 Found 72508 hits with Last Name = 'di' and Initial = 'l'
Found 72508 hits with Last Name = 'di' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50288820
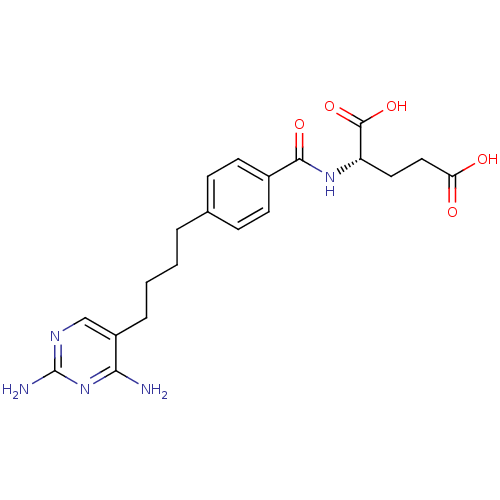
((S)-2-{(S)-4-[4-(2,4-Diamino-pyrimidin-5-yl)-butyl...)Show SMILES Nc1ncc(CCCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C20H25N5O5/c21-17-14(11-23-20(22)25-17)4-2-1-3-12-5-7-13(8-6-12)18(28)24-15(19(29)30)9-10-16(26)27/h5-8,11,15H,1-4,9-10H2,(H,24,28)(H,26,27)(H,29,30)(H4,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100528
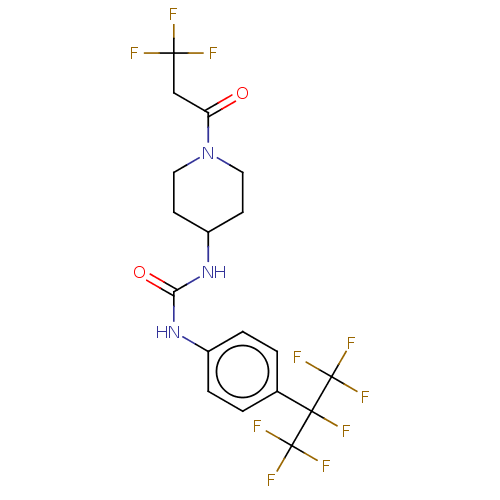
(CHEMBL3327081)Show SMILES FC(F)(F)CC(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H17F10N3O2/c19-15(20,21)9-13(32)31-7-5-12(6-8-31)30-14(33)29-11-3-1-10(2-4-11)16(22,17(23,24)25)18(26,27)28/h1-4,12H,5-9H2,(H2,29,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373939
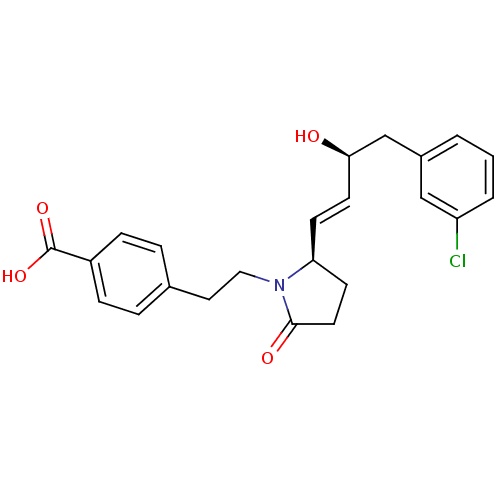
(CHEMBL258332)Show SMILES O[C@@H](Cc1cccc(Cl)c1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24ClNO4/c24-19-3-1-2-17(14-19)15-21(26)10-8-20-9-11-22(27)25(20)13-12-16-4-6-18(7-5-16)23(28)29/h1-8,10,14,20-21,26H,9,11-13,15H2,(H,28,29)/b10-8+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100535
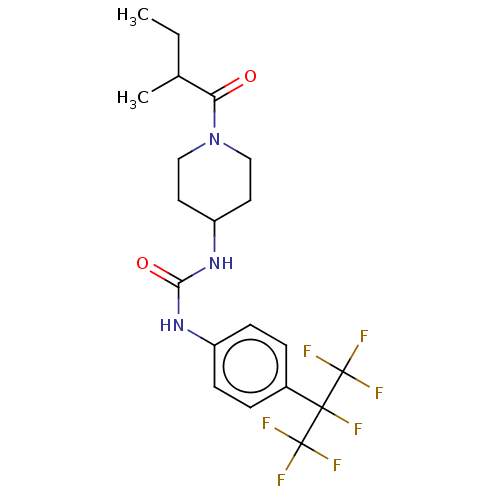
(CHEMBL3327073)Show SMILES CCC(C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H24F7N3O2/c1-3-12(2)16(31)30-10-8-15(9-11-30)29-17(32)28-14-6-4-13(5-7-14)18(21,19(22,23)24)20(25,26)27/h4-7,12,15H,3,8-11H2,1-2H3,(H2,28,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50232153
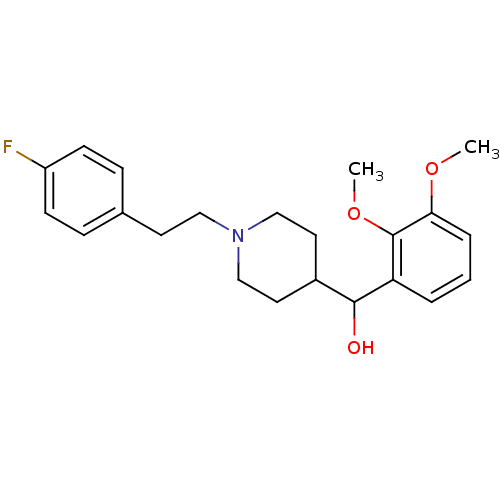
((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...)Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | -55.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding inhibition towards human serotonin transporter |
J Med Chem 48: 6023-34 (2005)
Article DOI: 10.1021/jm0503291
BindingDB Entry DOI: 10.7270/Q2PN96DN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM139371
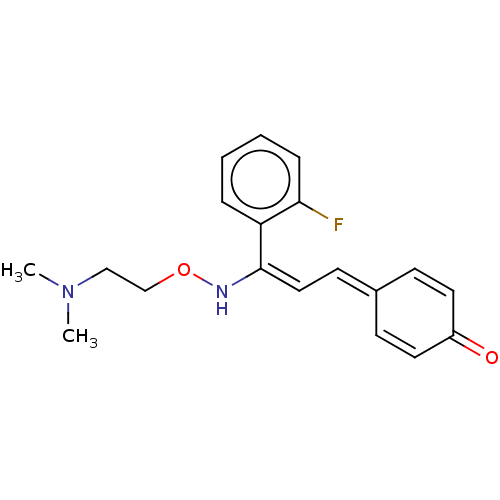
(eplivanserin)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]-[#8]-[#7]\[#6](=[#6]\[#6]=[#6]-1\[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccccc1F |c:11,15| Show InChI InChI=1S/C19H21FN2O2/c1-22(2)13-14-24-21-19(17-5-3-4-6-18(17)20)12-9-15-7-10-16(23)11-8-15/h3-12,21H,13-14H2,1-2H3/b19-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | -54.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283
