show InChI InChI=1S/C22H29FN4O/c1-14-7-18(27-22(24)8-14)10-16-12-25-13-21(16)28-6-5-26-20-11-19(20)15-3-2-4-17(23)9-15/h2-4,7-9,16,19-21,25-26H,5-6,10-13H2,1H3,(H2,24,27)/t16-,19-,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide by hemoglobin capture assay |
Bioorg Med Chem 21: 1333-43 (2013)
Article DOI: 10.1016/j.bmc.2012.12.019
BindingDB Entry DOI: 10.7270/Q20G3MG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330875

(CHEMBL1233717 | CHEMBL1278037 | N-{(3S,4R)-4-[(6-a...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341684

(6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...)Show InChI InChI=1S/C22H26N4/c1-15-10-19(25-21(23)12-15)8-6-17-4-3-5-18(14-17)7-9-20-11-16(2)13-22(24)26-20/h3-5,10-14H,6-9H2,1-2H3,(H2,23,25)(H2,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50255365

((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of nNOS |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330897
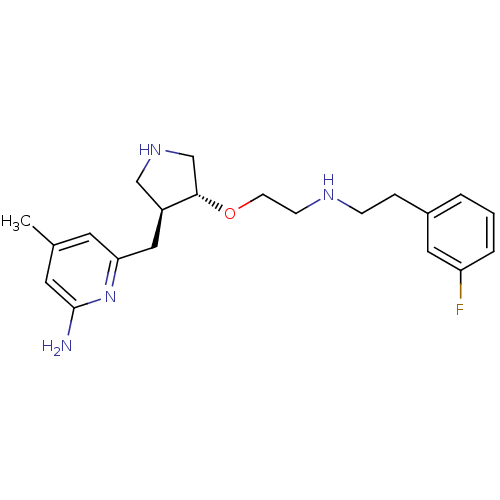
(6-(((3R,4S)-4-(2-(4-fluorobenzylamino)ethoxy)pyrro...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2OCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H29FN4O/c1-15-9-19(26-21(23)10-15)12-17-13-25-14-20(17)27-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-25H,5-8,12-14H2,1H3,(H2,23,26)/t17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50429228

(CHEMBL2332789)Show SMILES C[C@H](Cc1cccc(F)c1)NCCO[C@H]1CNC[C@H]1Cc1cc(C)cc(N)n1 |r| Show InChI InChI=1S/C22H31FN4O/c1-15-8-20(27-22(24)9-15)12-18-13-25-14-21(18)28-7-6-26-16(2)10-17-4-3-5-19(23)11-17/h3-5,8-9,11,16,18,21,25-26H,6-7,10,12-14H2,1-2H3,(H2,24,27)/t16-,18-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide by hemoglobin capture assay |
Bioorg Med Chem 21: 1333-43 (2013)
Article DOI: 10.1016/j.bmc.2012.12.019
BindingDB Entry DOI: 10.7270/Q20G3MG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50429228

(CHEMBL2332789)Show SMILES C[C@H](Cc1cccc(F)c1)NCCO[C@H]1CNC[C@H]1Cc1cc(C)cc(N)n1 |r| Show InChI InChI=1S/C22H31FN4O/c1-15-8-20(27-22(24)9-15)12-18-13-25-14-21(18)28-7-6-26-16(2)10-17-4-3-5-19(23)11-17/h3-5,8-9,11,16,18,21,25-26H,6-7,10,12-14H2,1-2H3,(H2,24,27)/t16-,18-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide by hemoglobin capture assay |
Bioorg Med Chem 21: 1333-43 (2013)
Article DOI: 10.1016/j.bmc.2012.12.019
BindingDB Entry DOI: 10.7270/Q20G3MG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50352521

