 Found 725 hits with Last Name = 'desai' and Initial = 'k'
Found 725 hits with Last Name = 'desai' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126919
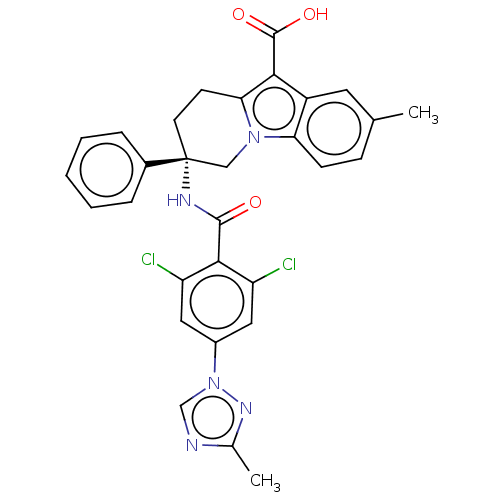
(CHEMBL3629114)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3c(C(O)=O)c4cc(C)ccc4n3C2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C30H25Cl2N5O3/c1-17-8-9-24-21(12-17)26(29(39)40)25-10-11-30(15-36(24)25,19-6-4-3-5-7-19)34-28(38)27-22(31)13-20(14-23(27)32)37-16-33-18(2)35-37/h3-9,12-14,16H,10-11,15H2,1-2H3,(H,34,38)(H,39,40)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126916
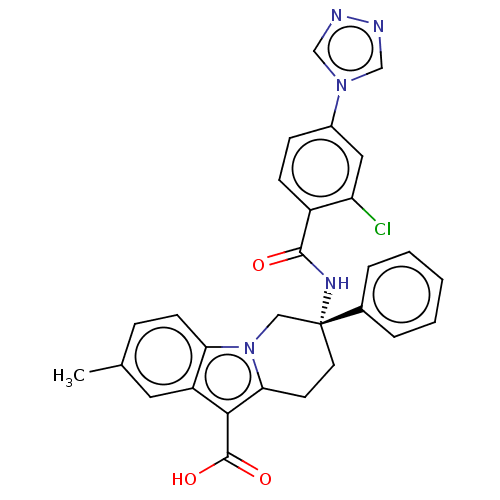
(CHEMBL3629111 | US10351558, Example 139)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(O)=O)c2c1)(NC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24ClN5O3/c1-18-7-10-24-22(13-18)26(28(37)38)25-11-12-29(15-35(24)25,19-5-3-2-4-6-19)33-27(36)21-9-8-20(14-23(21)30)34-16-31-32-17-34/h2-10,13-14,16-17H,11-12,15H2,1H3,(H,33,36)(H,37,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126872
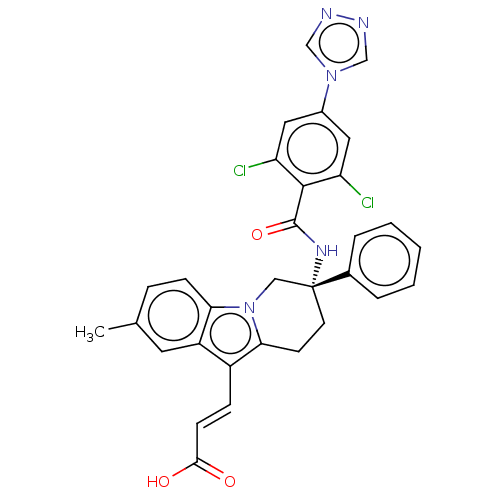
(CHEMBL3628964)Show SMILES Cc1ccc2n3C[C@@](CCc3c(\C=C\C(O)=O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C31H25Cl2N5O3/c1-19-7-9-26-23(13-19)22(8-10-28(39)40)27-11-12-31(16-38(26)27,20-5-3-2-4-6-20)36-30(41)29-24(32)14-21(15-25(29)33)37-17-34-35-18-37/h2-10,13-15,17-18H,11-12,16H2,1H3,(H,36,41)(H,39,40)/b10-8+/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human D2 dopamine receptor in CHO cells by [3H]-spiperone displacement. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125979
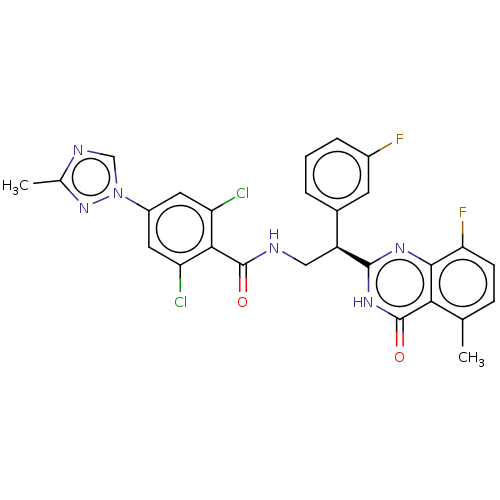
(CHEMBL3627899)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2nc3c(F)ccc(C)c3c(=O)[nH]2)c(Cl)c1 |r| Show InChI InChI=1S/C27H20Cl2F2N6O2/c1-13-6-7-21(31)24-22(13)27(39)35-25(34-24)18(15-4-3-5-16(30)8-15)11-32-26(38)23-19(28)9-17(10-20(23)29)37-12-33-14(2)36-37/h3-10,12,18H,11H2,1-2H3,(H,32,38)(H,34,35,39)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126956
(CHEMBL3628961 | US10351558, Example 174)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(N)=O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24Cl2N6O2/c1-17-7-8-23-20(11-17)25(27(32)38)24-9-10-29(14-37(23)24,18-5-3-2-4-6-18)35-28(39)26-21(30)12-19(13-22(26)31)36-15-33-34-16-36/h2-8,11-13,15-16H,9-10,14H2,1H3,(H2,32,38)(H,35,39)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126913