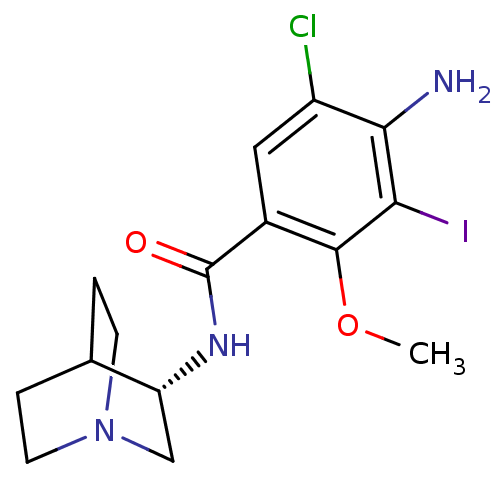
(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373942
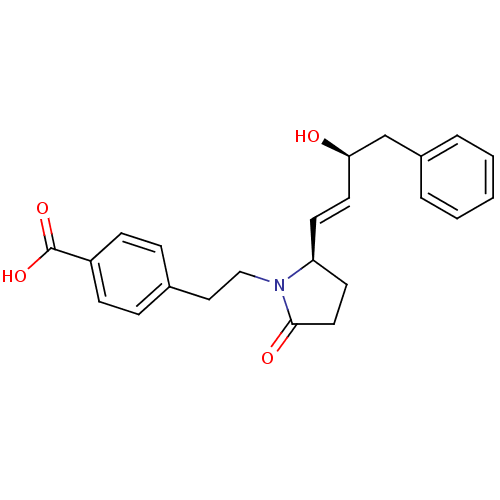
(CHEMBL272276)Show SMILES O[C@@H](Cc1ccccc1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO4/c25-21(16-18-4-2-1-3-5-18)12-10-20-11-13-22(26)24(20)15-14-17-6-8-19(9-7-17)23(27)28/h1-10,12,20-21,25H,11,13-16H2,(H,27,28)/b12-10+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283

(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its antagonistic activity against 5-hydroxytryptamine 3 receptor in rat CNS. |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283

(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor in whole rat brain using [125I]-DAIZAC as the radioligand. |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50235937
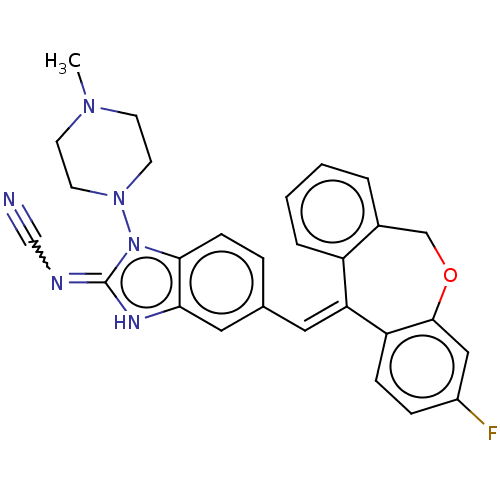
(CHEMBL4095917)Show SMILES CN1CCN(CC1)n1c2ccc(\C=C3/c4ccccc4COc4cc(F)ccc34)cc2[nH]c1=NC#N Show InChI InChI=1S/C28H25FN6O/c1-33-10-12-34(13-11-33)35-26-9-6-19(15-25(26)32-28(35)31-18-30)14-24-22-5-3-2-4-20(22)17-36-27-16-21(29)7-8-23(24)27/h2-9,14-16H,10-13,17H2,1H3,(H,31,32)/b24-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM-CSIC)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Aldosterone from MR overexpressed in human 293 cells measured after 2 hrs by Microbeta scintillation counting method |
J Med Chem 60: 2629-2650 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01065
BindingDB Entry DOI: 10.7270/Q2HD7XXB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0830 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-5
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50111741

(CHEMBL19666 | N-(5-Carbamimidoyl-thiophen-2-ylmeth...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1ccc(s1)C(N)=N Show InChI InChI=1S/C21H23N5O4S2/c1-14-7-9-17(25-32(29,30)13-15-5-3-2-4-6-15)21(28)26(14)12-19(27)24-11-16-8-10-18(31-16)20(22)23/h2-10,25H,11-13H2,1H3,(H3,22,23)(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory constant against human thrombin (FIIa). |
Bioorg Med Chem Lett 12: 1203-8 (2002)
BindingDB Entry DOI: 10.7270/Q2PK0FGB |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17759
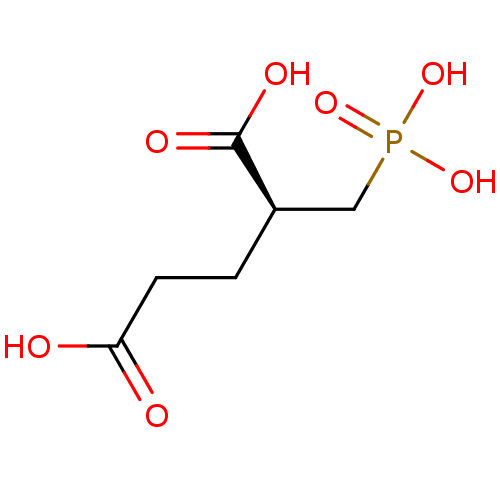
((2S)-2-(phosphonomethyl)pentanedioic acid | (S)-2-...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13)/t4-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50111728
