 Found 328 hits with Last Name = 'denton' and Initial = 's'
Found 328 hits with Last Name = 'denton' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287520

(CHEMBL4159883)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NC(C)c1cnn(C)n1 Show InChI InChI=1S/C19H20ClN5O4S/c1-12(16-11-21-25(2)23-16)22-19(26)13-4-9-17(29-3)18(10-13)30(27,28)24-15-7-5-14(20)6-8-15/h4-12,24H,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287557

(CHEMBL4159568)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(C)cc1)C(=O)NCc1nc(C)no1 Show InChI InChI=1S/C19H20N4O5S/c1-12-4-7-15(8-5-12)23-29(25,26)17-10-14(6-9-16(17)27-3)19(24)20-11-18-21-13(2)22-28-18/h4-10,23H,11H2,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287554

(CHEMBL4162926)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(C)cc2)n1 Show InChI InChI=1S/C19H20N4O4S/c1-12-4-8-16(9-5-12)23-28(25,26)17-10-15(7-6-13(17)2)19(24)20-11-18-21-14(3)22-27-18/h4-10,23H,11H2,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287553
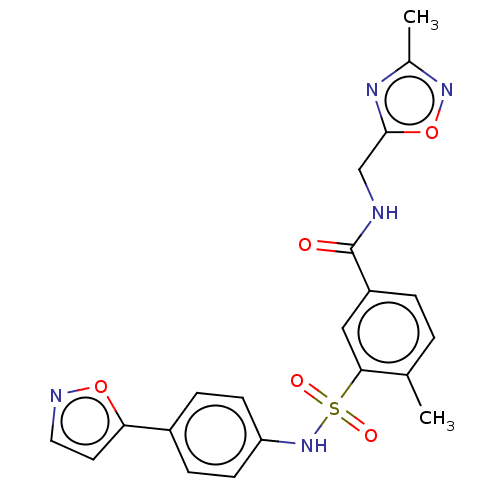
(CHEMBL4166791)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(cc2)-c2ccno2)n1 Show InChI InChI=1S/C21H19N5O5S/c1-13-3-4-16(21(27)22-12-20-24-14(2)25-31-20)11-19(13)32(28,29)26-17-7-5-15(6-8-17)18-9-10-23-30-18/h3-11,26H,12H2,1-2H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287559
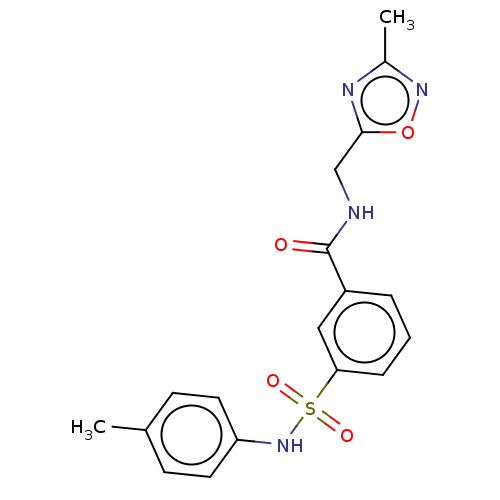
(CHEMBL4170197)Show SMILES Cc1noc(CNC(=O)c2cccc(c2)S(=O)(=O)Nc2ccc(C)cc2)n1 Show InChI InChI=1S/C18H18N4O4S/c1-12-6-8-15(9-7-12)22-27(24,25)16-5-3-4-14(10-16)18(23)19-11-17-20-13(2)21-26-17/h3-10,22H,11H2,1-2H3,(H,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287556

(CHEMBL4161489)Show SMILES Cc1ccc(cc1S(=O)(=O)Nc1ccc(cc1)-c1ccno1)C(=O)NCc1cn(C)nn1 Show InChI InChI=1S/C21H20N6O4S/c1-14-3-4-16(21(28)22-12-18-13-27(2)26-24-18)11-20(14)32(29,30)25-17-7-5-15(6-8-17)19-9-10-23-31-19/h3-11,13,25H,12H2,1-2H3,(H,22,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287552
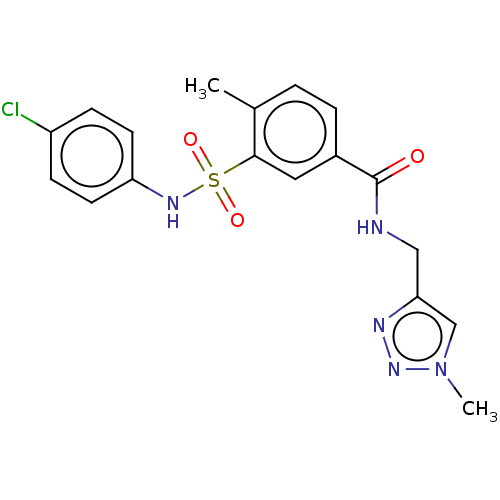