(CHEMBL3628966)Show SMILES Cc1ccc2n3C[C@@](CCc3c(-c3cncc(N)c3)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C33H27Cl2N7O/c1-20-7-8-28-25(11-20)30(21-12-23(36)16-37-15-21)29-9-10-33(17-42(28)29,22-5-3-2-4-6-22)40-32(43)31-26(34)13-24(14-27(31)35)41-18-38-39-19-41/h2-8,11-16,18-19H,9-10,17,36H2,1H3,(H,40,43)/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069035
((7R,9aS)-7-Phenoxymethyl-2-phenyl-octahydro-pyrido...)Show SMILES C(Oc1ccccc1)[C@@H]1CC[C@H]2CN(CCN2C1)c1ccccc1 Show InChI InChI=1S/C21H26N2O/c1-3-7-19(8-4-1)23-14-13-22-15-18(11-12-20(22)16-23)17-24-21-9-5-2-6-10-21/h1-10,18,20H,11-17H2/t18-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069037
((7R,9aS)-7-Phenoxymethyl-2-pyridin-2-yl-octahydro-...)Show SMILES C(Oc1ccccc1)[C@@H]1CC[C@H]2CN(CCN2C1)c1ccccn1 Show InChI InChI=1S/C20H25N3O/c1-2-6-19(7-3-1)24-16-17-9-10-18-15-23(13-12-22(18)14-17)20-8-4-5-11-21-20/h1-8,11,17-18H,9-10,12-16H2/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069036
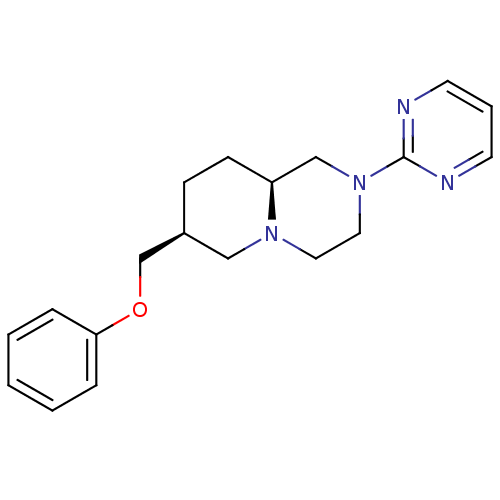
((7S,9aS)-7-Phenoxymethyl-2-pyrimidin-2-yl-octahydr...)Show SMILES C(Oc1ccccc1)[C@H]1CC[C@H]2CN(CCN2C1)c1ncccn1 Show InChI InChI=1S/C19H24N4O/c1-2-5-18(6-3-1)24-15-16-7-8-17-14-23(12-11-22(17)13-16)19-20-9-4-10-21-19/h1-6,9-10,16-17H,7-8,11-15H2/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069036

((7S,9aS)-7-Phenoxymethyl-2-pyrimidin-2-yl-octahydr...)Show SMILES C(Oc1ccccc1)[C@H]1CC[C@H]2CN(CCN2C1)c1ncccn1 Show InChI InChI=1S/C19H24N4O/c1-2-5-18(6-3-1)24-15-16-7-8-17-14-23(12-11-22(17)13-16)19-20-9-4-10-21-19/h1-6,9-10,16-17H,7-8,11-15H2/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126949
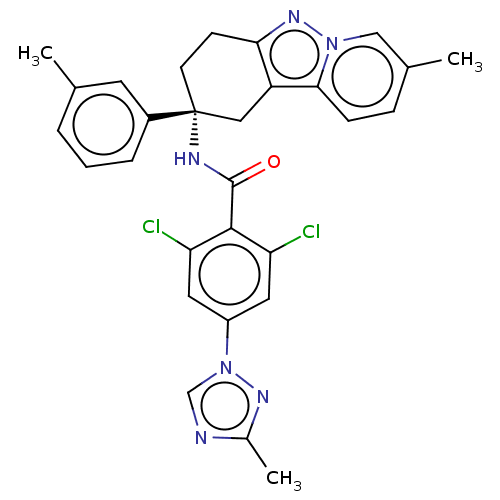
(CHEMBL3628954)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(C)c2)c(Cl)c1 |r| Show InChI InChI=1S/C29H26Cl2N6O/c1-17-5-4-6-20(11-17)29(10-9-25-22(14-29)26-8-7-18(2)15-36(26)35-25)33-28(38)27-23(30)12-21(13-24(27)31)37-16-32-19(3)34-37/h4-8,11-13,15-16H,9-10,14H2,1-3H3,(H,33,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069039
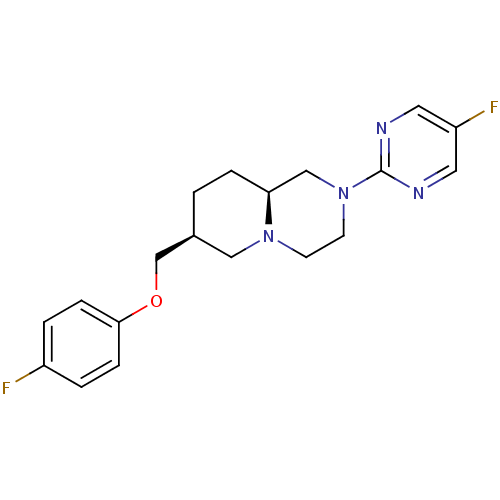
((7S,9aS)-7-(4-Fluoro-phenoxymethyl)-2-(5-fluoro-py...)Show SMILES Fc1ccc(OC[C@H]2CC[C@H]3CN(CCN3C2)c2ncc(F)cn2)cc1 Show InChI InChI=1S/C19H22F2N4O/c20-15-2-5-18(6-3-15)26-13-14-1-4-17-12-25(8-7-24(17)11-14)19-22-9-16(21)10-23-19/h2-3,5-6,9-10,14,17H,1,4,7-8,11-13H2/t14-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126941