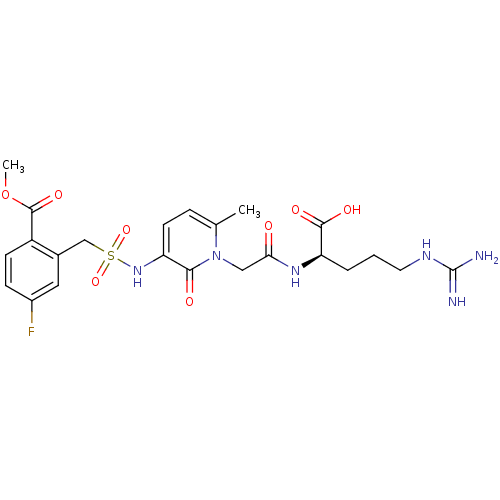
(4-Fluoro-2-({1-[((R)-1-formyl-4-guanidino-butylcar...)Show SMILES COC(=O)c1ccc(F)cc1CS(=O)(=O)Nc1ccc(C)n(CC(=O)N[C@H](CCCNC(N)=N)C(O)=O)c1=O Show InChI InChI=1S/C23H29FN6O8S/c1-13-5-8-17(29-39(36,37)12-14-10-15(24)6-7-16(14)22(35)38-2)20(32)30(13)11-19(31)28-18(21(33)34)4-3-9-27-23(25)26/h5-8,10,18,29H,3-4,9,11-12H2,1-2H3,(H,28,31)(H,33,34)(H4,25,26,27)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory constant against human thrombin (FIIa). |
Bioorg Med Chem Lett 12: 1203-8 (2002)
BindingDB Entry DOI: 10.7270/Q2PK0FGB |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | CHEMBL5172923

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50288821

((S)-2-{(S)-4-[3-(2,4-Diamino-pyrimidin-5-yl)-propy...)Show SMILES Nc1ncc(CCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C19H23N5O5/c20-16-13(10-22-19(21)24-16)3-1-2-11-4-6-12(7-5-11)17(27)23-14(18(28)29)8-9-15(25)26/h4-7,10,14H,1-3,8-9H2,(H,23,27)(H,25,26)(H,28,29)(H4,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50111729

(CHEMBL277695 | N-(5-Carbamimidoyl-thiophen-2-ylmet...)Show SMILES Cc1cnc(NCCc2ccccc2)c(=O)n1CC(=O)NCc1ccc(s1)C(N)=N Show InChI InChI=1S/C21H24N6O2S/c1-14-11-26-20(24-10-9-15-5-3-2-4-6-15)21(29)27(14)13-18(28)25-12-16-7-8-17(30-16)19(22)23/h2-8,11H,9-10,12-13H2,1H3,(H3,22,23)(H,24,26)(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory constant against human thrombin (FIIa). |
Bioorg Med Chem Lett 12: 1203-8 (2002)
BindingDB Entry DOI: 10.7270/Q2PK0FGB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50550643
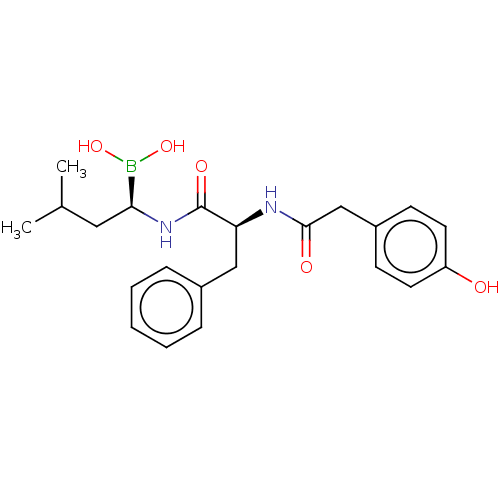
(CHEMBL4749207)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(O)cc1)B(O)O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 20S constitutive proteasome beta 5 subunit assessed as equilibrium constant using fluorogenic peptide Ac-WLA-AMC as substra... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01161
BindingDB Entry DOI: 10.7270/Q2K077W3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50111730

(2-[3-(2-Fluoro-phenylmethanesulfonylamino)-6-methy...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2F)c(=O)n1CC(=O)N[C@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C21H27FN6O6S/c1-13-8-9-16(27-35(33,34)12-14-5-2-3-6-15(14)22)19(30)28(13)11-18(29)26-17(20(31)32)7-4-10-25-21(23)24/h2-3,5-6,8-9,17,27H,4,7,10-12H2,1H3,(H,26,29)(H,31,32)(H4,23,24,25)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory constant against human thrombin (FIIa). |
