 Found 473 hits with Last Name = 'derian' and Initial = 'ck'
Found 473 hits with Last Name = 'derian' and Initial = 'ck' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162995
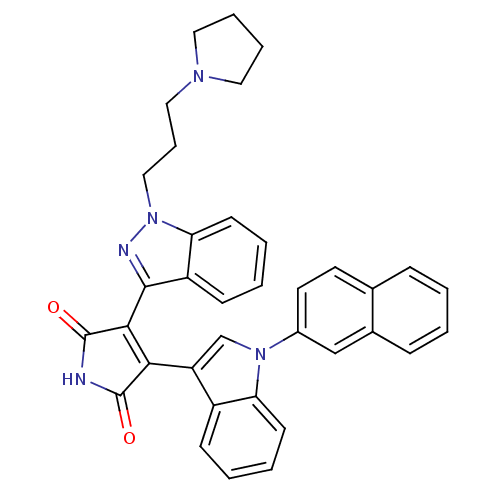
(3-[1-(1,2-Dihydro-pyridin-2-yl)-1H-indazol-3-yl]-4...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O2/c42-35-32(29-23-40(30-14-5-3-12-27(29)30)26-17-16-24-10-1-2-11-25(24)22-26)33(36(43)37-35)34-28-13-4-6-15-31(28)41(38-34)21-9-20-39-18-7-8-19-39/h1-6,10-17,22-23H,7-9,18-21H2,(H,37,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162996
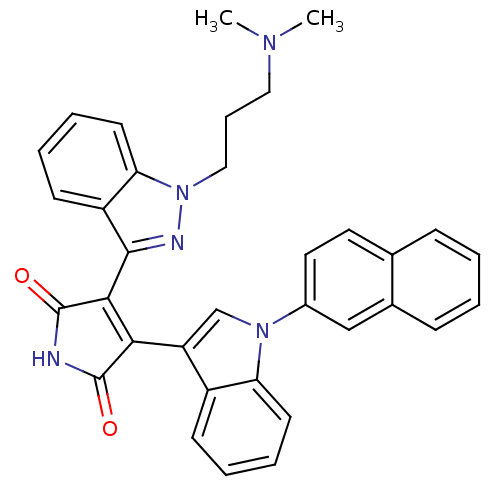
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162990
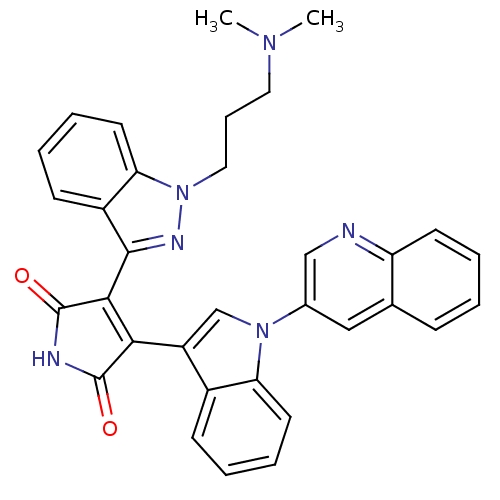
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50163000
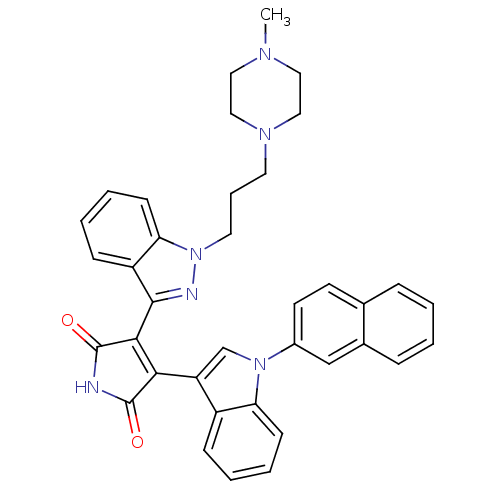
(3-(1-Piperazin-1-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES CN1CCN(CCCn2nc(C3=C(C(=O)NC3=O)c3cn(-c4ccc5ccccc5c4)c4ccccc34)c3ccccc23)CC1 |t:11| Show InChI InChI=1S/C37H34N6O2/c1-40-19-21-41(22-20-40)17-8-18-43-32-14-7-5-12-29(32)35(39-43)34-33(36(44)38-37(34)45)30-24-42(31-13-6-4-11-28(30)31)27-16-15-25-9-2-3-10-26(25)23-27/h2-7,9-16,23-24H,8,17-22H2,1H3,(H,38,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162992
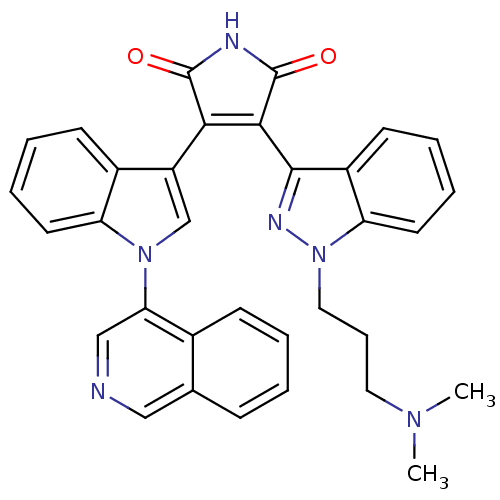
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cncc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-27-15-8-6-13-24(27)31(36-39)30-29(32(40)35-33(30)41)25-20-38(26-14-7-5-12-23(25)26)28-19-34-18-21-10-3-4-11-22(21)28/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162993
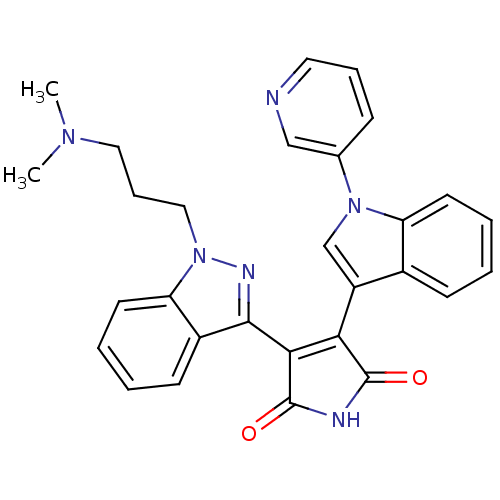