(CHEMBL1824858)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCC(F)(F)c2cc(F)cc(Cl)c2)c1 |r| Show InChI InChI=1S/C21H26ClF3N4O/c1-13-4-18(29-20(26)5-13)6-14-10-28-11-19(14)30-3-2-27-12-21(24,25)15-7-16(22)9-17(23)8-15/h4-5,7-9,14,19,27-28H,2-3,6,10-12H2,1H3,(H2,26,29)/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli assessed as reduction in formation of nitric oxide by hemoglobin capture assay |
J Med Chem 54: 6399-403 (2011)
Article DOI: 10.1021/jm200411j
BindingDB Entry DOI: 10.7270/Q2TX3FRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50341678

(6,6'-(2,2'-(5-Amino-1,3-phenylene)bis(ethane-2,1di...)Show SMILES Cc1cc(N)nc(CCc2cc(N)cc(CCc3cc(C)cc(N)n3)c2)c1 Show InChI InChI=1S/C22H27N5/c1-14-7-19(26-21(24)9-14)5-3-16-11-17(13-18(23)12-16)4-6-20-8-15(2)10-22(25)27-20/h7-13H,3-6,23H2,1-2H3,(H2,24,26)(H2,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Irvine
| Assay Description
Inhibition assay using nitric oxide synthases. |
Biochemistry 49: 10803-10 (2010)
Article DOI: 10.1021/bi1013479
BindingDB Entry DOI: 10.7270/Q2KS6Q51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM29234
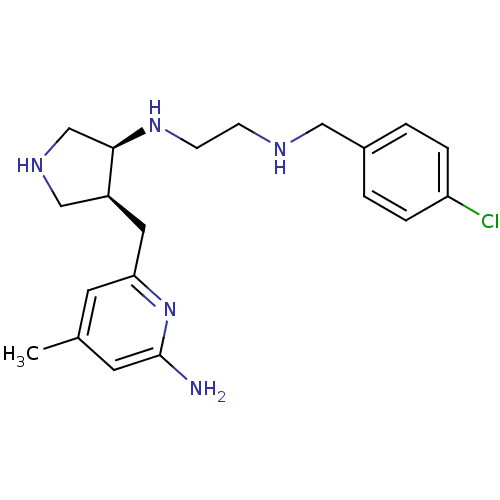
(CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2NCCNCc2ccc(Cl)cc2)c1 |r| Show InChI InChI=1S/C20H28ClN5/c1-14-8-18(26-20(22)9-14)10-16-12-24-13-19(16)25-7-6-23-11-15-2-4-17(21)5-3-15/h2-5,8-9,16,19,23-25H,6-7,10-13H2,1H3,(H2,22,26)/t16-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341678

(6,6'-(2,2'-(5-Amino-1,3-phenylene)bis(ethane-2,1di...)Show SMILES Cc1cc(N)nc(CCc2cc(N)cc(CCc3cc(C)cc(N)n3)c2)c1 Show InChI InChI=1S/C22H27N5/c1-14-7-19(26-21(24)9-14)5-3-16-11-17(13-18(23)12-16)4-6-20-8-15(2)10-22(25)27-20/h7-13H,3-6,23H2,1-2H3,(H2,24,26)(H2,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50352518