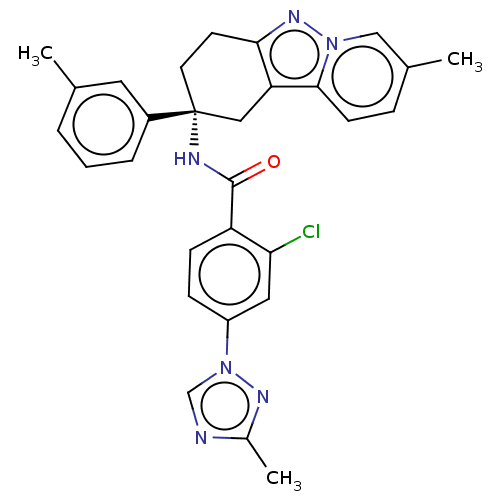
(CHEMBL3628838)Show SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(C)c2)c(Cl)c1 |r| Show InChI InChI=1S/C29H27ClN6O/c1-18-5-4-6-21(13-18)29(12-11-26-24(15-29)27-10-7-19(2)16-35(27)34-26)32-28(37)23-9-8-22(14-25(23)30)36-17-31-20(3)33-36/h4-10,13-14,16-17H,11-12,15H2,1-3H3,(H,32,37)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126912
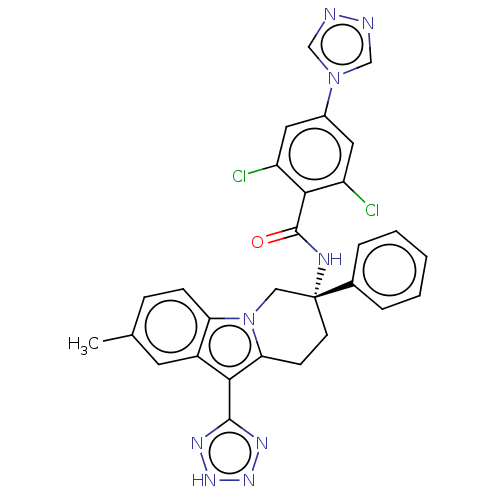
(CHEMBL3628965)Show SMILES Cc1ccc2n3C[C@@](CCc3c(-c3nn[nH]n3)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H23Cl2N9O/c1-17-7-8-23-20(11-17)25(27-35-37-38-36-27)24-9-10-29(14-40(23)24,18-5-3-2-4-6-18)34-28(41)26-21(30)12-19(13-22(26)31)39-15-32-33-16-39/h2-8,11-13,15-16H,9-10,14H2,1H3,(H,34,41)(H,35,36,37,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126855
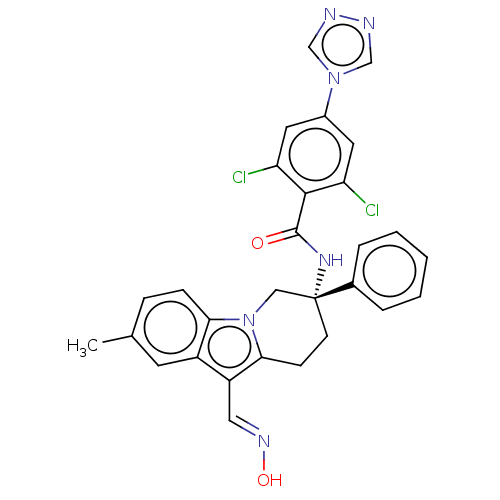
(CHEMBL3628962 | US10351558, Example 173)Show SMILES Cc1ccc2n3C[C@@](CCc3c(\C=N\O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24Cl2N6O2/c1-18-7-8-25-21(11-18)22(14-34-39)26-9-10-29(15-37(25)26,19-5-3-2-4-6-19)35-28(38)27-23(30)12-20(13-24(27)31)36-16-32-33-17-36/h2-8,11-14,16-17,39H,9-10,15H2,1H3,(H,35,38)/b34-14+/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126917
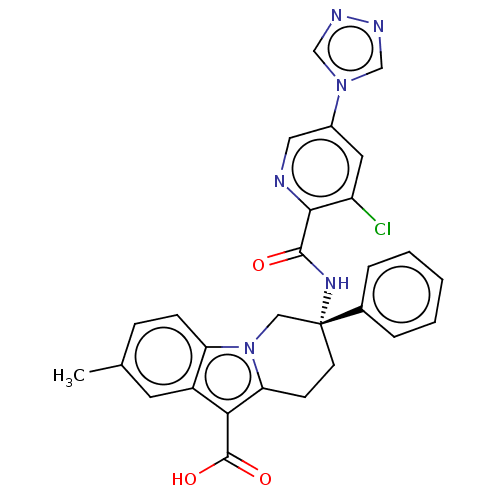
(CHEMBL3629112)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(O)=O)c2c1)(NC(=O)c1ncc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C28H23ClN6O3/c1-17-7-8-22-20(11-17)24(27(37)38)23-9-10-28(14-35(22)23,18-5-3-2-4-6-18)33-26(36)25-21(29)12-19(13-30-25)34-15-31-32-16-34/h2-8,11-13,15-16H,9-10,14H2,1H3,(H,33,36)(H,37,38)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126915
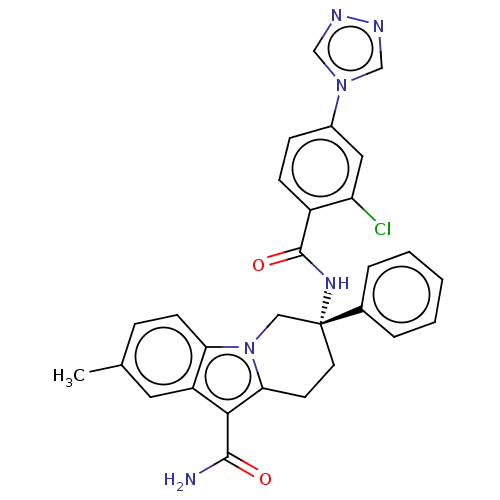
(CHEMBL3629110 | US10351558, Example 138)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(N)=O)c2c1)(NC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H25ClN6O2/c1-18-7-10-24-22(13-18)26(27(31)37)25-11-12-29(15-36(24)25,19-5-3-2-4-6-19)34-28(38)21-9-8-20(14-23(21)30)35-16-32-33-17-35/h2-10,13-14,16-17H,11-12,15H2,1H3,(H2,31,37)(H,34,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126926
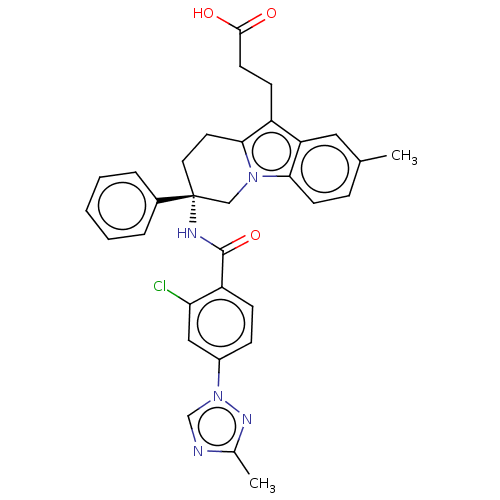