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccnc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H26N6O2/c1-33(2)15-8-16-35-24-13-6-4-11-21(24)27(32-35)26-25(28(36)31-29(26)37)22-18-34(19-9-7-14-30-17-19)23-12-5-3-10-20(22)23/h3-7,9-14,17-18H,8,15-16H2,1-2H3,(H,31,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50163002
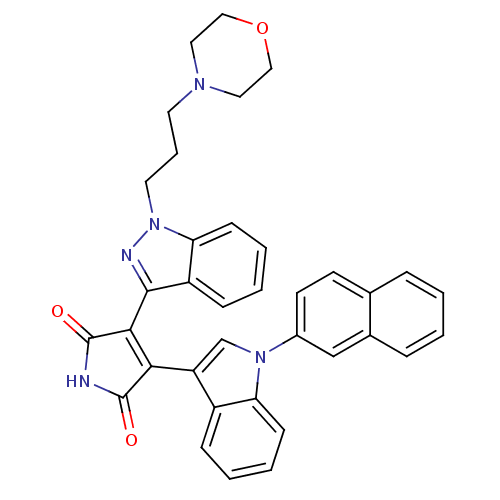
(3-(1-Morpholin-2-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCOCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O3/c42-35-32(29-23-40(30-12-5-3-10-27(29)30)26-15-14-24-8-1-2-9-25(24)22-26)33(36(43)37-35)34-28-11-4-6-13-31(28)41(38-34)17-7-16-39-18-20-44-21-19-39/h1-6,8-15,22-23H,7,16-21H2,(H,37,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050933

((S)-2-((S)-2-{(S)-2-[3-(4-Chloro-phenyl)-ureido]-4...)Show SMILES CSCC[C@H](NC(=O)Nc1ccc(Cl)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C27H35ClN4O5S/c1-17(2)15-22(25(34)31-23(26(35)36)16-18-7-5-4-6-8-18)30-24(33)21(13-14-38-3)32-27(37)29-20-11-9-19(28)10-12-20/h4-12,17,21-23H,13-16H2,1-3H3,(H,30,33)(H,31,34)(H,35,36)(H2,29,32,37)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050935
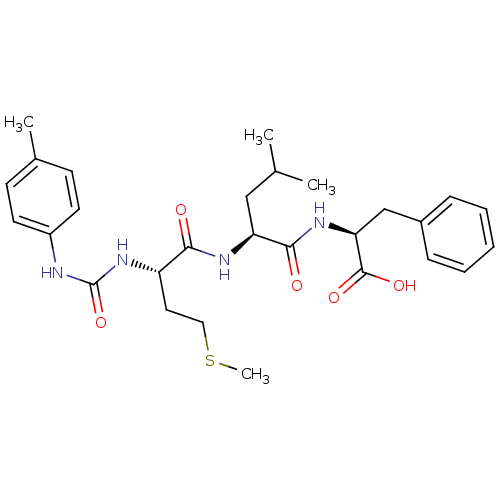
((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...)Show SMILES CSCC[C@H](NC(=O)Nc1ccc(C)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C28H38N4O5S/c1-18(2)16-23(26(34)31-24(27(35)36)17-20-8-6-5-7-9-20)30-25(33)22(14-15-38-4)32-28(37)29-21-12-10-19(3)11-13-21/h5-13,18,22-24H,14-17H2,1-4H3,(H,30,33)(H,31,34)(H,35,36)(H2,29,32,37)/t22-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050929