(CHEMBL1824855)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCC(F)(F)c2cccc(Cl)c2)c1 |r| Show InChI InChI=1S/C21H27ClF2N4O/c1-14-7-18(28-20(25)8-14)9-15-11-27-12-19(15)29-6-5-26-13-21(23,24)16-3-2-4-17(22)10-16/h2-4,7-8,10,15,19,26-27H,5-6,9,11-13H2,1H3,(H2,25,28)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli assessed as reduction in formation of nitric oxide by hemoglobin capture assay |
J Med Chem 54: 6399-403 (2011)
Article DOI: 10.1021/jm200411j
BindingDB Entry DOI: 10.7270/Q2TX3FRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330873
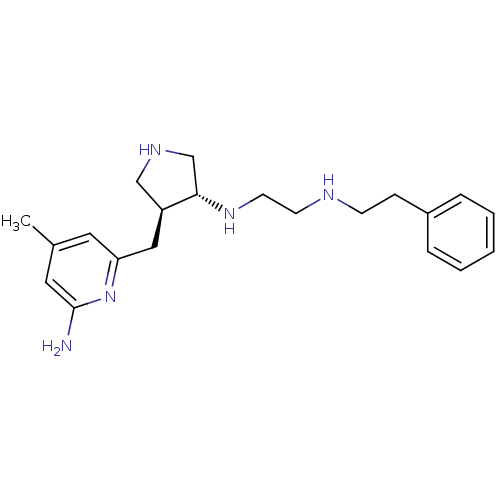
(CHEMBL1277786 | US9090589, 4 | trans rac-N1-(4-((6...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCCc2ccccc2)c1 |r| Show InChI InChI=1S/C21H31N5/c1-16-11-19(26-21(22)12-16)13-18-14-24-15-20(18)25-10-9-23-8-7-17-5-3-2-4-6-17/h2-6,11-12,18,20,23-25H,7-10,13-15H2,1H3,(H2,22,26)/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341685
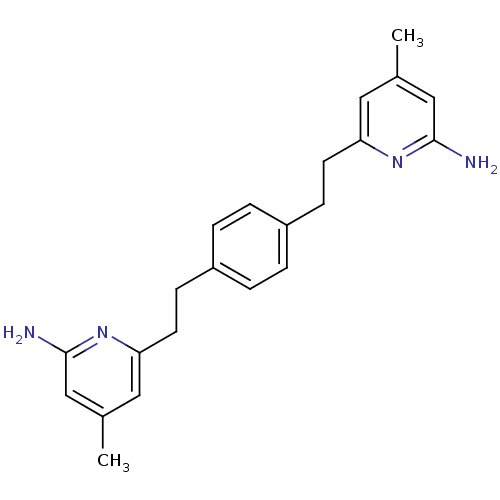
(6,6'-(2,2'-(1,4-Phenylene)bis(ethane-2,1-diyl))bis...)Show InChI InChI=1S/C22H26N4/c1-15-11-19(25-21(23)13-15)9-7-17-3-5-18(6-4-17)8-10-20-12-16(2)14-22(24)26-20/h3-6,11-14H,7-10H2,1-2H3,(H2,23,25)(H2,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341680