Bioorg Med Chem Lett 12: 1203-8 (2002)
BindingDB Entry DOI: 10.7270/Q2PK0FGB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123752
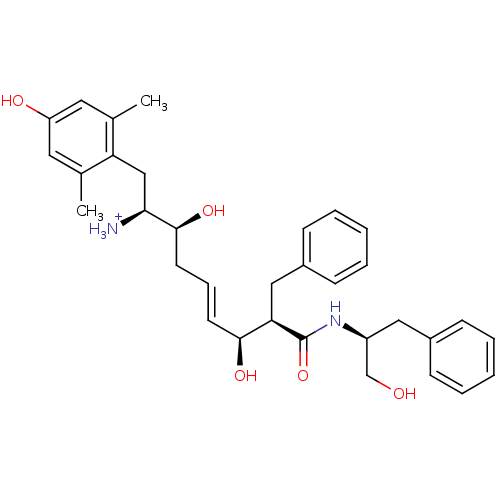
((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)N[C@H](CO)Cc1ccccc1 Show InChI InChI=1S/C33H42N2O5/c1-22-16-27(37)17-23(2)28(22)20-30(34)32(39)15-9-14-31(38)29(19-25-12-7-4-8-13-25)33(40)35-26(21-36)18-24-10-5-3-6-11-24/h3-14,16-17,26,29-32,36-39H,15,18-21,34H2,1-2H3,(H,35,40)/p+1/b14-9+/t26-,29+,30-,31-,32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50202406
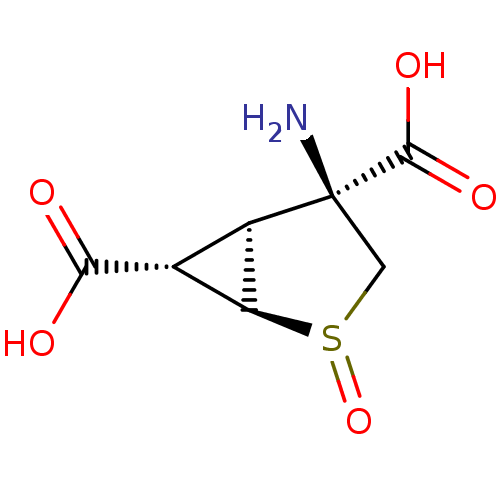
((+)-(1R,2R,4S,5S,6S)-4-amino-2-thiabicyclo[3.1.0]h...)Show SMILES N[C@]1(CS(=O)[C@H]2[C@@H]([C@@H]12)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C7H9NO5S/c8-7(6(11)12)1-14(13)4-2(3(4)7)5(9)10/h2-4H,1,8H2,(H,9,10)(H,11,12)/t2-,3-,4+,7+,14?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]LY341495 from human recombinant mGluR3 in RGT cells |
J Med Chem 50: 233-40 (2007)
Article DOI: 10.1021/jm060917u
BindingDB Entry DOI: 10.7270/Q21Z442N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50202406

((+)-(1R,2R,4S,5S,6S)-4-amino-2-thiabicyclo[3.1.0]h...)Show SMILES N[C@]1(CS(=O)[C@H]2[C@@H]([C@@H]12)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C7H9NO5S/c8-7(6(11)12)1-14(13)4-2(3(4)7)5(9)10/h2-4H,1,8H2,(H,9,10)(H,11,12)/t2-,3-,4+,7+,14?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]LY341495 from human recombinant mGluR3 in RGT cells |
J Med Chem 50: 233-40 (2007)
Article DOI: 10.1021/jm060917u
BindingDB Entry DOI: 10.7270/Q21Z442N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50505384
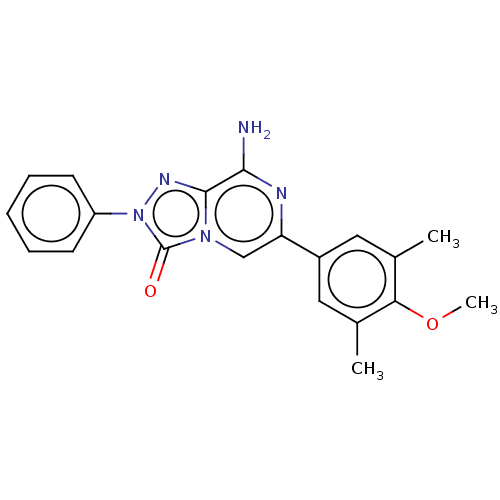
(CHEMBL4457393)Show SMILES COc1c(C)cc(cc1C)-c1cn2c(nn(-c3ccccc3)c2=O)c(N)n1 Show InChI InChI=1S/C20H19N5O2/c1-12-9-14(10-13(2)17(12)27-3)16-11-24-19(18(21)22-16)23-25(20(24)26)15-7-5-4-6-8-15/h4-11H,1-3H3,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human adenosine A2A receptor expressed in CHO cell membranes by radioligand competition assay |
J Med Chem 62: 8511-8531 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00778
BindingDB Entry DOI: 10.7270/Q2ST7T4D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123763