(2-((S)-2-{(S)-2-[3-(4-Methoxy-phenyl)-ureido]-4-me...)Show SMILES COc1ccc(NC(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)NC(Cc2ccccc2)C(O)=O)cc1 Show InChI InChI=1S/C28H38N4O6S/c1-18(2)16-23(26(34)31-24(27(35)36)17-19-8-6-5-7-9-19)30-25(33)22(14-15-39-4)32-28(37)29-20-10-12-21(38-3)13-11-20/h5-13,18,22-24H,14-17H2,1-4H3,(H,30,33)(H,31,34)(H,35,36)(H2,29,32,37)/t22-,23-,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50371215
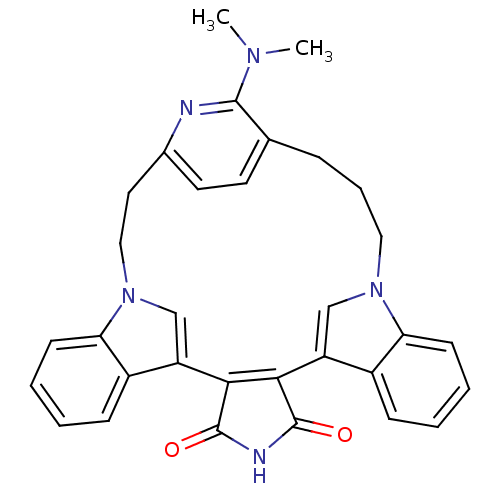
(CHEMBL1162963)Show SMILES CN(C)c1nc2CCn3cc(C4=C(C(=O)NC4=O)c4cn(CCCc1cc2)c1ccccc41)c1ccccc31 |t:11| Show InChI InChI=1S/C32H29N5O2/c1-35(2)30-20-8-7-16-36-18-24(22-9-3-5-11-26(22)36)28-29(32(39)34-31(28)38)25-19-37(17-15-21(33-30)14-13-20)27-12-6-4-10-23(25)27/h3-6,9-14,18-19H,7-8,15-17H2,1-2H3,(H,34,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
Bioorg Med Chem Lett 17: 2863-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.059
BindingDB Entry DOI: 10.7270/Q2SF2X1M |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132318

(3-(1-{2-[2-(2-Dimethylamino-ethoxy)-ethoxy]-ethyl}...)Show SMILES CN1CCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCCOCC1)c1ccccc31)c1ccccc21 |t:8| Show InChI InChI=1S/C30H32N4O4/c1-32-11-6-12-33-19-23(21-7-2-4-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-5-3-8-22(24)26)14-16-38-18-17-37-15-13-32/h2-5,7-10,19-20H,6,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132309
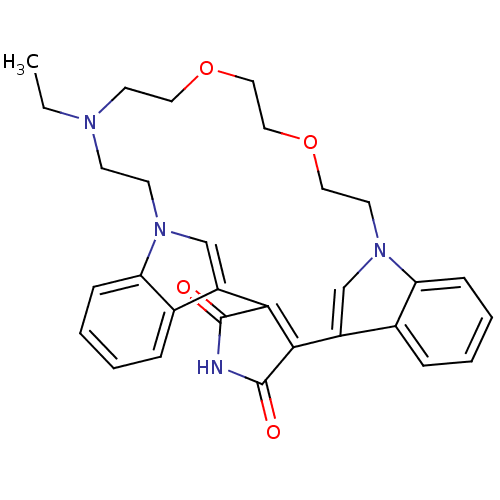
(23-ethyl-17,20-dioxa-4,14,23,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-12-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-6-4-8-22(24)26)14-16-38-18-17-37-15-13-32/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147472