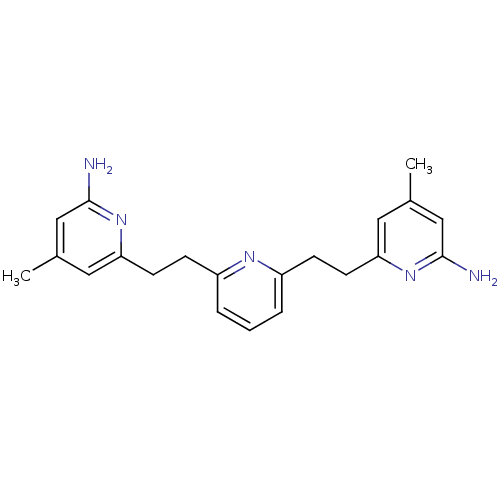
(6,6'-(2,2'-(Pyridine-2,6-diyl)bis(ethane-2,1-diyl)...)Show InChI InChI=1S/C21H25N5/c1-14-10-18(25-20(22)12-14)8-6-16-4-3-5-17(24-16)7-9-19-11-15(2)13-21(23)26-19/h3-5,10-13H,6-9H2,1-2H3,(H2,22,25)(H2,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341686
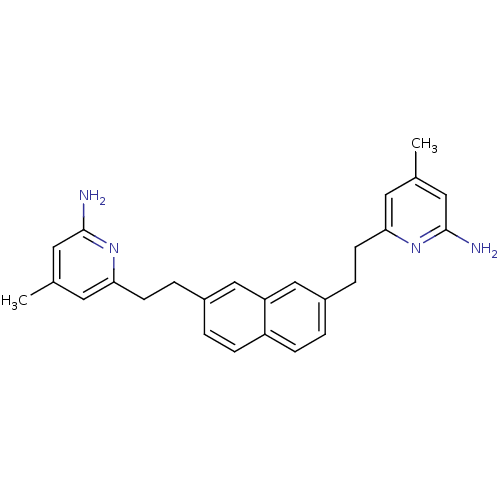
(6,6'-(2,2'-(Naphthalene-2,7-diyl)bis(ethane-2,1-di...)Show SMILES Cc1cc(N)nc(CCc2ccc3ccc(CCc4cc(C)cc(N)n4)cc3c2)c1 Show InChI InChI=1S/C26H28N4/c1-17-11-23(29-25(27)13-17)9-5-19-3-7-21-8-4-20(16-22(21)15-19)6-10-24-12-18(2)14-26(28)30-24/h3-4,7-8,11-16H,5-6,9-10H2,1-2H3,(H2,27,29)(H2,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50341681
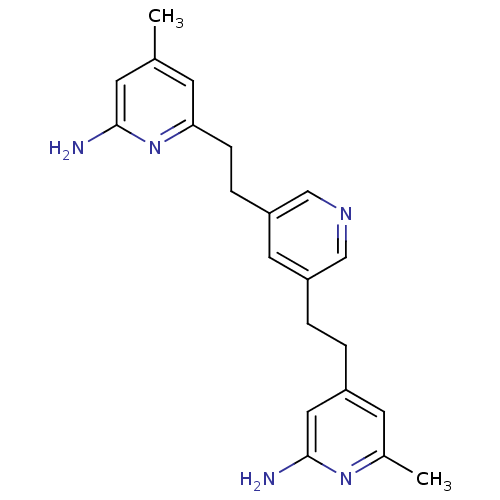
(4-(2-(5-(2-(6-Amino-4-methylpyridin-2-yl)ethyl)pyr...)Show InChI InChI=1S/C21H25N5/c1-14-7-19(26-20(22)8-14)6-5-18-10-17(12-24-13-18)4-3-16-9-15(2)25-21(23)11-16/h7-13H,3-6H2,1-2H3,(H2,22,26)(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Irvine
| Assay Description
Inhibition assay using nitric oxide synthases. |
Biochemistry 49: 10803-10 (2010)
Article DOI: 10.1021/bi1013479
BindingDB Entry DOI: 10.7270/Q2KS6Q51 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341681