(CHEMBL4175004)Show SMILES Cc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NCc1cn(C)nn1 Show InChI InChI=1S/C18H18ClN5O3S/c1-12-3-4-13(18(25)20-10-16-11-24(2)23-21-16)9-17(12)28(26,27)22-15-7-5-14(19)6-8-15/h3-9,11,22H,10H2,1-2H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287520

(CHEMBL4159883)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NC(C)c1cnn(C)n1 Show InChI InChI=1S/C19H20ClN5O4S/c1-12(16-11-21-25(2)23-16)22-19(26)13-4-9-17(29-3)18(10-13)30(27,28)24-15-7-5-14(20)6-8-15/h4-12,24H,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287558
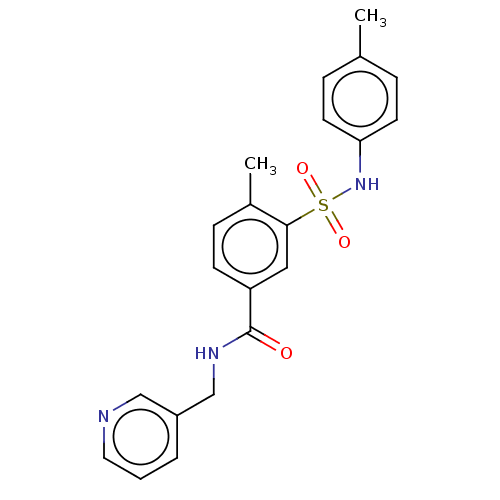
(CHEMBL1333494)Show SMILES Cc1ccc(NS(=O)(=O)c2cc(ccc2C)C(=O)NCc2cccnc2)cc1 Show InChI InChI=1S/C21H21N3O3S/c1-15-5-9-19(10-6-15)24-28(26,27)20-12-18(8-7-16(20)2)21(25)23-14-17-4-3-11-22-13-17/h3-13,24H,14H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287555
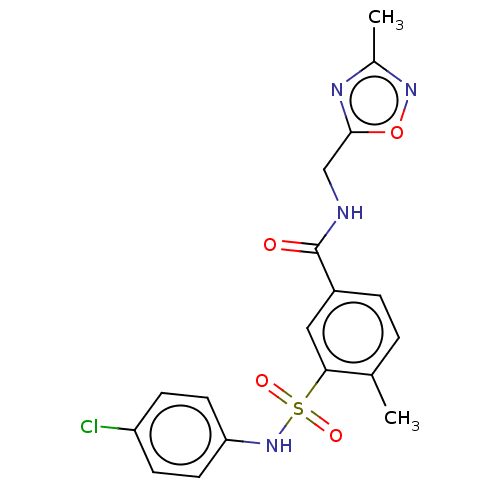
(CHEMBL4174695)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(Cl)cc2)n1 Show InChI InChI=1S/C18H17ClN4O4S/c1-11-3-4-13(18(24)20-10-17-21-12(2)22-27-17)9-16(11)28(25,26)23-15-7-5-14(19)6-8-15/h3-9,23H,10H2,1-2H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287519
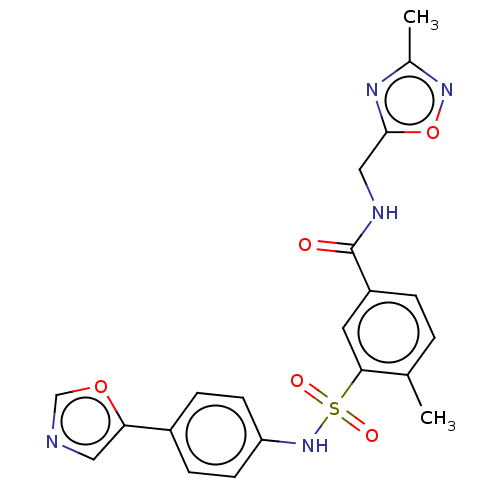
(CHEMBL4172084)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(cc2)-c2cnco2)n1 Show InChI InChI=1S/C21H19N5O5S/c1-13-3-4-16(21(27)23-11-20-24-14(2)25-31-20)9-19(13)32(28,29)26-17-7-5-15(6-8-17)18-10-22-12-30-18/h3-10,12,26H,11H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359779
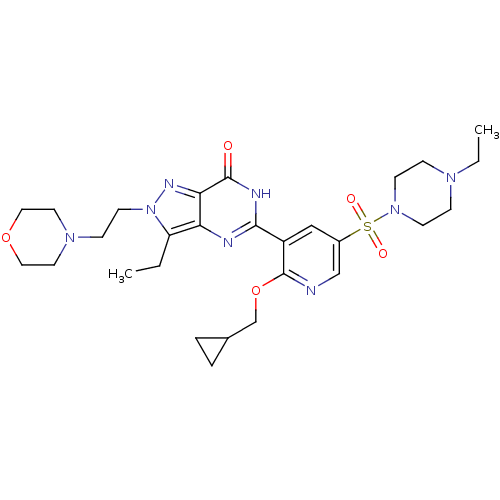
(CHEMBL1928271)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC2CC2)c(c1)-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C28H40N8O5S/c1-3-23-24-25(32-36(23)12-9-34-13-15-40-16-14-34)27(37)31-26(30-24)22-17-21(18-29-28(22)41-19-20-5-6-20)42(38,39)35-10-7-33(4-2)8-11-35/h17-18,20H,3-16,19H2,1-2H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359773
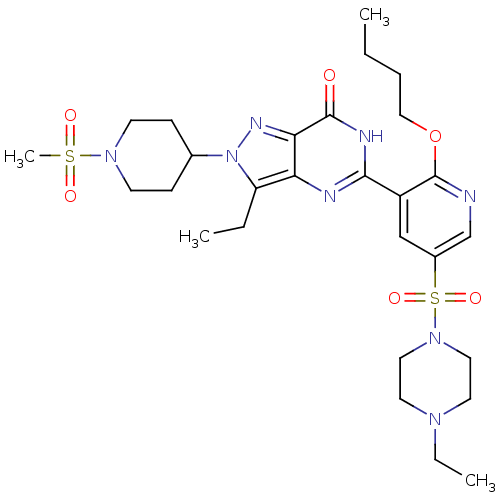
(CHEMBL1928265)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCN(CC1)S(C)(=O)=O)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C28H42N8O6S2/c1-5-8-17-42-28-22(18-21(19-29-28)44(40,41)35-15-13-33(7-3)14-16-35)26-30-24-23(6-2)36(32-25(24)27(37)31-26)20-9-11-34(12-10-20)43(4,38)39/h18-20H,5-17H2,1-4H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359767