(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCO)c2ncccc12 |t:9| Show InChI InChI=1S/C21H19N3O4/c1-28-16-8-3-2-6-14(16)17-18(21(27)23-20(17)26)15-12-24(10-5-11-25)19-13(15)7-4-9-22-19/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162996

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132317
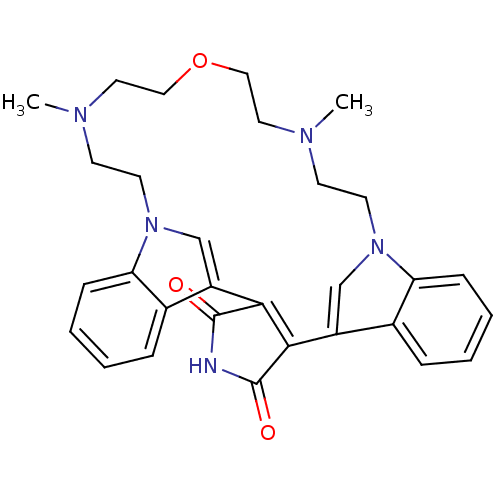
(17,23-dimethyl-20-oxa-4,14,17,23,26-pentaazahexacy...)Show SMILES CN1CCOCCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H33N5O3/c1-32-11-13-34-19-23(21-7-3-5-9-25(21)34)27-28(30(37)31-29(27)36)24-20-35(26-10-6-4-8-22(24)26)14-12-33(2)16-18-38-17-15-32/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Calcium/calmodulin-dependent protein kinase type II |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147465
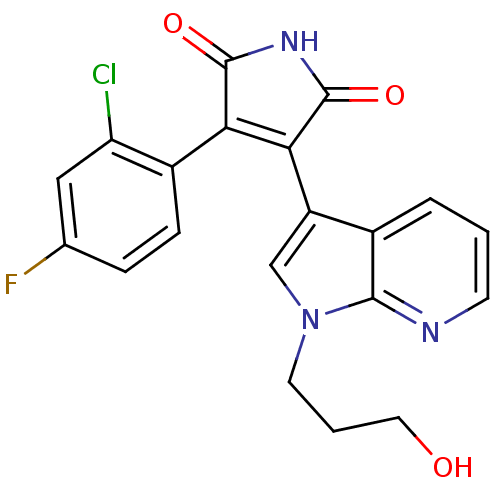
(3-(2-Chloro-4-fluoro-phenyl)-4-[1-(3-hydroxy-propy...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccc(F)cc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H15ClFN3O3/c21-15-9-11(22)4-5-13(15)16-17(20(28)24-19(16)27)14-10-25(7-2-8-26)18-12(14)3-1-6-23-18/h1,3-6,9-10,26H,2,7-8H2,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162993

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccnc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H26N6O2/c1-33(2)15-8-16-35-24-13-6-4-11-21(24)27(32-35)26-25(28(36)31-29(26)37)22-18-34(19-9-7-14-30-17-19)23-12-5-3-10-20(22)23/h3-7,9-14,17-18H,8,15-16H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147458
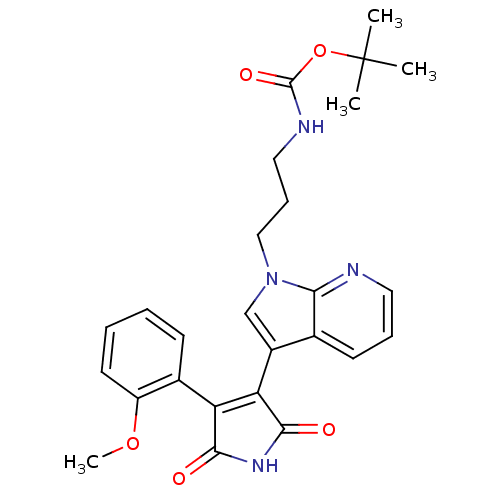
((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50207805