(CHEMBL3628835 | US10351558, Example 183)Show SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@]2(CCc3c(CCC(O)=O)c4cc(C)ccc4n3C2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C32H30ClN5O3/c1-20-8-12-28-26(16-20)24(11-13-30(39)40)29-14-15-32(18-37(28)29,22-6-4-3-5-7-22)35-31(41)25-10-9-23(17-27(25)33)38-19-34-21(2)36-38/h3-10,12,16-17,19H,11,13-15,18H2,1-2H3,(H,35,41)(H,39,40)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069048
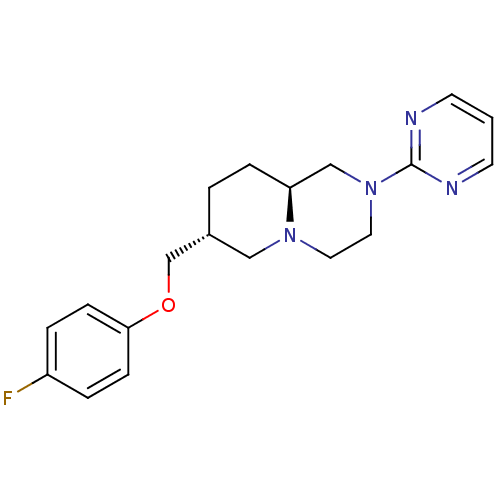
((7R,9aS)-7-(4-Fluoro-phenoxymethyl)-2-pyrimidin-2-...)Show SMILES Fc1ccc(OC[C@@H]2CC[C@H]3CN(CCN3C2)c2ncccn2)cc1 Show InChI InChI=1S/C19H23FN4O/c20-16-3-6-18(7-4-16)25-14-15-2-5-17-13-24(11-10-23(17)12-15)19-21-8-1-9-22-19/h1,3-4,6-9,15,17H,2,5,10-14H2/t15-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125972
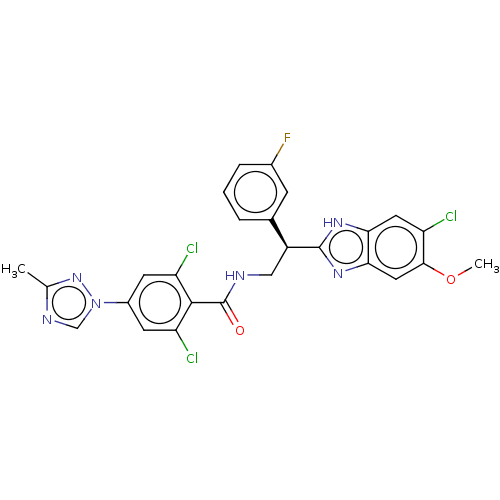
(CHEMBL3627894)Show SMILES COc1cc2nc([nH]c2cc1Cl)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnc(C)n1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O2/c1-13-32-12-36(35-13)16-7-19(28)24(20(29)8-16)26(37)31-11-17(14-4-3-5-15(30)6-14)25-33-21-9-18(27)23(38-2)10-22(21)34-25/h3-10,12,17H,11H2,1-2H3,(H,31,37)(H,33,34)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126856
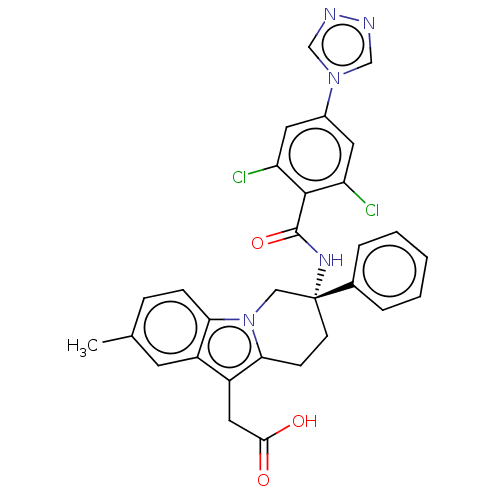
(CHEMBL3628963)Show SMILES Cc1ccc2n3C[C@@](CCc3c(CC(O)=O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C30H25Cl2N5O3/c1-18-7-8-25-21(11-18)22(14-27(38)39)26-9-10-30(15-37(25)26,19-5-3-2-4-6-19)35-29(40)28-23(31)12-20(13-24(28)32)36-16-33-34-17-36/h2-8,11-13,16-17H,9-10,14-15H2,1H3,(H,35,40)(H,38,39)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126942
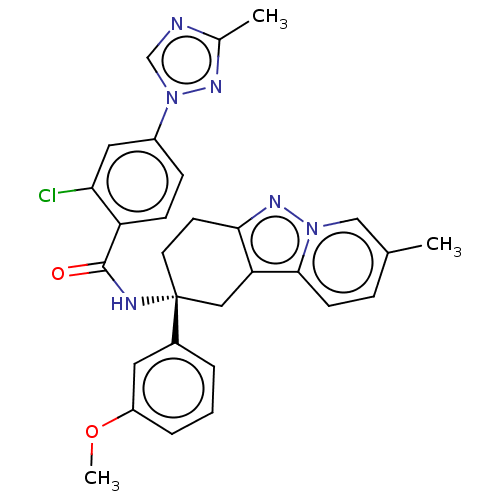
(CHEMBL3628947)Show SMILES COc1cccc(c1)[C@]1(CCc2nn3cc(C)ccc3c2C1)NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1 |r| Show InChI InChI=1S/C29H27ClN6O2/c1-18-7-10-27-24-15-29(12-11-26(24)34-35(27)16-18,20-5-4-6-22(13-20)38-3)32-28(37)23-9-8-21(14-25(23)30)36-17-31-19(2)33-36/h4-10,13-14,16-17H,11-12,15H2,1-3H3,(H,32,37)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126944
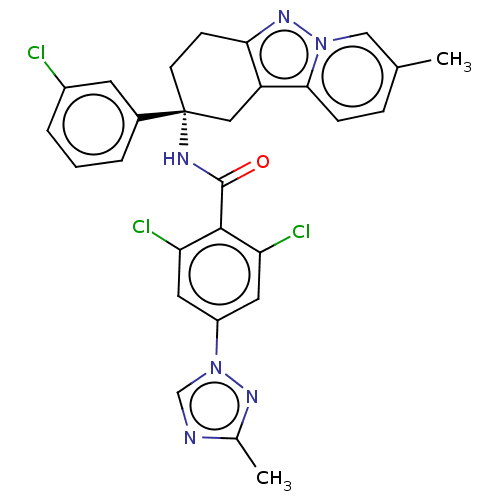
(CHEMBL3628949)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(Cl)c2)c(Cl)c1 |r| Show InChI InChI=1S/C28H23Cl3N6O/c1-16-6-7-25-21-13-28(18-4-3-5-19(29)10-18,9-8-24(21)35-36(25)14-16)33-27(38)26-22(30)11-20(12-23(26)31)37-15-32-17(2)34-37/h3-7,10-12,14-15H,8-9,13H2,1-2H3,(H,33,38)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069034
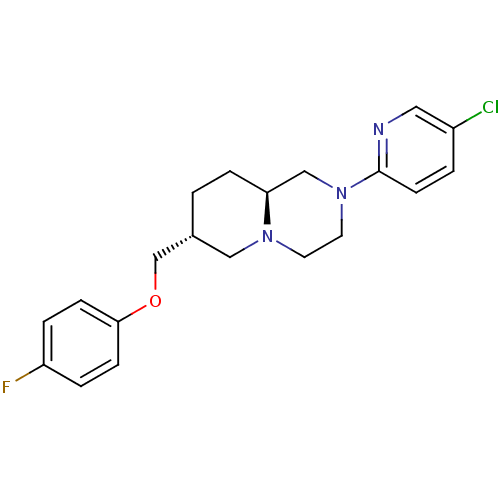