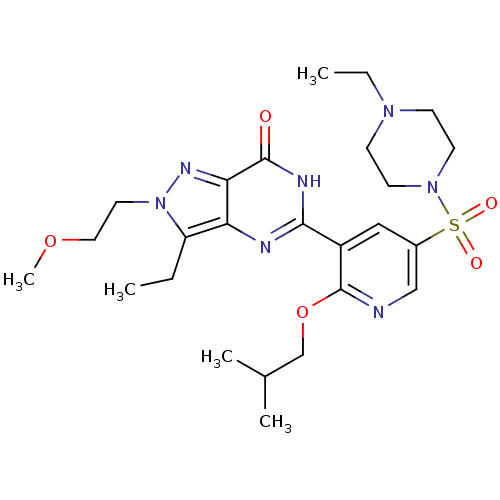
(CHEMBL1928259)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC(C)C)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H37N7O5S/c1-6-20-21-22(29-32(20)12-13-36-5)24(33)28-23(27-21)19-14-18(15-26-25(19)37-16-17(3)4)38(34,35)31-10-8-30(7-2)9-11-31/h14-15,17H,6-13,16H2,1-5H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359795
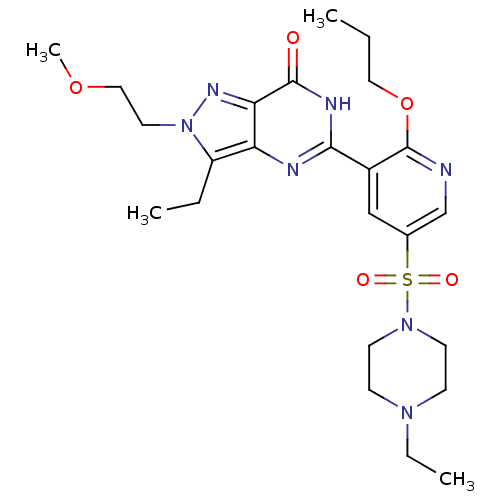
(CHEMBL1928258)Show SMILES CCCOc1ncc(cc1-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C24H35N7O5S/c1-5-13-36-24-18(15-17(16-25-24)37(33,34)30-10-8-29(7-3)9-11-30)22-26-20-19(6-2)31(12-14-35-4)28-21(20)23(32)27-22/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359774

(CHEMBL1928266)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCN(C)CC1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C28H42N8O4S/c1-5-8-17-40-28-22(18-21(19-29-28)41(38,39)35-15-13-34(7-3)14-16-35)26-30-24-23(6-2)36(32-25(24)27(37)31-26)20-9-11-33(4)12-10-20/h18-20H,5-17H2,1-4H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359768
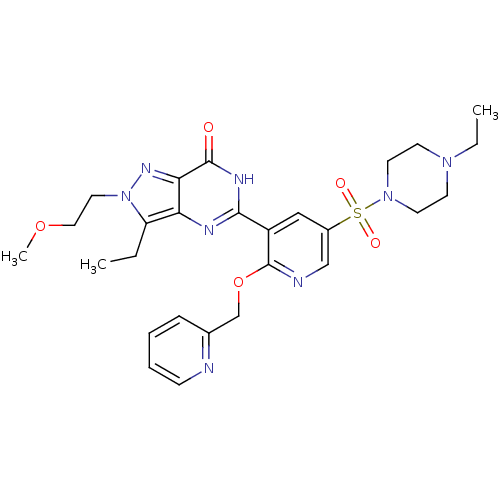
(CHEMBL1928260)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCc2ccccn2)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H34N8O5S/c1-4-22-23-24(32-35(22)14-15-39-3)26(36)31-25(30-23)21-16-20(41(37,38)34-12-10-33(5-2)11-13-34)17-29-27(21)40-18-19-8-6-7-9-28-19/h6-9,16-17H,4-5,10-15,18H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359777
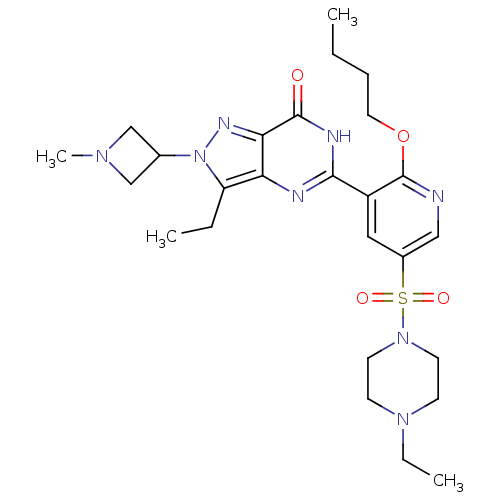
(CHEMBL1928269)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CN(C)C1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H38N8O4S/c1-5-8-13-38-26-20(14-19(15-27-26)39(36,37)33-11-9-32(7-3)10-12-33)24-28-22-21(6-2)34(18-16-31(4)17-18)30-23(22)25(35)29-24/h14-15,18H,5-13,16-17H2,1-4H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359778
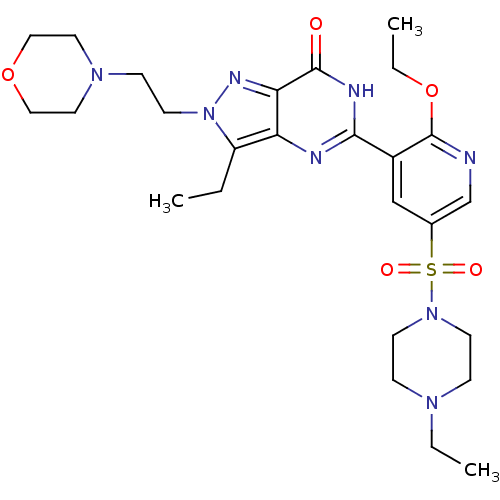