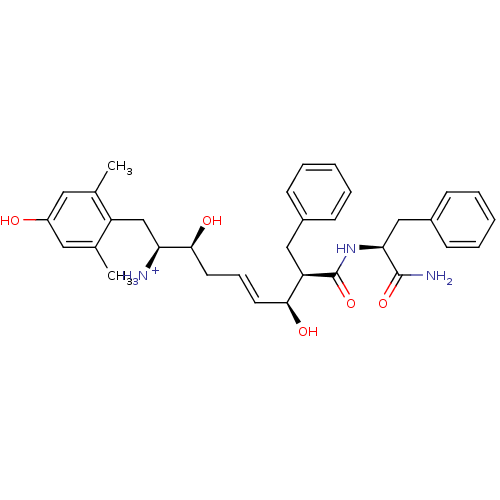
((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C33H41N3O5/c1-21-16-25(37)17-22(2)26(21)20-28(34)31(39)15-9-14-30(38)27(18-23-10-5-3-6-11-23)33(41)36-29(32(35)40)19-24-12-7-4-8-13-24/h3-14,16-17,27-31,37-39H,15,18-20,34H2,1-2H3,(H2,35,40)(H,36,41)/p+1/b14-9+/t27-,28+,29+,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50000066
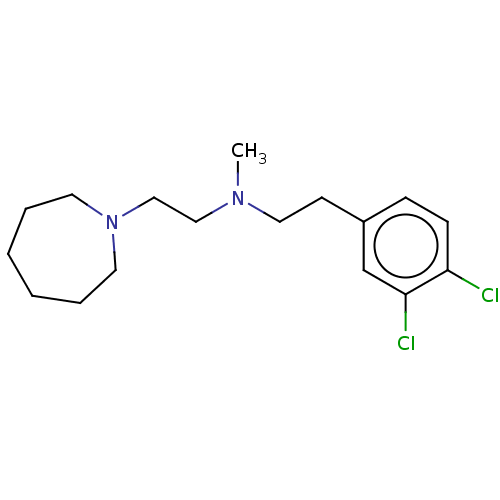
((2-Azepan-1-yl-ethyl)-[2-(3,4-dichloro-phenyl)-eth...)Show InChI InChI=1S/C17H26Cl2N2/c1-20(12-13-21-9-4-2-3-5-10-21)11-8-15-6-7-16(18)17(19)14-15/h6-7,14H,2-5,8-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+) -3PPP from sigma receptor in guinea pig brain membranes |
J Med Chem 35: 38-47 (1992)
BindingDB Entry DOI: 10.7270/Q2BP01RW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50173715
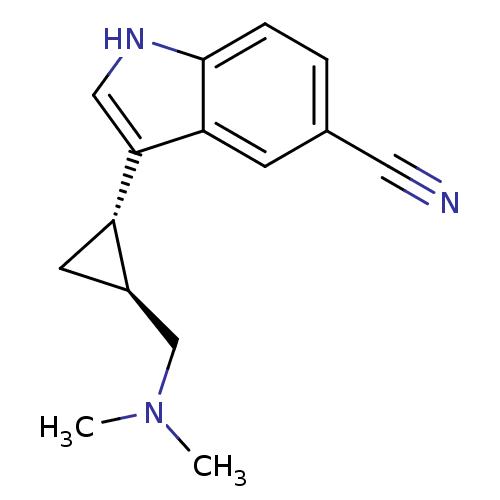
((+)3-((1S,2S)-2-Dimethylaminomethyl-cyclopropyl)-1...)Show InChI InChI=1S/C15H17N3/c1-18(2)9-11-6-12(11)14-8-17-15-4-3-10(7-16)5-13(14)15/h3-5,8,11-12,17H,6,9H2,1-2H3/t11-,12+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding inhibition towards human serotonin transporter |
J Med Chem 48: 6023-34 (2005)
Article DOI: 10.1021/jm0503291
BindingDB Entry DOI: 10.7270/Q2PN96DN |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100521
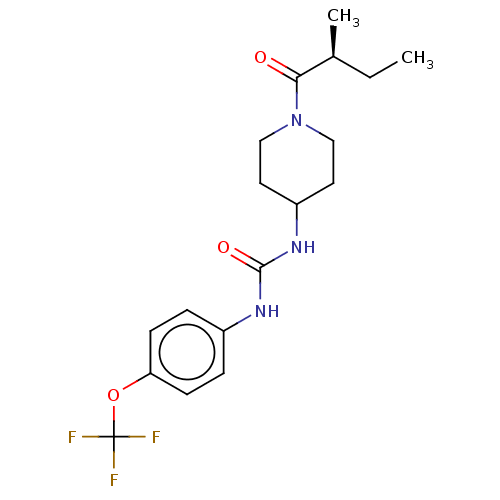
(CHEMBL3327078 | US10377744, Compound No. 2696)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C18H24F3N3O3/c1-3-12(2)16(25)24-10-8-14(9-11-24)23-17(26)22-13-4-6-15(7-5-13)27-18(19,20)21/h4-7,12,14H,3,8-11H2,1-2H3,(H2,22,23,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM26236
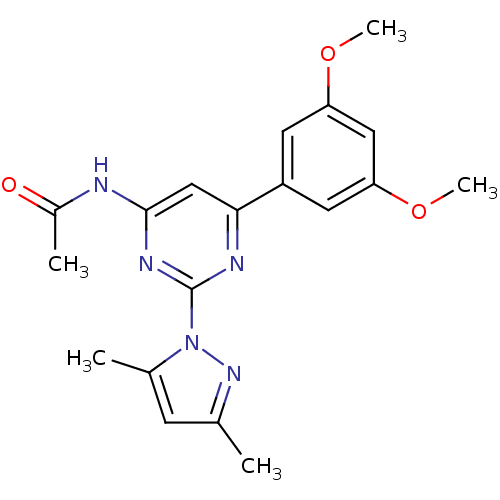
(CHEMBL487569 | N-[6-(3,5-dimethoxyphenyl)-2-(3,5-d...)Show SMILES COc1cc(OC)cc(c1)-c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C Show InChI InChI=1S/C19H21N5O3/c1-11-6-12(2)24(23-11)19-21-17(10-18(22-19)20-13(3)25)14-7-15(26-4)9-16(8-14)27-5/h6-10H,1-5H3,(H,20,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Neurocrine Bioscience
| Assay Description
The membranes prepared from HEK cells transfected with adenosine receptors were used in binding assays. Nonspecific binding was determined in the pre... |
J Med Chem 51: 7099-7110 (2008)
Article DOI: 10.1021/jm800851u
BindingDB Entry DOI: 10.7270/Q20R9MQG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86311