((7R,9aS)-2-(5-Chloro-pyridin-2-yl)-7-(4-fluoro-phe...)Show SMILES Fc1ccc(OC[C@@H]2CC[C@H]3CN(CCN3C2)c2ccc(Cl)cn2)cc1 Show InChI InChI=1S/C20H23ClFN3O/c21-16-2-8-20(23-11-16)25-10-9-24-12-15(1-5-18(24)13-25)14-26-19-6-3-17(22)4-7-19/h2-4,6-8,11,15,18H,1,5,9-10,12-14H2/t15-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125980
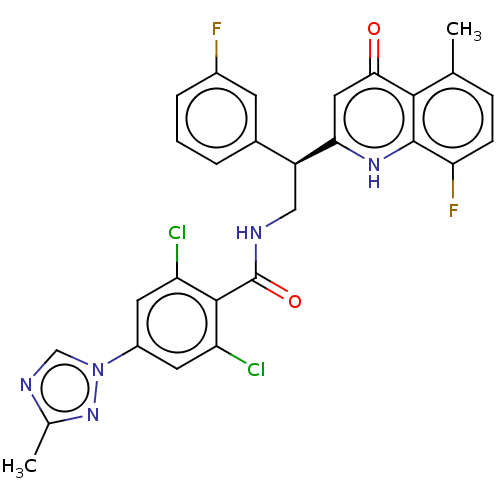
(CHEMBL3627900)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cc(=O)c3c(C)ccc(F)c3[nH]2)c(Cl)c1 |r| Show InChI InChI=1S/C28H21Cl2F2N5O2/c1-14-6-7-22(32)27-25(14)24(38)11-23(35-27)19(16-4-3-5-17(31)8-16)12-33-28(39)26-20(29)9-18(10-21(26)30)37-13-34-15(2)36-37/h3-11,13,19H,12H2,1-2H3,(H,33,39)(H,35,38)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069041
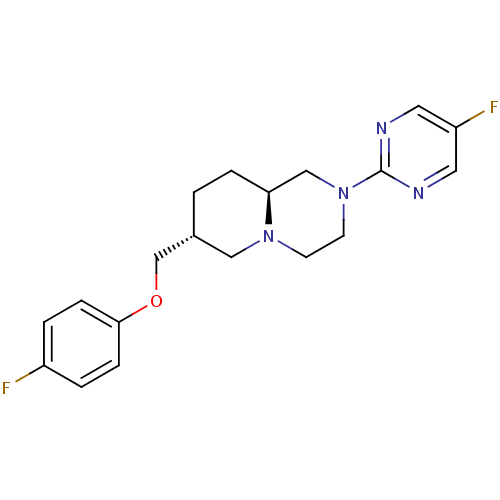
((7R,9aS)-7-(4-Fluoro-phenoxymethyl)-2-(5-fluoro-py...)Show SMILES Fc1ccc(OC[C@@H]2CC[C@H]3CN(CCN3C2)c2ncc(F)cn2)cc1 Show InChI InChI=1S/C19H22F2N4O/c20-15-2-5-18(6-3-15)26-13-14-1-4-17-12-25(8-7-24(17)11-14)19-22-9-16(21)10-23-19/h2-3,5-6,9-10,14,17H,1,4,7-8,11-13H2/t14-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069047
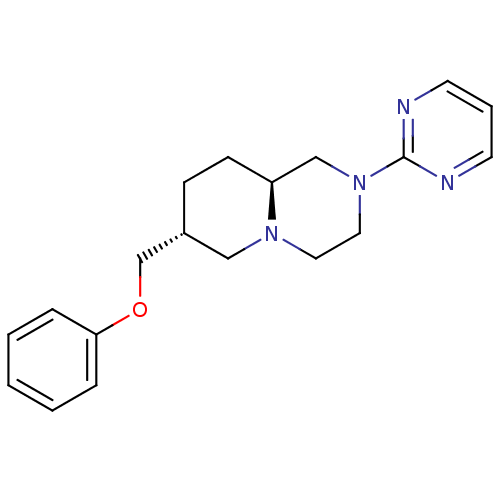
((7R,9aS)-7-Phenoxymethyl-2-pyrimidin-2-yl-octahydr...)Show SMILES C(Oc1ccccc1)[C@@H]1CC[C@H]2CN(CCN2C1)c1ncccn1 Show InChI InChI=1S/C19H24N4O/c1-2-5-18(6-3-1)24-15-16-7-8-17-14-23(12-11-22(17)13-16)19-20-9-4-10-21-19/h1-6,9-10,16-17H,7-8,11-15H2/t16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125978
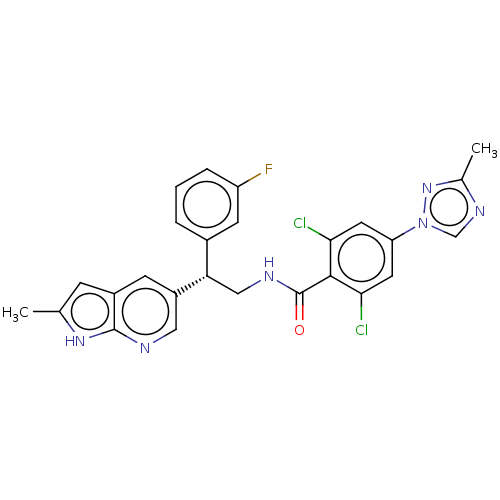
(CHEMBL3627898 | US10189819, Example 77)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cnc3[nH]c(C)cc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-6-17-7-18(11-30-25(17)33-14)21(16-4-3-5-19(29)8-16)12-31-26(36)24-22(27)9-20(10-23(24)28)35-13-32-15(2)34-35/h3-11,13,21H,12H2,1-2H3,(H,30,33)(H,31,36)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069038
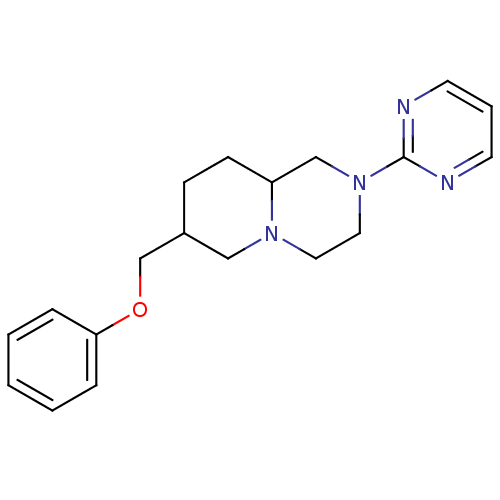
(7-Phenoxymethyl-2-pyrimidin-2-yl-octahydro-pyrido[...)Show InChI InChI=1S/C19H24N4O/c1-2-5-18(6-3-1)24-15-16-7-8-17-14-23(12-11-22(17)13-16)19-20-9-4-10-21-19/h1-6,9-10,16-17H,7-8,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126946
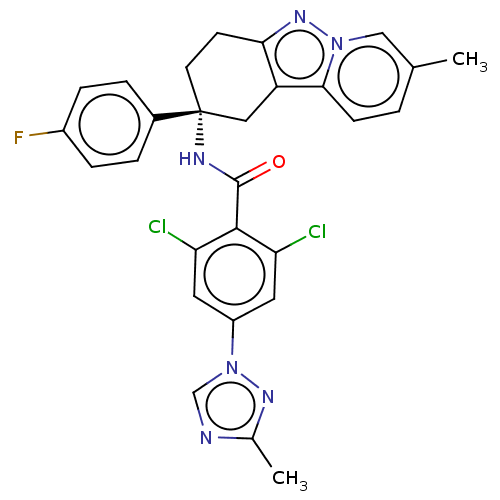
(CHEMBL3628951)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2ccc(F)cc2)c(Cl)c1 |r| Show InChI InChI=1S/C28H23Cl2FN6O/c1-16-3-8-25-21-13-28(18-4-6-19(31)7-5-18,10-9-24(21)35-36(25)14-16)33-27(38)26-22(29)11-20(12-23(26)30)37-15-32-17(2)34-37/h3-8,11-12,14-15H,9-10,13H2,1-2H3,(H,33,38)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126945