(CHEMBL1928270)Show SMILES CCOc1ncc(cc1-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H38N8O5S/c1-4-21-22-23(30-34(21)12-9-32-13-15-38-16-14-32)25(35)29-24(28-22)20-17-19(18-27-26(20)39-6-3)40(36,37)33-10-7-31(5-2)8-11-33/h17-18H,4-16H2,1-3H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359789
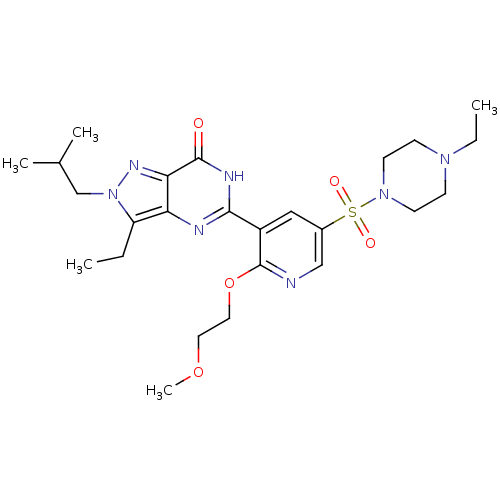
(CHEMBL1928252)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CC(C)C)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H37N7O5S/c1-6-20-21-22(29-32(20)16-17(3)4)24(33)28-23(27-21)19-14-18(15-26-25(19)37-13-12-36-5)38(34,35)31-10-8-30(7-2)9-11-31/h14-15,17H,6-13,16H2,1-5H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359771
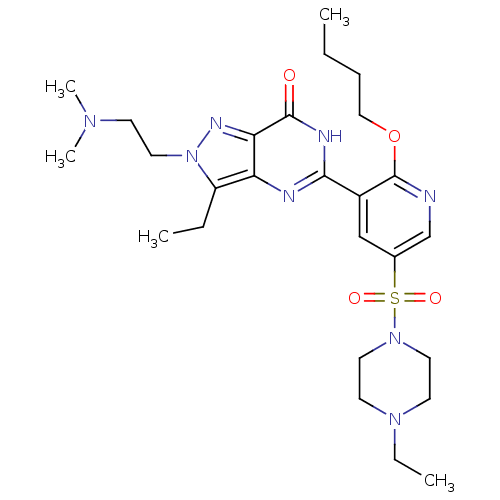
(CHEMBL1928263)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(CCN(C)C)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H40N8O4S/c1-6-9-16-38-26-20(17-19(18-27-26)39(36,37)33-13-11-32(8-3)12-14-33)24-28-22-21(7-2)34(15-10-31(4)5)30-23(22)25(35)29-24/h17-18H,6-16H2,1-5H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359790

(CHEMBL1928253)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CC3CC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H35N7O5S/c1-4-20-21-22(29-32(20)16-17-6-7-17)24(33)28-23(27-21)19-14-18(15-26-25(19)37-13-12-36-3)38(34,35)31-10-8-30(5-2)9-11-31/h14-15,17H,4-13,16H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50246483
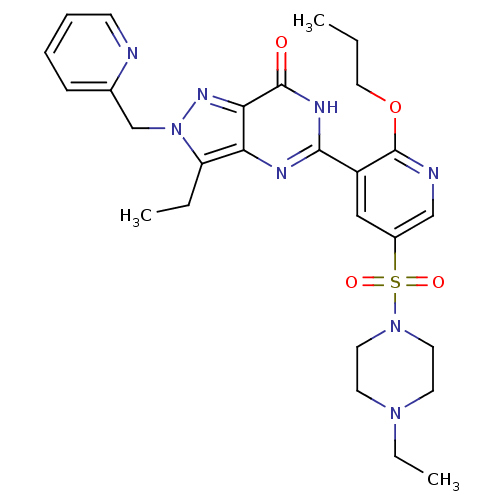
(3-ethyl-5-(5-(4-ethylpiperazin-1-ylsulfonyl)-2-pro...)Show SMILES CCCOc1ncc(cc1-c1nc2c(CC)n(Cc3ccccn3)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C27H34N8O4S/c1-4-15-39-27-21(16-20(17-29-27)40(37,38)34-13-11-33(6-3)12-14-34)25-30-23-22(5-2)35(32-24(23)26(36)31-25)18-19-9-7-8-10-28-19/h7-10,16-17H,4-6,11-15,18H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359770
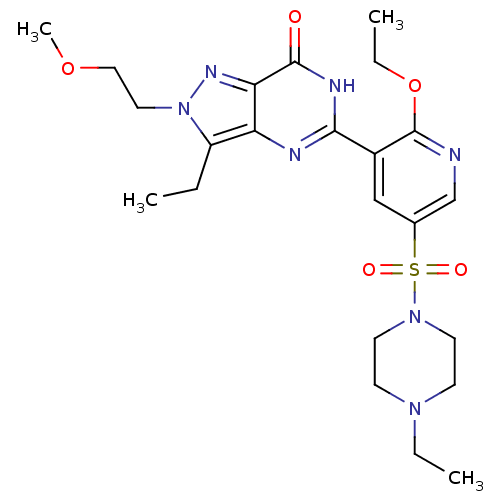
(CHEMBL1928262)Show SMILES CCOc1ncc(cc1-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H33N7O5S/c1-5-18-19-20(27-30(18)12-13-34-4)22(31)26-21(25-19)17-14-16(15-24-23(17)35-7-3)36(32,33)29-10-8-28(6-2)9-11-29/h14-15H,5-13H2,1-4H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359780
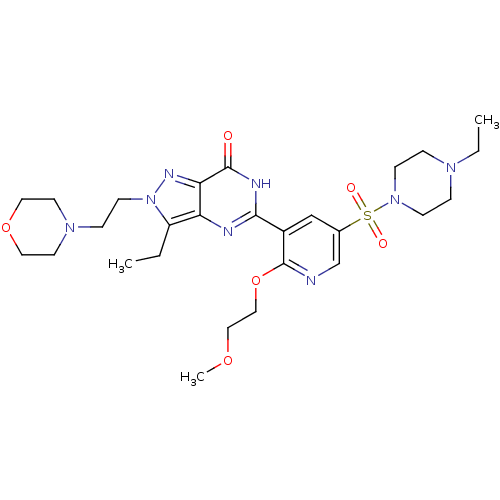