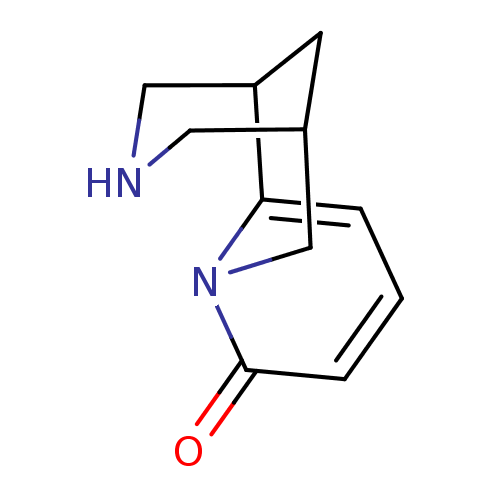
(CAS_485-35-8 | Cytisine | Cytisine-(-) | NSC_22407...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29098
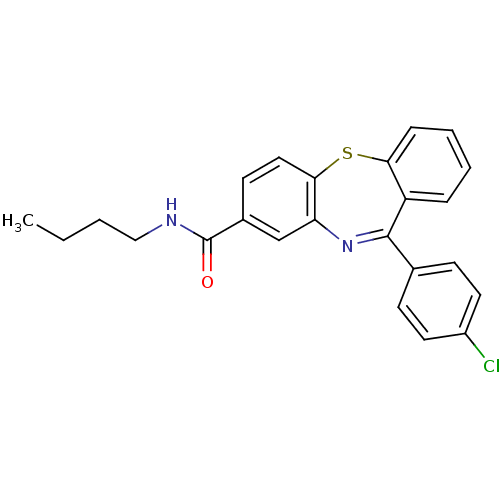
(dibenzothiazepine, 12e)Show SMILES CCCCNC(=O)c1ccc2Sc3ccccc3C(=Nc2c1)c1ccc(Cl)cc1 |c:19| Show InChI InChI=1S/C24H21ClN2OS/c1-2-3-14-26-24(28)17-10-13-22-20(15-17)27-23(16-8-11-18(25)12-9-16)19-6-4-5-7-21(19)29-22/h4-13,15H,2-3,14H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29100

(dibenzothiazepine, 12h)Show SMILES CCCCNC(=O)c1ccc2Sc3ccccc3C(=Nc2c1)c1ccc(F)c(Cl)c1 |c:19| Show InChI InChI=1S/C24H20ClFN2OS/c1-2-3-12-27-24(29)16-9-11-22-20(14-16)28-23(15-8-10-19(26)18(25)13-15)17-6-4-5-7-21(17)30-22/h4-11,13-14H,2-3,12H2,1H3,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
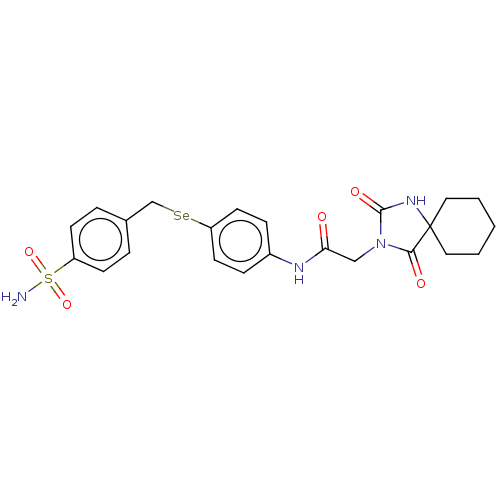
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50513286
(CHEMBL4470319)Show SMILES [#7]S(=O)(=O)c1ccc(-[#6][Se;v2]c2ccc(-[#7]-[#6](=O)-[#6]-[#7]-3-[#6](=O)-[#7]C4([#6]-[#6]-[#6]-[#6]-[#6]4)[#6]-3=O)cc2)cc1 Show InChI InChI=1S/C23H26N4O5SSe/c24-33(31,32)18-8-4-16(5-9-18)15-34-19-10-6-17(7-11-19)25-20(28)14-27-21(29)23(26-22(27)30)12-2-1-3-13-23/h4-11H,1-3,12-15H2,(H,25,28)(H,26,30)(H2,24,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped-flow CO2 hydration assay |
Eur J Med Chem 177: 188-197 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.058
BindingDB Entry DOI: 10.7270/Q2T156ZB |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
The binding affinity was measured on 5-hydroxytryptamine 2 receptor in rat brain tissue |
J Med Chem 35: 552-8 (1992)
BindingDB Entry DOI: 10.7270/Q2PR7WMW |
More data for this
Ligand-Target Pair | |
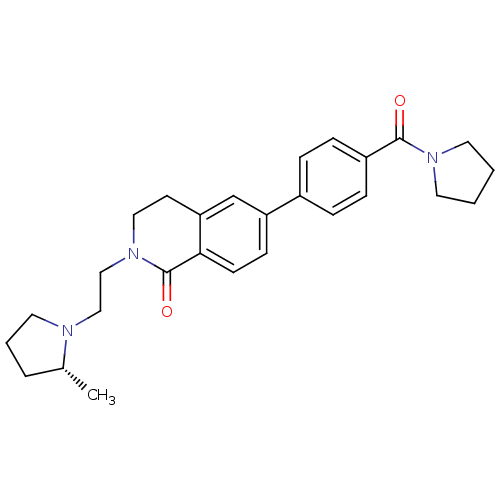
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383163
(CHEMBL2031885)Show SMILES C[C@@H]1CCCN1CCN1CCc2cc(ccc2C1=O)-c1ccc(cc1)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C27H33N3O2/c1-20-5-4-15-28(20)17-18-30-16-12-24-19-23(10-11-25(24)27(30)32)21-6-8-22(9-7-21)26(31)29-13-2-3-14-29/h6-11,19-20H,2-5,12-18H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum |
J Med Chem 35: 552-8 (1992)
BindingDB Entry DOI: 10.7270/Q2PR7WMW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM17689
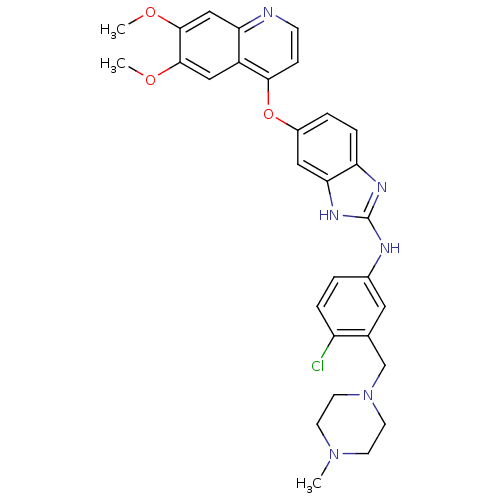