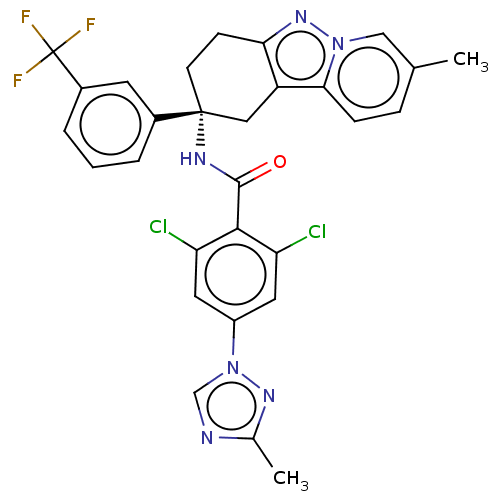
(CHEMBL3628950)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(c2)C(F)(F)F)c(Cl)c1 |r| Show InChI InChI=1S/C29H23Cl2F3N6O/c1-16-6-7-25-21-13-28(9-8-24(21)38-39(25)14-16,18-4-3-5-19(10-18)29(32,33)34)36-27(41)26-22(30)11-20(12-23(26)31)40-15-35-17(2)37-40/h3-7,10-12,14-15H,8-9,13H2,1-2H3,(H,36,41)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126948
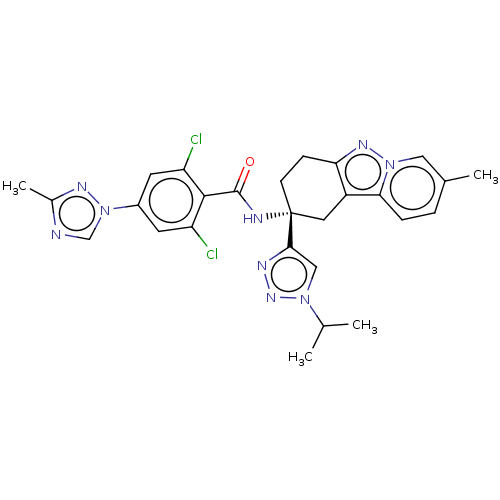
(CHEMBL3628953)Show SMILES CC(C)n1cc(nn1)[C@]1(CCc2nn3cc(C)ccc3c2C1)NC(=O)c1c(Cl)cc(cc1Cl)-n1cnc(C)n1 |r| Show InChI InChI=1S/C27H27Cl2N9O/c1-15(2)36-13-24(32-35-36)27(8-7-22-19(11-27)23-6-5-16(3)12-37(23)34-22)31-26(39)25-20(28)9-18(10-21(25)29)38-14-30-17(4)33-38/h5-6,9-10,12-15H,7-8,11H2,1-4H3,(H,31,39)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126955
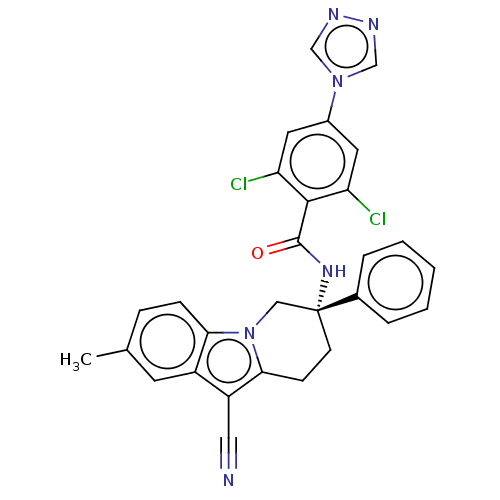
(CHEMBL3628960 | US10351558, Example 152)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C#N)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H22Cl2N6O/c1-18-7-8-25-21(11-18)22(14-32)26-9-10-29(15-37(25)26,19-5-3-2-4-6-19)35-28(38)27-23(30)12-20(13-24(27)31)36-16-33-34-17-36/h2-8,11-13,16-17H,9-10,15H2,1H3,(H,35,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069042
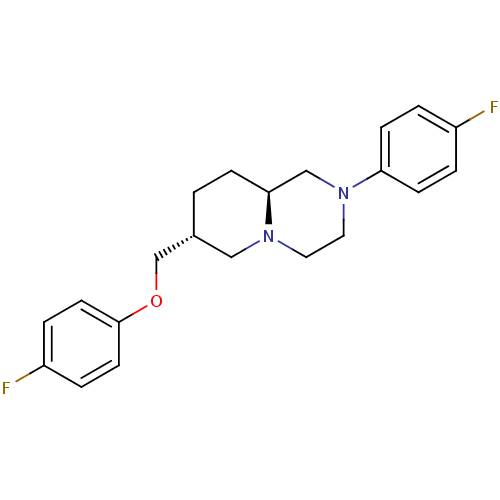
((7R,9aS)-7-(4-Fluoro-phenoxymethyl)-2-(4-fluoro-ph...)Show SMILES Fc1ccc(OC[C@@H]2CC[C@H]3CN(CCN3C2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H24F2N2O/c22-17-2-7-19(8-3-17)25-12-11-24-13-16(1-6-20(24)14-25)15-26-21-9-4-18(23)5-10-21/h2-5,7-10,16,20H,1,6,11-15H2/t16-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126914
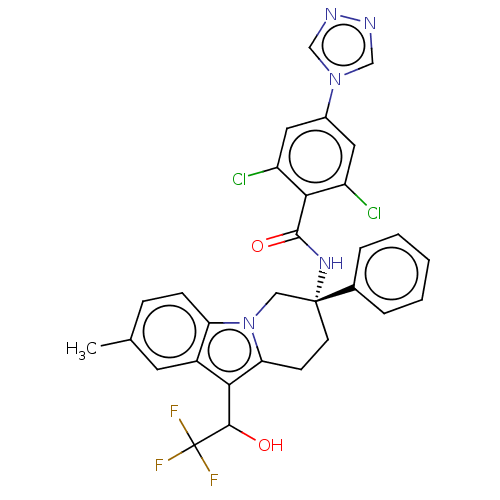
(CHEMBL3629109)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(O)C(F)(F)F)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C30H24Cl2F3N5O2/c1-17-7-8-23-20(11-17)25(27(41)30(33,34)35)24-9-10-29(14-40(23)24,18-5-3-2-4-6-18)38-28(42)26-21(31)12-19(13-22(26)32)39-15-36-37-16-39/h2-8,11-13,15-16,27,41H,9-10,14H2,1H3,(H,38,42)/t27?,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125960
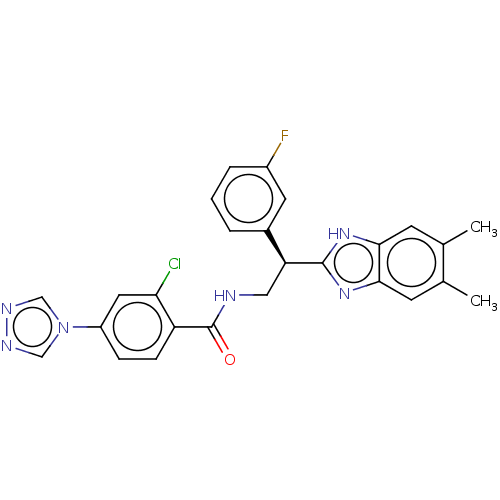