(CHEMBL1928272)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H40N8O6S/c1-4-22-23-24(31-35(22)11-8-33-12-14-40-15-13-33)26(36)30-25(29-23)21-18-20(19-28-27(21)41-17-16-39-3)42(37,38)34-9-6-32(5-2)7-10-34/h18-19H,4-17H2,1-3H3,(H,29,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359787
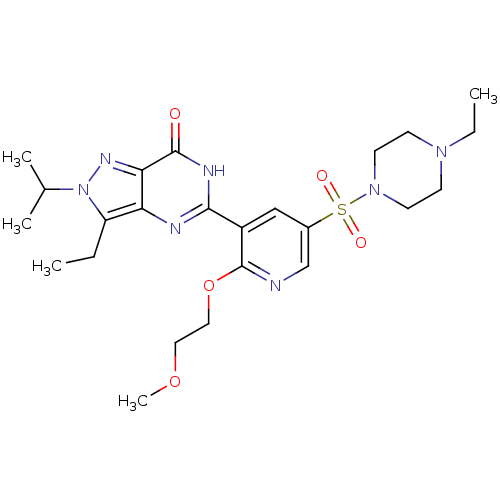
(CHEMBL1928250)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C(C)C Show InChI InChI=1S/C24H35N7O5S/c1-6-19-20-21(28-31(19)16(3)4)23(32)27-22(26-20)18-14-17(15-25-24(18)36-13-12-35-5)37(33,34)30-10-8-29(7-2)9-11-30/h14-16H,6-13H2,1-5H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359769

(CHEMBL1928261)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C24H35N7O6S/c1-5-19-20-21(28-31(19)11-12-35-3)23(32)27-22(26-20)18-15-17(16-25-24(18)37-14-13-36-4)38(33,34)30-9-7-29(6-2)8-10-30/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359791

(CHEMBL1928254)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C26H37N7O6S/c1-4-21-22-23(30-33(21)18-6-12-38-13-7-18)25(34)29-24(28-22)20-16-19(17-27-26(20)39-15-14-37-3)40(35,36)32-10-8-31(5-2)9-11-32/h16-18H,4-15H2,1-3H3,(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359788
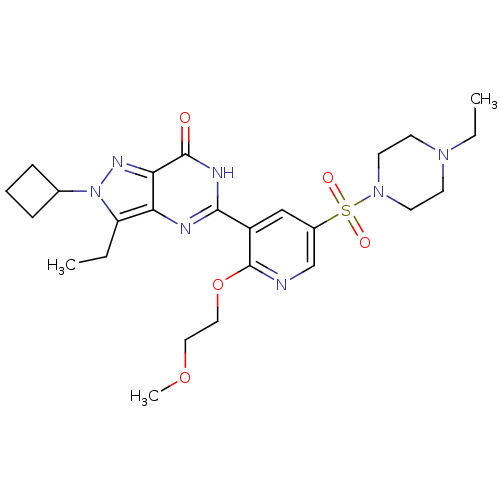
(CHEMBL1928251)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCC1 Show InChI InChI=1S/C25H35N7O5S/c1-4-20-21-22(29-32(20)17-7-6-8-17)24(33)28-23(27-21)19-15-18(16-26-25(19)37-14-13-36-3)38(34,35)31-11-9-30(5-2)10-12-31/h15-17H,4-14H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359786

(CHEMBL1928249)Show SMILES CCCn1nc2c(nc([nH]c2=O)-c2cc(cnc2OCCOC)S(=O)(=O)N2CCN(CC)CC2)c1CC Show InChI InChI=1S/C24H35N7O5S/c1-5-8-31-19(6-2)20-21(28-31)23(32)27-22(26-20)18-15-17(16-25-24(18)36-14-13-35-4)37(33,34)30-11-9-29(7-3)10-12-30/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257167
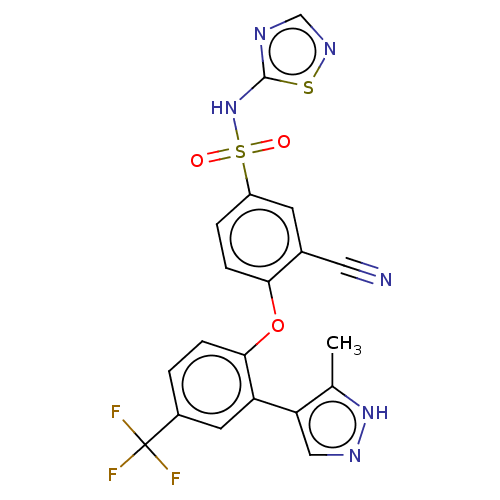
(CHEMBL2325619)Show SMILES Cc1[nH]ncc1-c1cc(ccc1Oc1ccc(cc1C#N)S(=O)(=O)Nc1ncns1)C(F)(F)F Show InChI InChI=1S/C20H13F3N6O3S2/c1-11-16(9-26-28-11)15-7-13(20(21,22)23)2-4-18(15)32-17-5-3-14(6-12(17)8-24)34(30,31)29-19-25-10-27-33-19/h2-7,9-10H,1H3,(H,26,28)(H,25,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359772