(20-(dimethylamino)-4,14,19,26-tetraazaheptacyclo[2...)Show SMILES CN(C)c1cc2CCCn3cc(C4=C(C(=O)NC4=O)c4cn(CCCc(c2)n1)c1ccccc41)c1ccccc31 |t:12| Show InChI InChI=1S/C33H31N5O2/c1-36(2)29-18-21-9-7-15-37-19-25(23-11-3-5-13-27(23)37)30-31(33(40)35-32(30)39)26-20-38(16-8-10-22(17-21)34-29)28-14-6-4-12-24(26)28/h3-6,11-14,17-20H,7-10,15-16H2,1-2H3,(H,35,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
Bioorg Med Chem Lett 17: 2863-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.059
BindingDB Entry DOI: 10.7270/Q2SF2X1M |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296
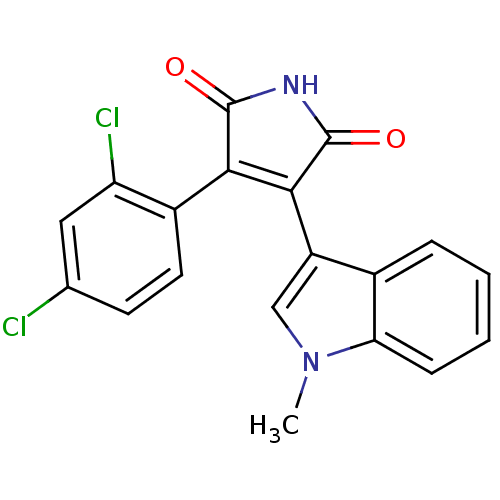
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Mus musculus) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1 in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132316
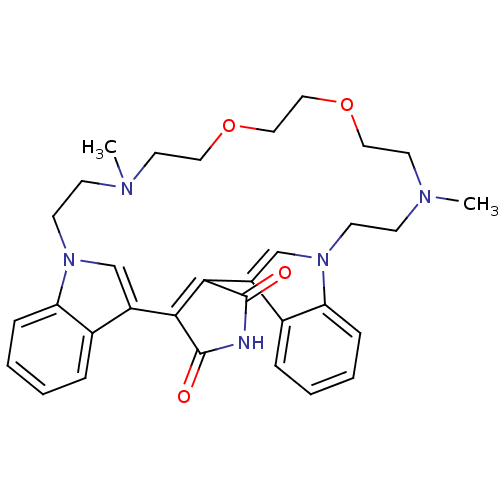
(17,26-dimethyl-20,23-dioxa-4,14,17,26,29-pentaazah...)Show SMILES CN1CCOCCOCCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:17| Show InChI InChI=1S/C32H37N5O4/c1-34-11-13-36-21-25(23-7-3-5-9-27(23)36)29-30(32(39)33-31(29)38)26-22-37(28-10-6-4-8-24(26)28)14-12-35(2)16-18-41-20-19-40-17-15-34/h3-10,21-22H,11-20H2,1-2H3,(H,33,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147464

(CHEMBL109977 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H20N4O4/c1-30-17-8-3-2-6-15(17)18-19(22(29)25-21(18)28)16-12-26(11-5-9-23-13-27)20-14(16)7-4-10-24-20/h2-4,6-8,10,12-13H,5,9,11H2,1H3,(H,23,27)(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132310
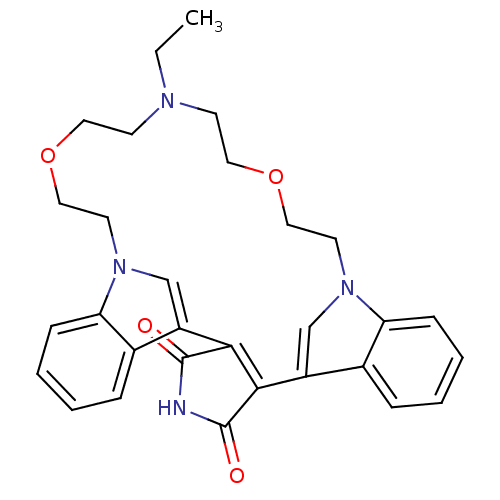