(CHEMBL3627871)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-8-23-24(9-16(15)2)33-25(32-23)21(17-4-3-5-18(28)10-17)12-29-26(35)20-7-6-19(11-22(20)27)34-13-30-31-14-34/h3-11,13-14,21H,12H2,1-2H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126923
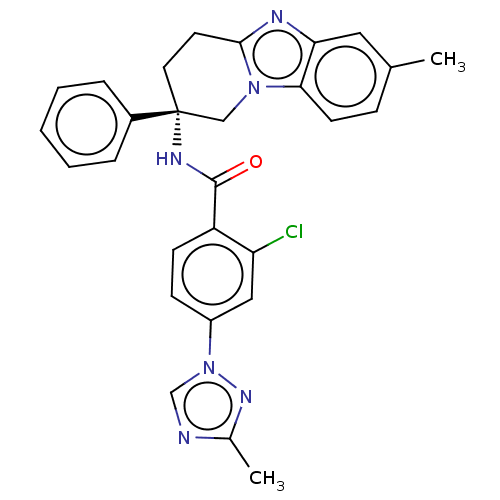
(CHEMBL3628832 | US10351558, Example 67)Show SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@]2(CCc3nc4cc(C)ccc4n3C2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C28H25ClN6O/c1-18-8-11-25-24(14-18)31-26-12-13-28(16-34(25)26,20-6-4-3-5-7-20)32-27(36)22-10-9-21(15-23(22)29)35-17-30-19(2)33-35/h3-11,14-15,17H,12-13,16H2,1-2H3,(H,32,36)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125976
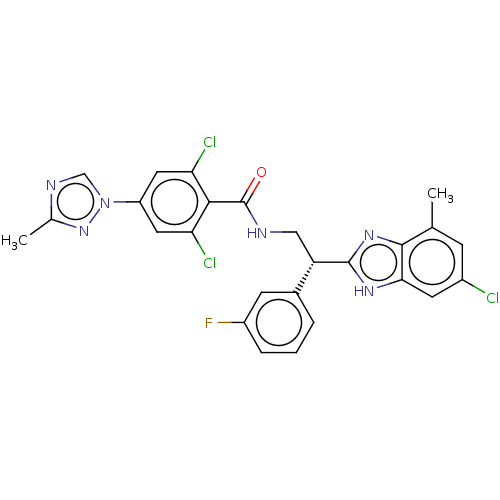
(CHEMBL3627896)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3c(C)cc(Cl)cc3[nH]2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O/c1-13-6-16(27)8-22-24(13)34-25(33-22)19(15-4-3-5-17(30)7-15)11-31-26(37)23-20(28)9-18(10-21(23)29)36-12-32-14(2)35-36/h3-10,12,19H,11H2,1-2H3,(H,31,37)(H,33,34)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126952
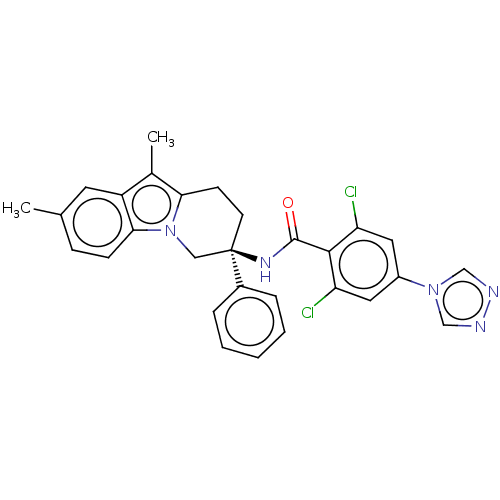
(CHEMBL3628957)Show SMILES Cc1c2CC[C@](Cn2c2ccc(C)cc12)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H25Cl2N5O/c1-18-8-9-26-22(12-18)19(2)25-10-11-29(15-36(25)26,20-6-4-3-5-7-20)34-28(37)27-23(30)13-21(14-24(27)31)35-16-32-33-17-35/h3-9,12-14,16-17H,10-11,15H2,1-2H3,(H,34,37)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126943
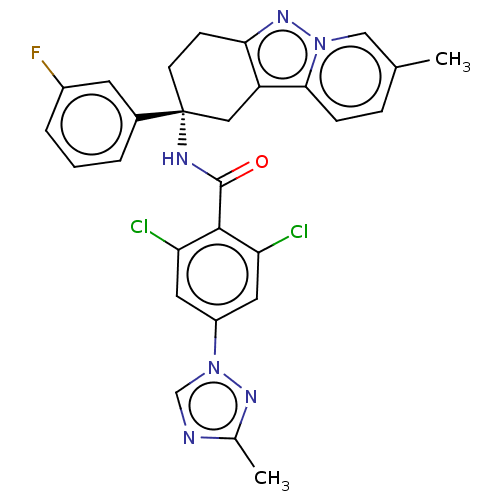
(CHEMBL3628948)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C28H23Cl2FN6O/c1-16-6-7-25-21-13-28(18-4-3-5-19(31)10-18,9-8-24(21)35-36(25)14-16)33-27(38)26-22(29)11-20(12-23(26)30)37-15-32-17(2)34-37/h3-7,10-12,14-15H,8-9,13H2,1-2H3,(H,33,38)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126922
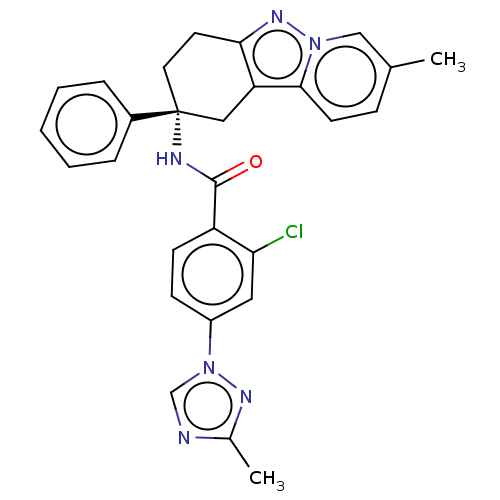
(CHEMBL3628831)Show SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C28H25ClN6O/c1-18-8-11-26-23-15-28(20-6-4-3-5-7-20,13-12-25(23)33-34(26)16-18)31-27(36)22-10-9-21(14-24(22)29)35-17-30-19(2)32-35/h3-11,14,16-17H,12-13,15H2,1-2H3,(H,31,36)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125965
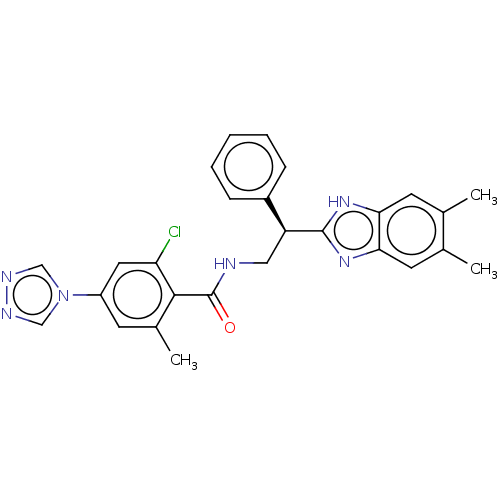
(CHEMBL3627866)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(C)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C27H25ClN6O/c1-16-10-23-24(11-17(16)2)33-26(32-23)21(19-7-5-4-6-8-19)13-29-27(35)25-18(3)9-20(12-22(25)28)34-14-30-31-15-34/h4-12,14-15,21H,13H2,1-3H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126953