(CHEMBL1928264)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC(C)C)c(c1)-c1nc2c(CC)n(CCCN(C)C)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H42N8O4S/c1-7-22-23-24(31-35(22)11-9-10-32(5)6)26(36)30-25(29-23)21-16-20(17-28-27(21)39-18-19(3)4)40(37,38)34-14-12-33(8-2)13-15-34/h16-17,19H,7-15,18H2,1-6H3,(H,29,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359782

(CHEMBL1928274)Show SMILES CCCOc1ncc(cc1-c1nc2c3CCCCn3nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H31N7O4S/c1-3-13-34-23-17(14-16(15-24-23)35(32,33)29-11-9-28(4-2)10-12-29)21-25-19-18-7-5-6-8-30(18)27-20(19)22(31)26-21/h14-15H,3-13H2,1-2H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359785
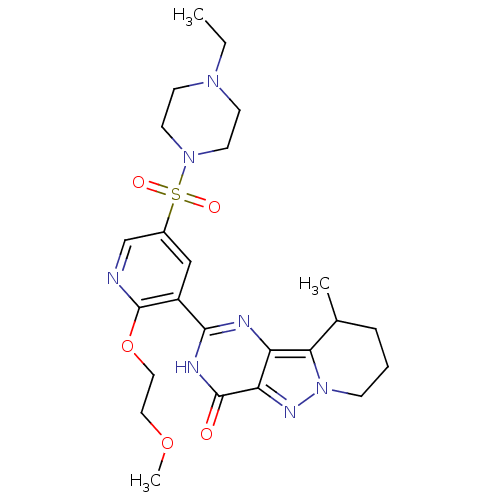
(CHEMBL1928277)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c3C(C)CCCn3nc2c(=O)[nH]1 Show InChI InChI=1S/C24H33N7O5S/c1-4-29-8-10-30(11-9-29)37(33,34)17-14-18(24(25-15-17)36-13-12-35-3)22-26-19-20(23(32)27-22)28-31-7-5-6-16(2)21(19)31/h14-16H,4-13H2,1-3H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359793

(CHEMBL1928256)Show SMILES CCc1n(CC(C)C)nc2c1nc([nH]c2=O)-c1cc(cnc1OCCOC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C24H35N7O5S/c1-6-19-20-21(28-31(19)15-16(2)3)23(32)27-22(26-20)18-13-17(14-25-24(18)36-12-11-35-5)37(33,34)30-9-7-29(4)8-10-30/h13-14,16H,6-12,15H2,1-5H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257179
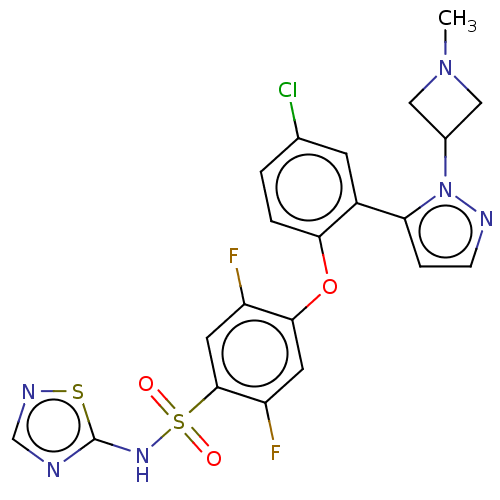
(CHEMBL2325622)Show SMILES CN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C21H17ClF2N6O3S2/c1-29-9-13(10-29)30-17(4-5-26-30)14-6-12(22)2-3-18(14)33-19-7-16(24)20(8-15(19)23)35(31,32)28-21-25-11-27-34-21/h2-8,11,13H,9-10H2,1H3,(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359784
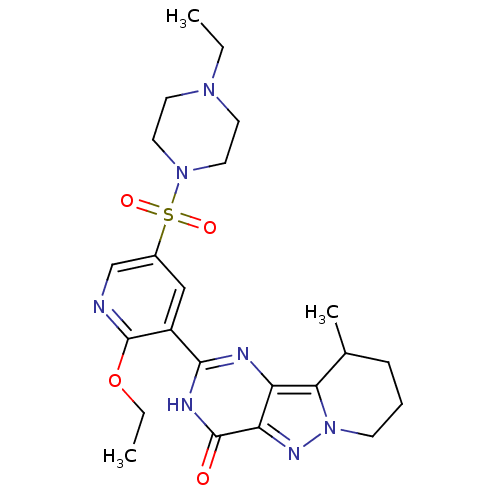
(CHEMBL1928276)Show SMILES CCOc1ncc(cc1-c1nc2c3C(C)CCCn3nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H31N7O4S/c1-4-28-9-11-29(12-10-28)35(32,33)16-13-17(23(24-14-16)34-5-2)21-25-18-19(22(31)26-21)27-30-8-6-7-15(3)20(18)30/h13-15H,4-12H2,1-3H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257166