(2-aminobenzimidazole, 14 | N-{4-chloro-3-[(4-methy...)Show SMILES COc1cc2nccc(Oc3ccc4nc(Nc5ccc(Cl)c(CN6CCN(C)CC6)c5)[nH]c4c3)c2cc1OC Show InChI InChI=1S/C30H31ClN6O3/c1-36-10-12-37(13-11-36)18-19-14-20(4-6-23(19)31)33-30-34-24-7-5-21(15-26(24)35-30)40-27-8-9-32-25-17-29(39-3)28(38-2)16-22(25)27/h4-9,14-17H,10-13,18H2,1-3H3,(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen
| Assay Description
The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... |
J Med Chem 50: 4351-4373 (2007)
Article DOI: 10.1021/jm070034i
BindingDB Entry DOI: 10.7270/Q21V5C74 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100519
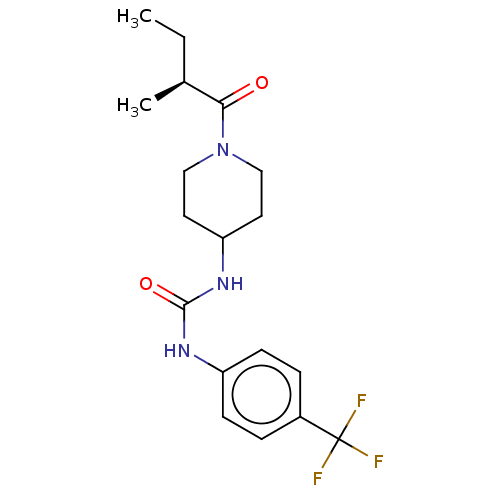
(CHEMBL3327067 | US10377744, Compound No. 2391)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C18H24F3N3O2/c1-3-12(2)16(25)24-10-8-15(9-11-24)23-17(26)22-14-6-4-13(5-7-14)18(19,20)21/h4-7,12,15H,3,8-11H2,1-2H3,(H2,22,23,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100531
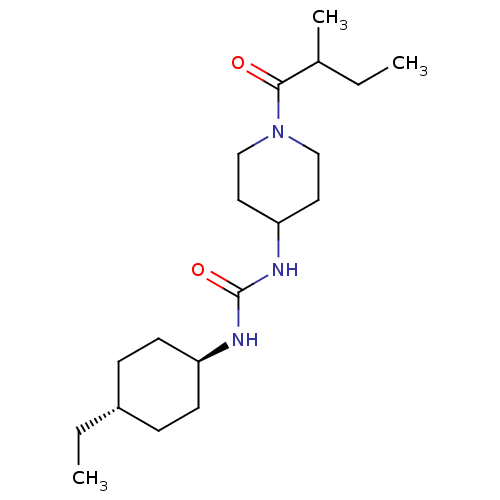
(CHEMBL3325465)Show SMILES CCC(C)C(=O)N1CCC(CC1)NC(=O)N[C@H]1CC[C@H](CC)CC1 |r,wU:16.16,wD:19.20,(21.72,-50.53,;20.39,-49.76,;20.39,-48.22,;19.05,-47.45,;21.72,-47.45,;21.72,-45.91,;23.06,-48.22,;24.38,-47.45,;25.72,-48.22,;25.72,-49.76,;24.38,-50.53,;23.06,-49.76,;27.06,-50.53,;28.39,-49.76,;29.72,-50.53,;28.39,-48.22,;29.72,-47.45,;29.72,-45.91,;31.06,-45.14,;32.39,-45.91,;33.72,-45.14,;35.06,-45.91,;32.39,-47.45,;31.06,-48.22,)| Show InChI InChI=1S/C19H35N3O2/c1-4-14(3)18(23)22-12-10-17(11-13-22)21-19(24)20-16-8-6-15(5-2)7-9-16/h14-17H,4-13H2,1-3H3,(H2,20,21,24)/t14?,15-,16- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50235934
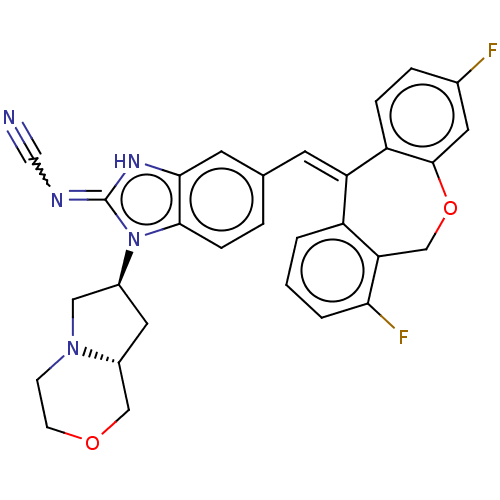
(CHEMBL4086416)Show SMILES [H][C@]12C[C@@H](CN1CCOC2)n1c2ccc(\C=C3\c4ccc(F)cc4OCc4c(F)cccc34)cc2[nH]c1=NC#N |r| Show InChI InChI=1S/C30H25F2N5O2/c31-19-5-6-23-24(22-2-1-3-26(32)25(22)16-39-29(23)12-19)10-18-4-7-28-27(11-18)35-30(34-17-33)37(28)20-13-21-15-38-9-8-36(21)14-20/h1-7,10-12,20-21H,8-9,13-16H2,(H,34,35)/b24-10+/t20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM-CSIC)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Aldosterone from MR overexpressed in human 293 cells measured after 2 hrs by Microbeta scintillation counting method |
J Med Chem 60: 2629-2650 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01065
BindingDB Entry DOI: 10.7270/Q2HD7XXB |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418366