(20-ethyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-15-37-17-13-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(14-18-38-16-12-32)26-10-6-4-8-22(24)26/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132310

(20-ethyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-15-37-17-13-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(14-18-38-16-12-32)26-10-6-4-8-22(24)26/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132311
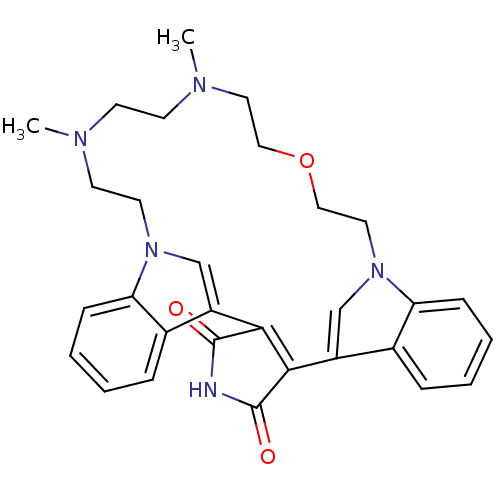
(20,23-dimethyl-17-oxa-4,14,20,23,26-pentaazahexacy...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C30H33N5O3/c1-32-11-12-33(2)15-17-38-18-16-35-20-24(22-8-4-6-10-26(22)35)28-27(29(36)31-30(28)37)23-19-34(14-13-32)25-9-5-3-7-21(23)25/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147473
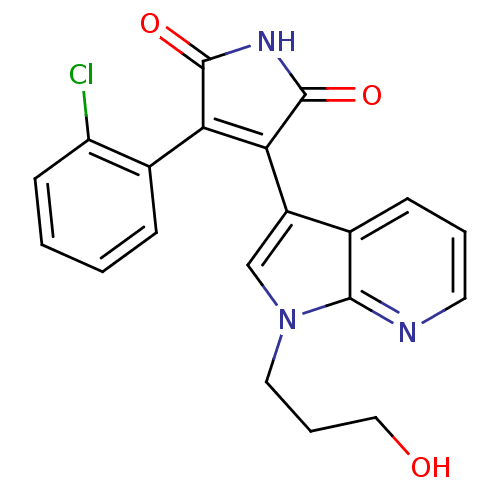
(3-(2-Chloro-phenyl)-4-[1-(3-hydroxy-propyl)-1H-pyr...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H16ClN3O3/c21-15-7-2-1-5-13(15)16-17(20(27)23-19(16)26)14-11-24(9-4-10-25)18-12(14)6-3-8-22-18/h1-3,5-8,11,25H,4,9-10H2,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162998
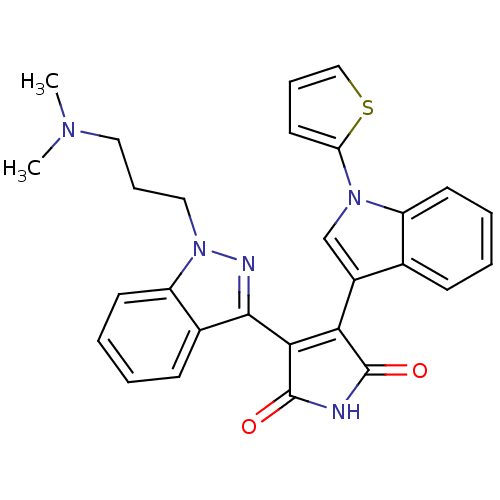
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccs3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C28H25N5O2S/c1-31(2)14-8-15-33-22-12-6-4-10-19(22)26(30-33)25-24(27(34)29-28(25)35)20-17-32(23-13-7-16-36-23)21-11-5-3-9-18(20)21/h3-7,9-13,16-17H,8,14-15H2,1-2H3,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50162990

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C alpha using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50207804