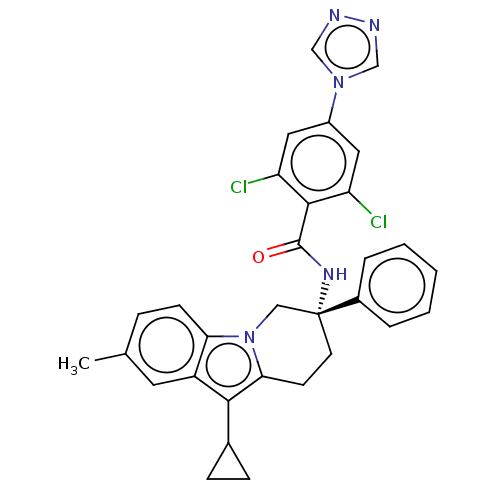
(CHEMBL3628958)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C3CC3)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C31H27Cl2N5O/c1-19-7-10-26-23(13-19)28(20-8-9-20)27-11-12-31(16-38(26)27,21-5-3-2-4-6-21)36-30(39)29-24(32)14-22(15-25(29)33)37-17-34-35-18-37/h2-7,10,13-15,17-18,20H,8-9,11-12,16H2,1H3,(H,36,39)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126954
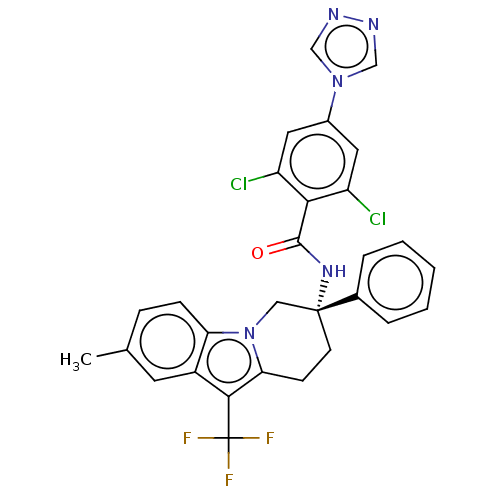
(CHEMBL3628959)Show SMILES Cc1ccc2n3C[C@@](CCc3c(c2c1)C(F)(F)F)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H22Cl2F3N5O/c1-17-7-8-23-20(11-17)26(29(32,33)34)24-9-10-28(14-39(23)24,18-5-3-2-4-6-18)37-27(40)25-21(30)12-19(13-22(25)31)38-15-35-36-16-38/h2-8,11-13,15-16H,9-10,14H2,1H3,(H,37,40)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125973
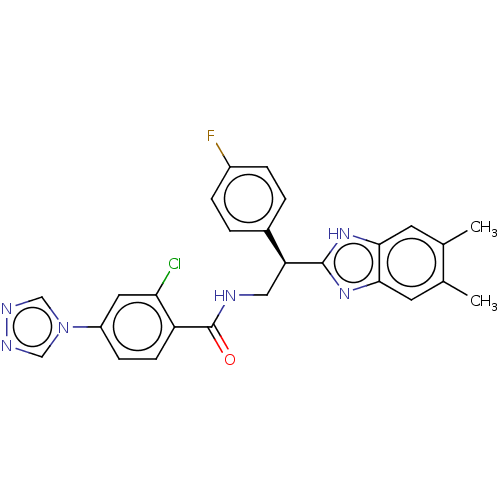
(CHEMBL3627872)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-9-23-24(10-16(15)2)33-25(32-23)21(17-3-5-18(28)6-4-17)12-29-26(35)20-8-7-19(11-22(20)27)34-13-30-31-14-34/h3-11,13-14,21H,12H2,1-2H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126918
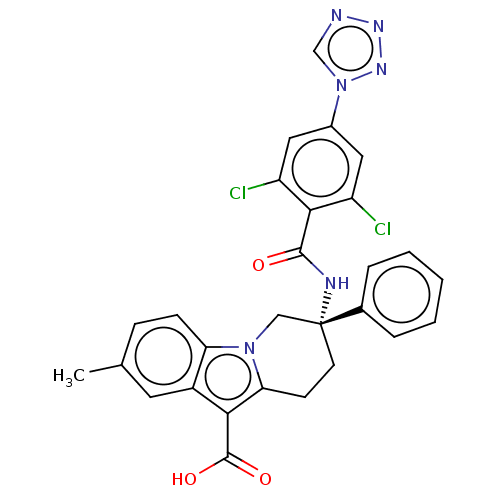
(CHEMBL3629113)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(O)=O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnn1)c1ccccc1 |r| Show InChI InChI=1S/C28H22Cl2N6O3/c1-16-7-8-22-19(11-16)24(27(38)39)23-9-10-28(14-35(22)23,17-5-3-2-4-6-17)32-26(37)25-20(29)12-18(13-21(25)30)36-15-31-33-34-36/h2-8,11-13,15H,9-10,14H2,1H3,(H,32,37)(H,38,39)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125977
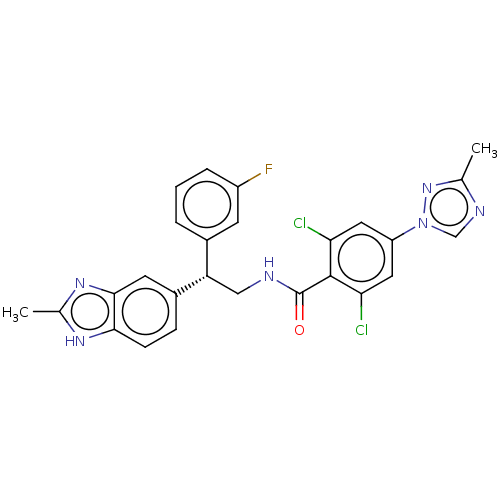
(CHEMBL3627897)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-31-13-35(34-14)19-10-21(27)25(22(28)11-19)26(36)30-12-20(16-4-3-5-18(29)8-16)17-6-7-23-24(9-17)33-15(2)32-23/h3-11,13,20H,12H2,1-2H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125974
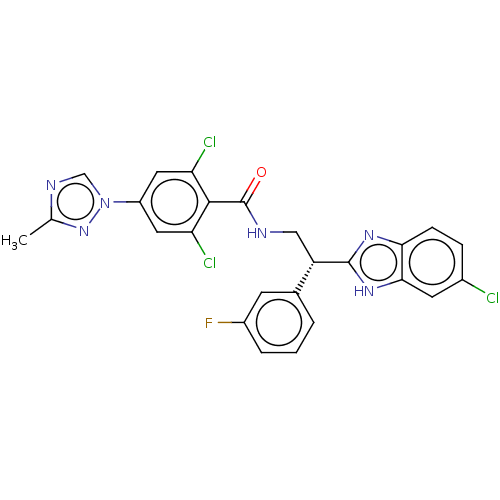
(CHEMBL3627895 | US10189819, Example 46)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3ccc(Cl)cc3[nH]2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C25H18Cl3FN6O/c1-13-31-12-35(34-13)17-9-19(27)23(20(28)10-17)25(36)30-11-18(14-3-2-4-16(29)7-14)24-32-21-6-5-15(26)8-22(21)33-24/h2-10,12,18H,11H2,1H3,(H,30,36)(H,32,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data