(4-(2-(5-(2-(6-Amino-4-methylpyridin-2-yl)ethyl)pyr...)Show InChI InChI=1S/C21H25N5/c1-14-7-19(26-20(22)8-14)6-5-18-10-17(12-24-13-18)4-3-16-9-15(2)25-21(23)11-16/h7-13H,3-6H2,1-2H3,(H2,22,26)(H2,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50352517
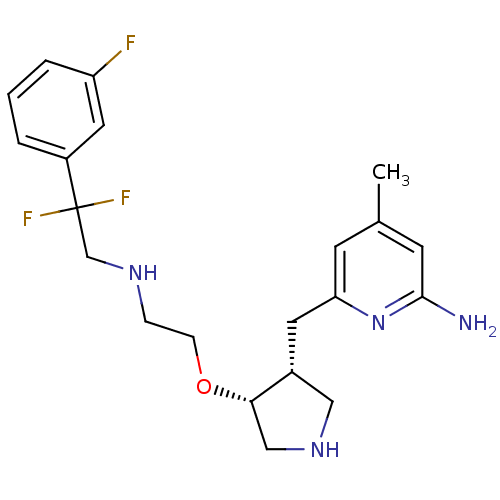
(CHEMBL1614782)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCC(F)(F)c2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H27F3N4O/c1-14-7-18(28-20(25)8-14)9-15-11-27-12-19(15)29-6-5-26-13-21(23,24)16-3-2-4-17(22)10-16/h2-4,7-8,10,15,19,26-27H,5-6,9,11-13H2,1H3,(H2,25,28)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli assessed as reduction in formation of nitric oxide by hemoglobin capture assay |
J Med Chem 54: 6399-403 (2011)
Article DOI: 10.1021/jm200411j
BindingDB Entry DOI: 10.7270/Q2TX3FRR |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330874
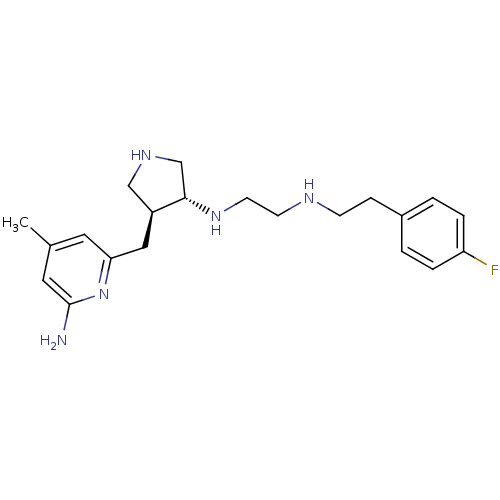
(CHEMBL1277787 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCCc2ccc(F)cc2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-10-19(27-21(23)11-15)12-17-13-25-14-20(17)26-9-8-24-7-6-16-2-4-18(22)5-3-16/h2-5,10-11,17,20,24-26H,6-9,12-14H2,1H3,(H2,23,27)/t17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50429230
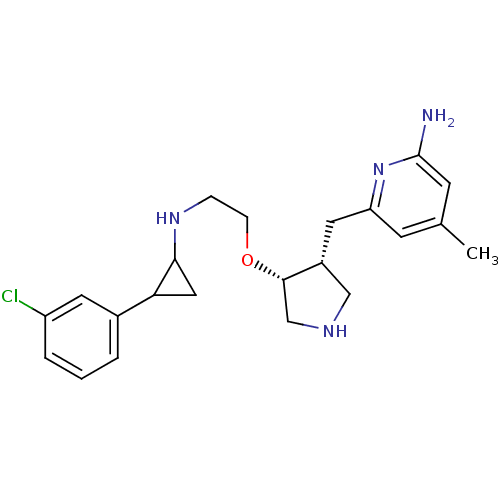
(CHEMBL2332791)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNC2CC2c2cccc(Cl)c2)c1 |r| Show InChI InChI=1S/C22H29ClN4O/c1-14-7-18(27-22(24)8-14)10-16-12-25-13-21(16)28-6-5-26-20-11-19(20)15-3-2-4-17(23)9-15/h2-4,7-9,16,19-21,25-26H,5-6,10-13H2,1H3,(H2,24,27)/t16-,19?,20?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide by hemoglobin capture assay |
Bioorg Med Chem 21: 1333-43 (2013)
Article DOI: 10.1016/j.bmc.2012.12.019
BindingDB Entry DOI: 10.7270/Q20G3MG9 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50352516