(20-(pyrrolidin-1-yl)-4,14,19,26-tetraazaheptacyclo...)Show SMILES O=C1NC(=O)C2=C1c1cn(CCCc3cc(CCCn4cc2c2ccccc42)nc(c3)N2CCCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C35H33N5O2/c41-34-32-27-21-39(29-13-3-1-11-25(27)29)17-7-9-23-19-24(36-31(20-23)38-15-5-6-16-38)10-8-18-40-22-28(33(32)35(42)37-34)26-12-2-4-14-30(26)40/h1-4,11-14,19-22H,5-10,15-18H2,(H,37,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
Bioorg Med Chem Lett 17: 2863-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.059
BindingDB Entry DOI: 10.7270/Q2SF2X1M |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C epsilon |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50163001
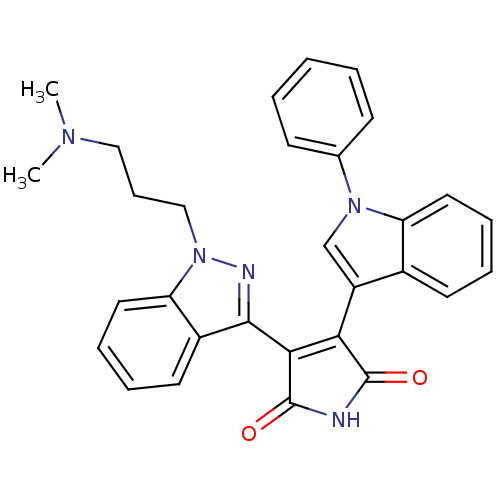
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccccc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C30H27N5O2/c1-33(2)17-10-18-35-25-16-9-7-14-22(25)28(32-35)27-26(29(36)31-30(27)37)23-19-34(20-11-4-3-5-12-20)24-15-8-6-13-21(23)24/h3-9,11-16,19H,10,17-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2 |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147459
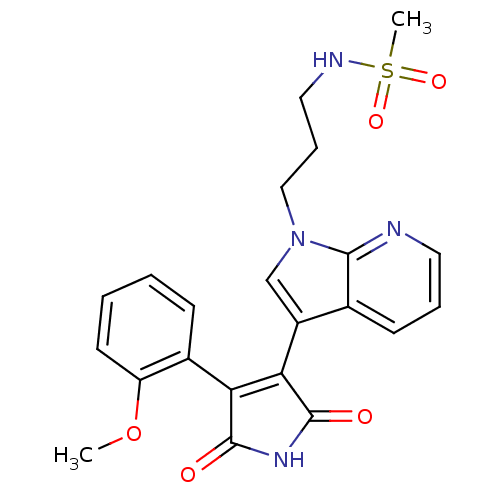
(CHEMBL326208 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNS(C)(=O)=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H22N4O5S/c1-31-17-9-4-3-7-15(17)18-19(22(28)25-21(18)27)16-13-26(12-6-11-24-32(2,29)30)20-14(16)8-5-10-23-20/h3-5,7-10,13,24H,6,11-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147461
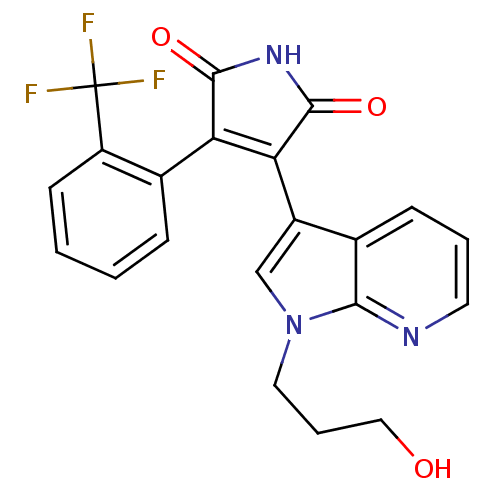
(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2C(F)(F)F)c2cccnc12 |t:7| Show InChI InChI=1S/C21H16F3N3O3/c22-21(23,24)15-7-2-1-5-13(15)16-17(20(30)26-19(16)29)14-11-27(9-4-10-28)18-12(14)6-3-8-25-18/h1-3,5-8,11,28H,4,9-10H2,(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162998