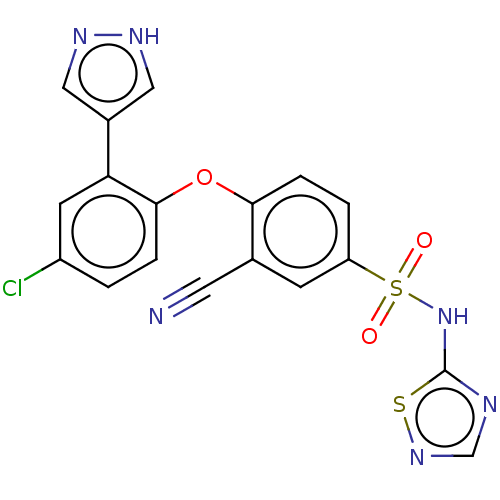
(CHEMBL2325330)Show SMILES Clc1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1cn[nH]c1 Show InChI InChI=1S/C18H11ClN6O3S2/c19-13-1-3-17(15(6-13)12-8-22-23-9-12)28-16-4-2-14(5-11(16)7-20)30(26,27)25-18-21-10-24-29-18/h1-6,8-10H,(H,22,23)(H,21,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359792
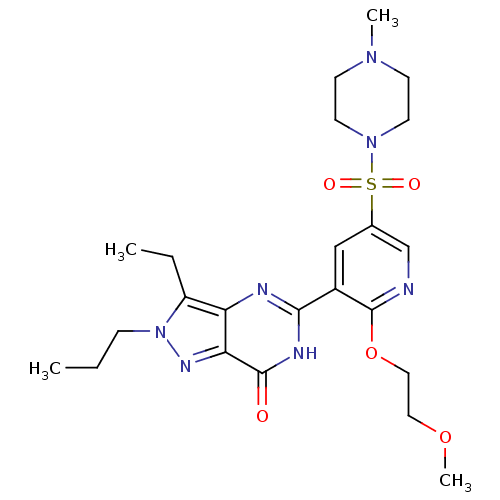
(CHEMBL1928255)Show SMILES CCCn1nc2c(nc([nH]c2=O)-c2cc(cnc2OCCOC)S(=O)(=O)N2CCN(C)CC2)c1CC Show InChI InChI=1S/C23H33N7O5S/c1-5-7-30-18(6-2)19-20(27-30)22(31)26-21(25-19)17-14-16(15-24-23(17)35-13-12-34-4)36(32,33)29-10-8-28(3)9-11-29/h14-15H,5-13H2,1-4H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257216
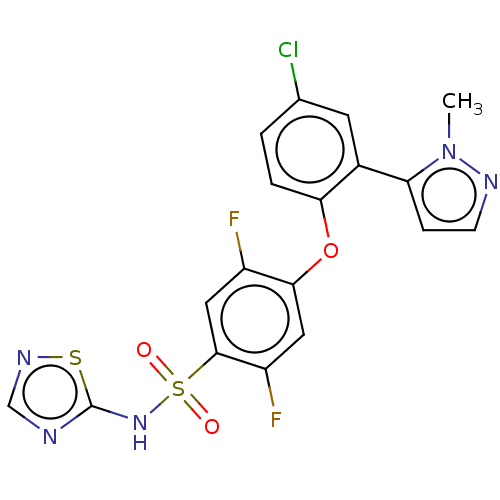
(CHEMBL2325350)Show SMILES Cn1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C18H12ClF2N5O3S2/c1-26-14(4-5-23-26)11-6-10(19)2-3-15(11)29-16-7-13(21)17(8-12(16)20)31(27,28)25-18-22-9-24-30-18/h2-9H,1H3,(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257180
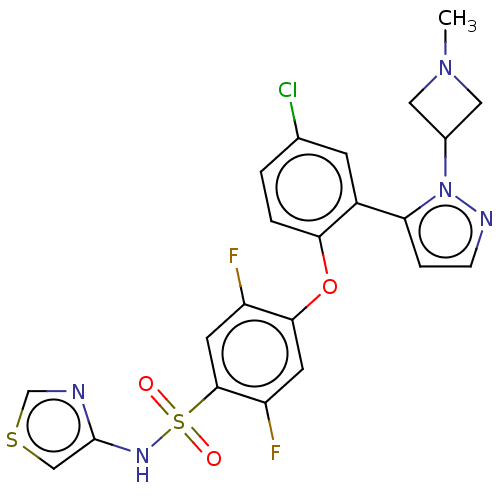
(CHEMBL2325317)Show SMILES CN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C22H18ClF2N5O3S2/c1-29-9-14(10-29)30-18(4-5-27-30)15-6-13(23)2-3-19(15)33-20-7-17(25)21(8-16(20)24)35(31,32)28-22-11-34-12-26-22/h2-8,11-12,14,28H,9-10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257178
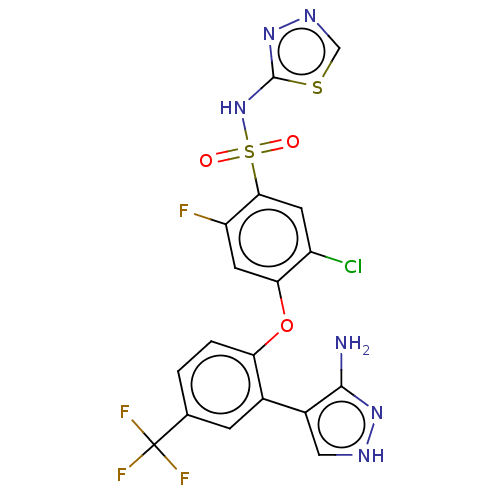
(CHEMBL2325627)Show SMILES Nc1n[nH]cc1-c1cc(ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1nncs1)C(F)(F)F Show InChI InChI=1S/C18H11ClF4N6O3S2/c19-11-4-15(34(30,31)29-17-28-26-7-33-17)12(20)5-14(11)32-13-2-1-8(18(21,22)23)3-9(13)10-6-25-27-16(10)24/h1-7H,(H,28,29)(H3,24,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359776

(CHEMBL1928268)Show SMILES CCOc1ncc(cc1C(=O)Nc1c(CC)n(nc1C(N)=O)C1CN(C)C1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C24H36N8O5S/c1-5-19-20(21(22(25)33)28-32(19)16-14-29(4)15-16)27-23(34)18-12-17(13-26-24(18)37-7-3)38(35,36)31-10-8-30(6-2)9-11-31/h12-13,16H,5-11,14-15H2,1-4H3,(H2,25,33)(H,27,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359783