(CHEMBL1614783)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCC(F)(F)c2ccc(F)cc2)c1 |r| Show InChI InChI=1S/C21H27F3N4O/c1-14-8-18(28-20(25)9-14)10-15-11-27-12-19(15)29-7-6-26-13-21(23,24)16-2-4-17(22)5-3-16/h2-5,8-9,15,19,26-27H,6-7,10-13H2,1H3,(H2,25,28)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli assessed as reduction in formation of nitric oxide by hemoglobin capture assay |
J Med Chem 54: 6399-403 (2011)
Article DOI: 10.1021/jm200411j
BindingDB Entry DOI: 10.7270/Q2TX3FRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341689

(4-(4-(2-(6-Amino-4-methylpyridin-2-yl)ethyl)phenet...)Show InChI InChI=1S/C22H26N4/c1-15-11-20(26-21(23)12-15)10-9-18-5-3-17(4-6-18)7-8-19-13-16(2)25-22(24)14-19/h3-6,11-14H,7-10H2,1-2H3,(H2,23,26)(H2,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50352519
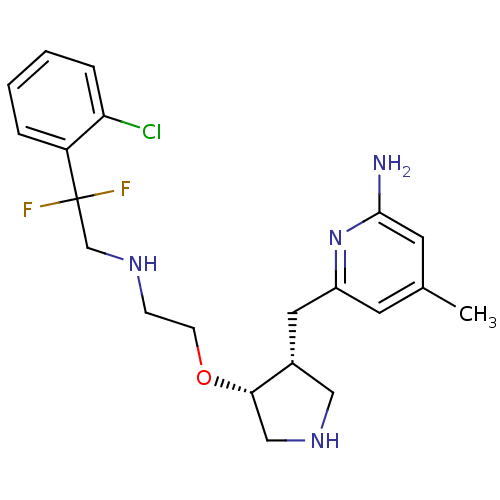
(CHEMBL1824856)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCC(F)(F)c2ccccc2Cl)c1 |r| Show InChI InChI=1S/C21H27ClF2N4O/c1-14-8-16(28-20(25)9-14)10-15-11-27-12-19(15)29-7-6-26-13-21(23,24)17-4-2-3-5-18(17)22/h2-5,8-9,15,19,26-27H,6-7,10-13H2,1H3,(H2,25,28)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli assessed as reduction in formation of nitric oxide by hemoglobin capture assay |
J Med Chem 54: 6399-403 (2011)
Article DOI: 10.1021/jm200411j
BindingDB Entry DOI: 10.7270/Q2TX3FRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50429229

(CHEMBL2332793)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCN[C@H]2C[C@@H]2c2cccc(F)c2)c1 |r| Show InChI InChI=1S/C22H29FN4O/c1-14-7-18(27-22(24)8-14)10-16-12-25-13-21(16)28-6-5-26-20-11-19(20)15-3-2-4-17(23)9-15/h2-4,7-9,16,19-21,25-26H,5-6,10-13H2,1H3,(H2,24,27)/t16-,19-,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide by hemoglobin capture assay |
Bioorg Med Chem 21: 1333-43 (2013)
Article DOI: 10.1016/j.bmc.2012.12.019
BindingDB Entry DOI: 10.7270/Q20G3MG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50429231

(CHEMBL2332792)Show SMILES Cc1cccc(c1)C1CC1NCCO[C@H]1CNC[C@H]1Cc1cc(C)cc(N)n1 |r| Show InChI InChI=1S/C23H32N4O/c1-15-4-3-5-17(8-15)20-12-21(20)26-6-7-28-22-14-25-13-18(22)11-19-9-16(2)10-23(24)27-19/h3-5,8-10,18,20-22,25-26H,6-7,11-14H2,1-2H3,(H2,24,27)/t18-,20?,21?,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide by hemoglobin capture assay |
Bioorg Med Chem 21: 1333-43 (2013)
Article DOI: 10.1016/j.bmc.2012.12.019
BindingDB Entry DOI: 10.7270/Q20G3MG9 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50352522
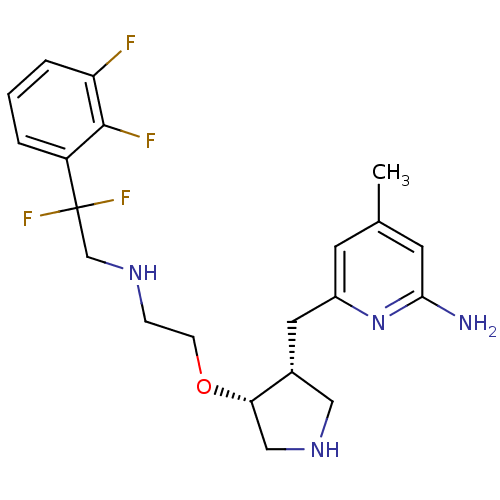
(CHEMBL1824859)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCC(F)(F)c2cccc(F)c2F)c1 |r| Show InChI InChI=1S/C21H26F4N4O/c1-13-7-15(29-19(26)8-13)9-14-10-28-11-18(14)30-6-5-27-12-21(24,25)16-3-2-4-17(22)20(16)23/h2-4,7-8,14,18,27-28H,5-6,9-12H2,1H3,(H2,26,29)/t14-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS expressed in Escherichia coli assessed as reduction in formation of nitric oxide by hemoglobin capture assay |
J Med Chem 54: 6399-403 (2011)
Article DOI: 10.1021/jm200411j
BindingDB Entry DOI: 10.7270/Q2TX3FRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50341679