(CHEMBL1774023)Show SMILES Clc1ccc(C[C@@H](NC(=O)Cc2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C24H27Cl2N3O2/c25-17-5-1-15(2-6-17)11-22(29-23(30)12-16-3-7-18(26)8-4-16)24(31)28-21-13-19-9-10-20(14-21)27-19/h1-8,19-22,27H,9-14H2,(H,28,31)(H,29,30)/t19-,20+,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123756
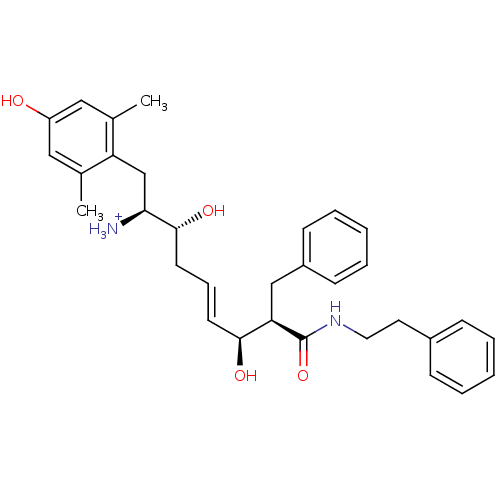
((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C32H40N2O4/c1-22-18-26(35)19-23(2)27(22)21-29(33)31(37)15-9-14-30(36)28(20-25-12-7-4-8-13-25)32(38)34-17-16-24-10-5-3-6-11-24/h3-14,18-19,28-31,35-37H,15-17,20-21,33H2,1-2H3,(H,34,38)/p+1/b14-9+/t28-,29+,30+,31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50341392
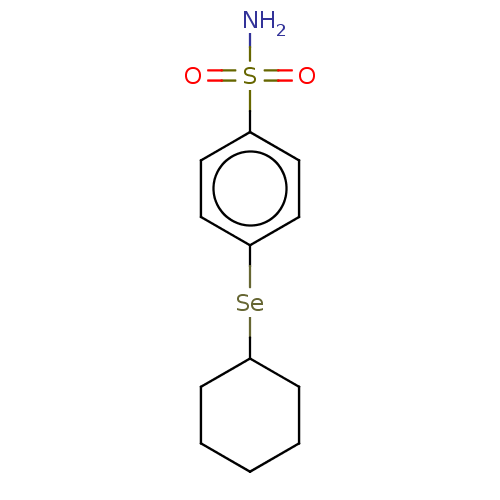
(CHEMBL4171524)Show SMILES [#7]S(=O)(=O)c1ccc([Se;v2][#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C12H17NO2SSe/c13-16(14,15)10-6-8-12(9-7-10)17-11-4-2-1-3-5-11/h6-9,11H,1-5H2,(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 7 after 15 mins by stopped flow carbon dioxide hydration assay |
ACS Med Chem Lett 9: 462-467 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00076
BindingDB Entry DOI: 10.7270/Q2319ZFQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334730
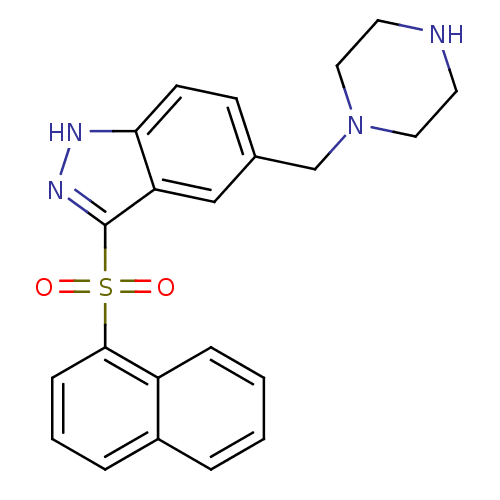
(3-(Naphthalen-1-ylsulfonyl)-5-(piperazin-1-ylmethy...)Show SMILES O=S(=O)(c1n[nH]c2ccc(CN3CCNCC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-7-3-5-17-4-1-2-6-18(17)21)22-19-14-16(8-9-20(19)24-25-22)15-26-12-10-23-11-13-26/h1-9,14,23H,10-13,15H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123754
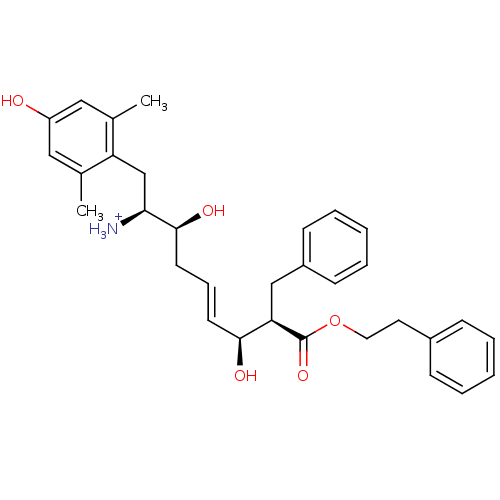
((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)OCCc1ccccc1 Show InChI InChI=1S/C32H39NO5/c1-22-18-26(34)19-23(2)27(22)21-29(33)31(36)15-9-14-30(35)28(20-25-12-7-4-8-13-25)32(37)38-17-16-24-10-5-3-6-11-24/h3-14,18-19,28-31,34-36H,15-17,20-21,33H2,1-2H3/p+1/b14-9+/t28-,29+,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50341395
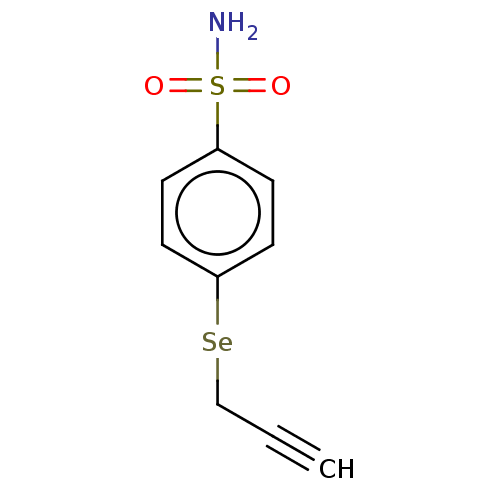
(CHEMBL4175369)Show InChI InChI=1S/C9H9NO2SSe/c1-2-7-14-9-5-3-8(4-6-9)13(10,11)12/h1,3-6H,7H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 7 after 15 mins by stopped flow carbon dioxide hydration assay |
ACS Med Chem Lett 9: 462-467 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00076
BindingDB Entry DOI: 10.7270/Q2319ZFQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data