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccs3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C28H25N5O2S/c1-31(2)14-8-15-33-22-12-6-4-10-19(22)26(30-33)25-24(27(34)29-28(25)35)20-17-32(23-13-7-16-36-23)21-11-5-3-9-18(20)21/h3-7,9-13,16-17H,8,14-15H2,1-2H3,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 1 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50162992

(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cncc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-27-15-8-6-13-24(27)31(36-39)30-29(32(40)35-33(30)41)25-20-38(26-14-7-5-12-23(25)26)28-19-34-18-21-10-3-4-11-22(21)28/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 using [gamma-33P]-ATP |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147462
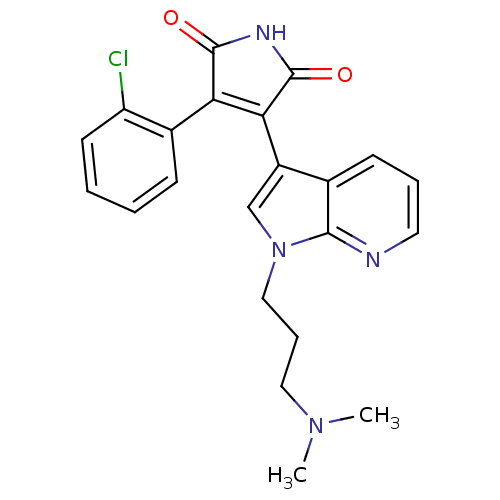
(3-(2-Chloro-phenyl)-4-[1-(3-dimethylamino-propyl)-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:9| Show InChI InChI=1S/C22H21ClN4O2/c1-26(2)11-6-12-27-13-16(14-8-5-10-24-20(14)27)19-18(21(28)25-22(19)29)15-7-3-4-9-17(15)23/h3-5,7-10,13H,6,11-12H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132319
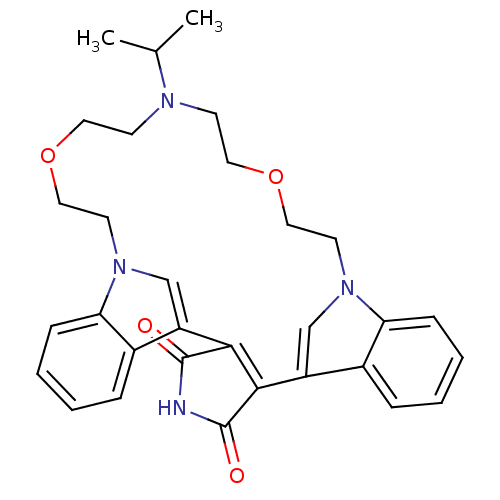
(20-isopropyl-17,23-dioxa-4,14,20,26-tetraazahexacy...)Show SMILES CC(C)N1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:12| Show InChI InChI=1S/C31H34N4O4/c1-21(2)33-11-15-38-17-13-34-19-24(22-7-3-5-9-26(22)34)28-29(31(37)32-30(28)36)25-20-35(14-18-39-16-12-33)27-10-6-4-8-23(25)27/h3-10,19-21H,11-18H2,1-2H3,(H,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data