(4-(2-(6-(2-(6-Amino-4-methylpyridin-2-yl)ethyl)pyr...)Show InChI InChI=1S/C21H25N5/c1-14-10-19(26-20(22)11-14)9-8-18-5-3-4-17(25-18)7-6-16-12-15(2)24-21(23)13-16/h3-5,10-13H,6-9H2,1-2H3,(H2,22,26)(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Irvine
| Assay Description
Inhibition assay using nitric oxide synthases. |
Biochemistry 49: 10803-10 (2010)
Article DOI: 10.1021/bi1013479
BindingDB Entry DOI: 10.7270/Q2KS6Q51 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341679

(4-(2-(6-(2-(6-Amino-4-methylpyridin-2-yl)ethyl)pyr...)Show InChI InChI=1S/C21H25N5/c1-14-10-19(26-20(22)11-14)9-8-18-5-3-4-17(25-18)7-6-16-12-15(2)24-21(23)13-16/h3-5,10-13H,6-9H2,1-2H3,(H2,22,26)(H2,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50341687
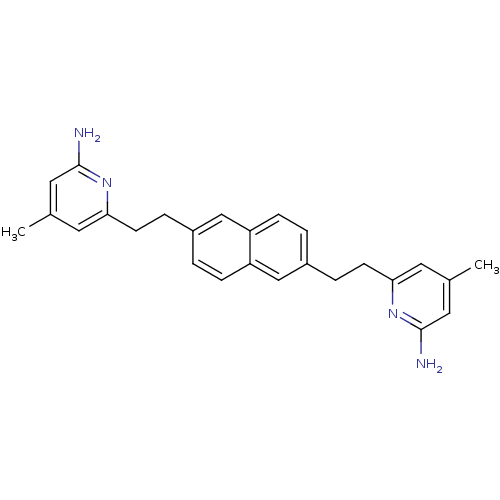
(6,6'-(2,2'-(Naphthalene-2,6-diyl)bis(ethane-2,1-di...)Show SMILES Cc1cc(N)nc(CCc2ccc3cc(CCc4cc(C)cc(N)n4)ccc3c2)c1 Show InChI InChI=1S/C26H28N4/c1-17-11-23(29-25(27)13-17)9-5-19-3-7-22-16-20(4-8-21(22)15-19)6-10-24-12-18(2)14-26(28)30-24/h3-4,7-8,11-16H,5-6,9-10H2,1-2H3,(H2,27,29)(H2,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM29235
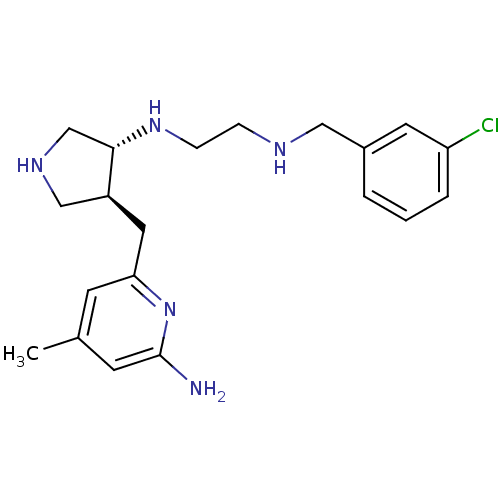
(aminopyridine-pyrrolidine, 7)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2cccc(Cl)c2)c1 |r| Show InChI InChI=1S/C20H28ClN5/c1-14-7-18(26-20(22)8-14)10-16-12-24-13-19(16)25-6-5-23-11-15-3-2-4-17(21)9-15/h2-4,7-9,16,19,23-25H,5-6,10-13H2,1H3,(H2,22,26)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM29233