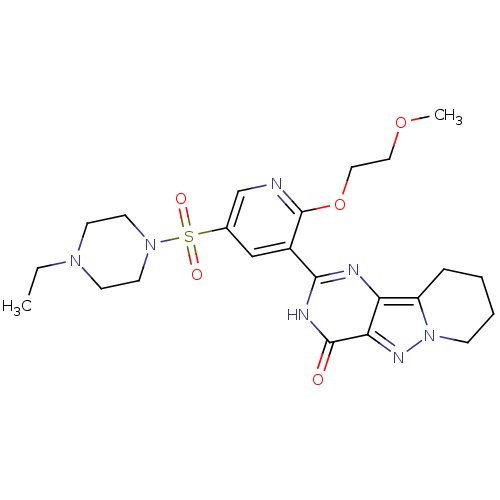
(CHEMBL1928275)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c3CCCCn3nc2c(=O)[nH]1 Show InChI InChI=1S/C23H31N7O5S/c1-3-28-8-10-29(11-9-28)36(32,33)16-14-17(23(24-15-16)35-13-12-34-2)21-25-19-18-6-4-5-7-30(18)27-20(19)22(31)26-21/h14-15H,3-13H2,1-2H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257168
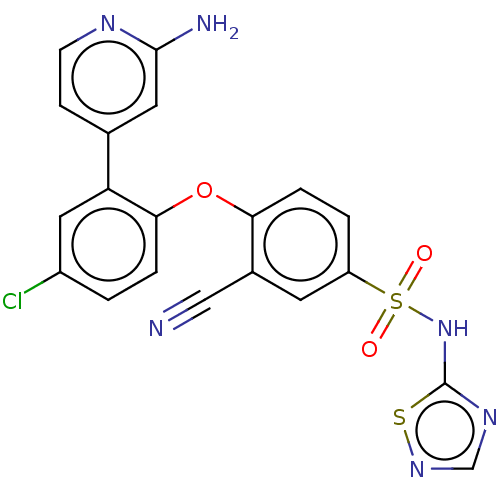
(CHEMBL2325013)Show SMILES Nc1cc(ccn1)-c1cc(Cl)ccc1Oc1ccc(cc1C#N)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C20H13ClN6O3S2/c21-14-1-3-18(16(9-14)12-5-6-24-19(23)8-12)30-17-4-2-15(7-13(17)10-22)32(28,29)27-20-25-11-26-31-20/h1-9,11H,(H2,23,24)(H,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257214
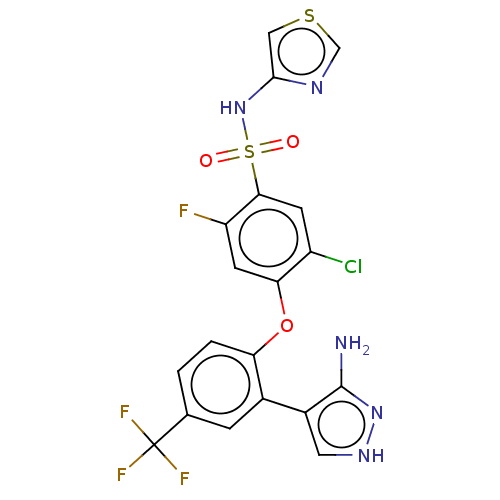
(CHEMBL2325601)Show SMILES Nc1n[nH]cc1-c1cc(ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1cscn1)C(F)(F)F Show InChI InChI=1S/C19H12ClF4N5O3S2/c20-12-4-16(34(30,31)29-17-7-33-8-26-17)13(21)5-15(12)32-14-2-1-9(19(22,23)24)3-10(14)11-6-27-28-18(11)25/h1-8,29H,(H3,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257181
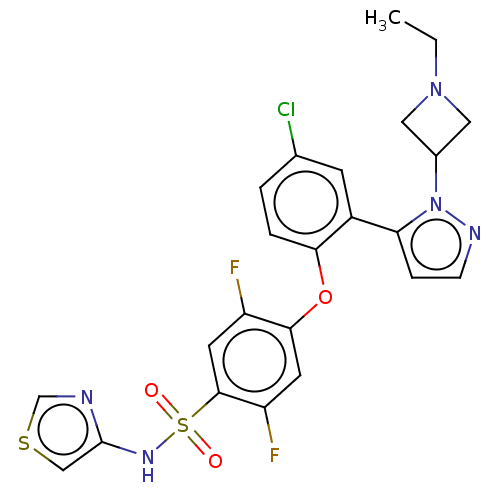
(CHEMBL2325020)Show SMILES CCN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C23H20ClF2N5O3S2/c1-2-30-10-15(11-30)31-19(5-6-28-31)16-7-14(24)3-4-20(16)34-21-8-18(26)22(9-17(21)25)36(32,33)29-23-12-35-13-27-23/h3-9,12-13,15,29H,2,10-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50240267
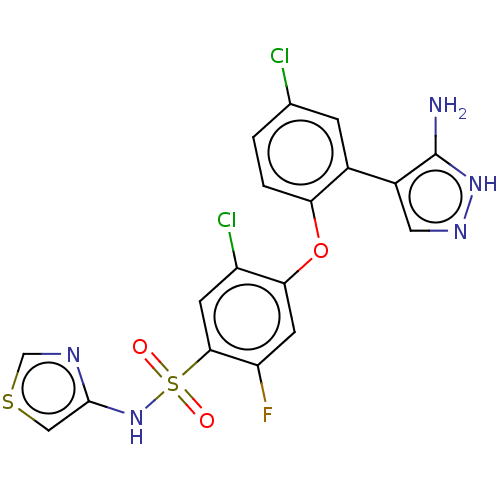
(CHEMBL2325014)Show SMILES Nc1[nH]ncc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C18H12Cl2FN5O3S2/c19-9-1-2-14(10(3-9)11-6-24-25-18(11)22)29-15-5-13(21)16(4-12(15)20)31(27,28)26-17-7-30-8-23-17/h1-8,26H,(H3,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257211
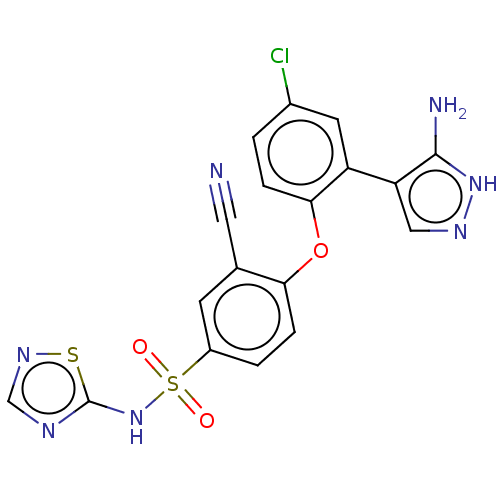
(CHEMBL2325038)Show SMILES Nc1[nH]ncc1-c1cc(Cl)ccc1Oc1ccc(cc1C#N)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C18H12ClN7O3S2/c19-11-1-3-16(13(6-11)14-8-23-25-17(14)21)29-15-4-2-12(5-10(15)7-20)31(27,28)26-18-22-9-24-30-18/h1-6,8-9H,(H3,21,23,25)(H,22,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data