(CHEMBL508014 | aminopyridine-pyrrolidine, 5)Show InChI InChI=1S/C12H21N5/c13-4-5-16-11-8-15-7-9(11)6-10-2-1-3-12(14)17-10/h1-3,9,11,15-16H,4-8,13H2,(H2,14,17)/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330869
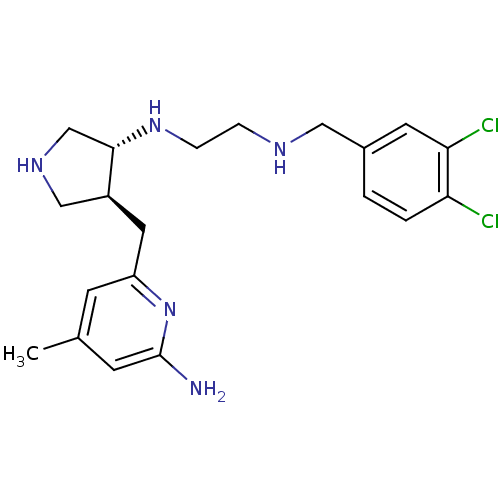
(CHEMBL1277601 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2ccc(Cl)c(Cl)c2)c1 |r| Show InChI InChI=1S/C20H27Cl2N5/c1-13-6-16(27-20(23)7-13)9-15-11-25-12-19(15)26-5-4-24-10-14-2-3-17(21)18(22)8-14/h2-3,6-8,15,19,24-26H,4-5,9-12H2,1H3,(H2,23,27)/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 414 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330870
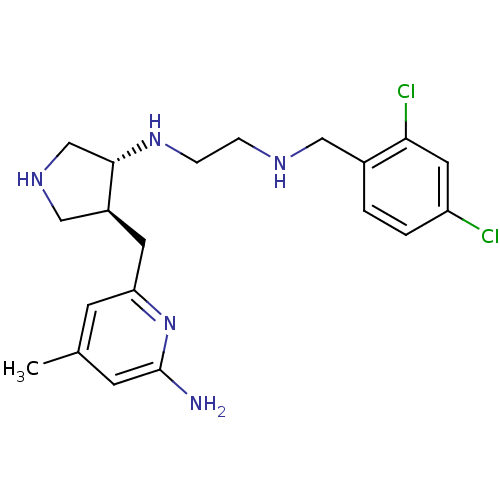
(CHEMBL1277602 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2ccc(Cl)cc2Cl)c1 |r| Show InChI InChI=1S/C20H27Cl2N5/c1-13-6-17(27-20(23)7-13)8-15-11-25-12-19(15)26-5-4-24-10-14-2-3-16(21)9-18(14)22/h2-3,6-7,9,15,19,24-26H,4-5,8,10-12H2,1H3,(H2,23,27)/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330895

(CHEMBL1277327 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2ccc(Cl)cc2)c1 |r| Show InChI InChI=1S/C20H28ClN5/c1-14-8-18(26-20(22)9-14)10-16-12-24-13-19(16)25-7-6-23-11-15-2-4-17(21)5-3-15/h2-5,8-9,16,19,23-25H,6-7,10-13H2,1H3,(H2,22,26)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50225106
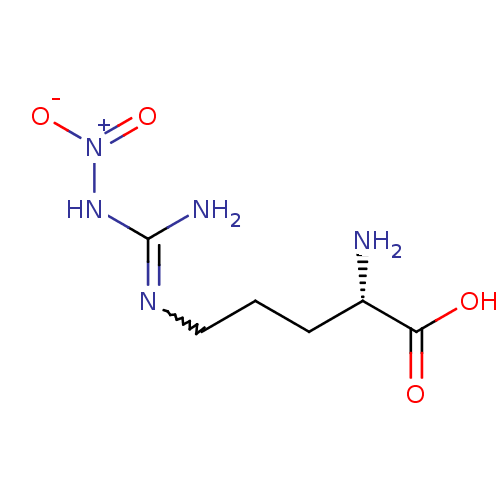
((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data