 Found 31 hits with Last Name = 'di cera' and Initial = 'e'
Found 31 hits with Last Name = 'di cera' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Chymase
(Homo sapiens (Human)) | BDBM50208224
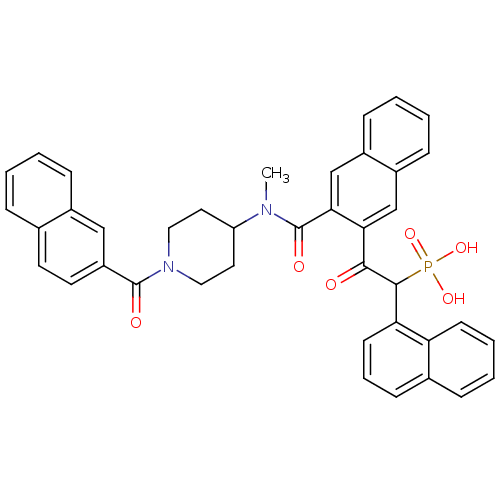
(2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...)Show SMILES CN(C1CCN(CC1)C(=O)c1ccc2ccccc2c1)C(=O)c1cc2ccccc2cc1C(=O)C(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C40H35N2O6P/c1-41(32-19-21-42(22-20-32)39(44)31-18-17-26-9-2-3-11-28(26)23-31)40(45)36-25-30-13-5-4-12-29(30)24-35(36)37(43)38(49(46,47)48)34-16-8-14-27-10-6-7-15-33(27)34/h2-18,23-25,32,38H,19-22H2,1H3,(H2,46,47,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM50208222

((E)-2-(3-chloro-5-fluorostyrylamino)-1-(5-chlorobe...)Show SMILES CP(O)(=O)C(C(=O)NC=Cc1cc(F)cc(Cl)c1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C19H15Cl2FNO3PS/c1-27(25,26)18(16-10-28-17-3-2-12(20)9-15(16)17)19(24)23-5-4-11-6-13(21)8-14(22)7-11/h2-10,18H,1H3,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208228
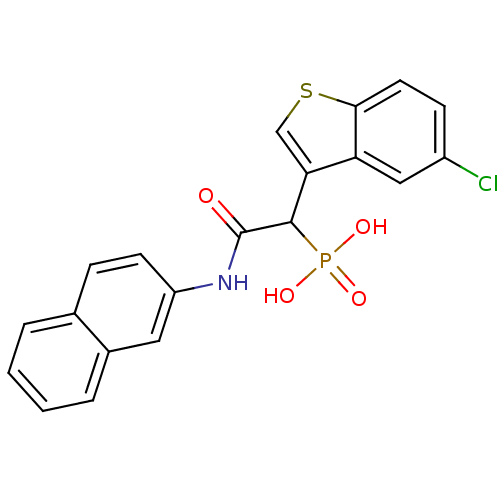
(1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...)Show SMILES OP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1csc2ccc(Cl)cc12 Show InChI InChI=1S/C20H15ClNO4PS/c21-14-6-8-18-16(10-14)17(11-28-18)19(27(24,25)26)20(23)22-15-7-5-12-3-1-2-4-13(12)9-15/h1-11,19H,(H,22,23)(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin G
(Homo sapiens (Human)) | BDBM50208224

(2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...)Show SMILES CN(C1CCN(CC1)C(=O)c1ccc2ccccc2c1)C(=O)c1cc2ccccc2cc1C(=O)C(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C40H35N2O6P/c1-41(32-19-21-42(22-20-32)39(44)31-18-17-26-9-2-3-11-28(26)23-31)40(45)36-25-30-13-5-4-12-29(30)24-35(36)37(43)38(49(46,47)48)34-16-8-14-27-10-6-7-15-33(27)34/h2-18,23-25,32,38H,19-22H2,1H3,(H2,46,47,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil Cat G |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin G
(Homo sapiens (Human)) | BDBM50208228

(1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...)Show SMILES OP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1csc2ccc(Cl)cc12 Show InChI InChI=1S/C20H15ClNO4PS/c21-14-6-8-18-16(10-14)17(11-28-18)19(27(24,25)26)20(23)22-15-7-5-12-3-1-2-4-13(12)9-15/h1-11,19H,(H,22,23)(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil Cat G |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208225
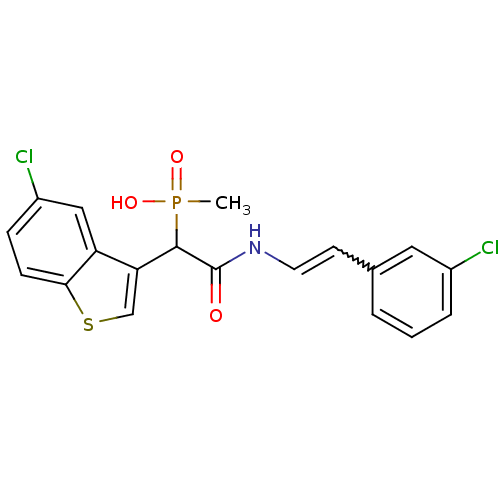
((E)-2-(3-chlorostyrylamino)-1-(5-chlorobenzo[b]thi...)Show SMILES CP(O)(=O)C(C(=O)NC=Cc1cccc(Cl)c1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C19H16Cl2NO3PS/c1-26(24,25)18(16-11-27-17-6-5-14(21)10-15(16)17)19(23)22-8-7-12-3-2-4-13(20)9-12/h2-11,18H,1H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208224

(2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...)Show SMILES CN(C1CCN(CC1)C(=O)c1ccc2ccccc2c1)C(=O)c1cc2ccccc2cc1C(=O)C(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C40H35N2O6P/c1-41(32-19-21-42(22-20-32)39(44)31-18-17-26-9-2-3-11-28(26)23-31)40(45)36-25-30-13-5-4-12-29(30)24-35(36)37(43)38(49(46,47)48)34-16-8-14-27-10-6-7-15-33(27)34/h2-18,23-25,32,38H,19-22H2,1H3,(H2,46,47,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM50208236
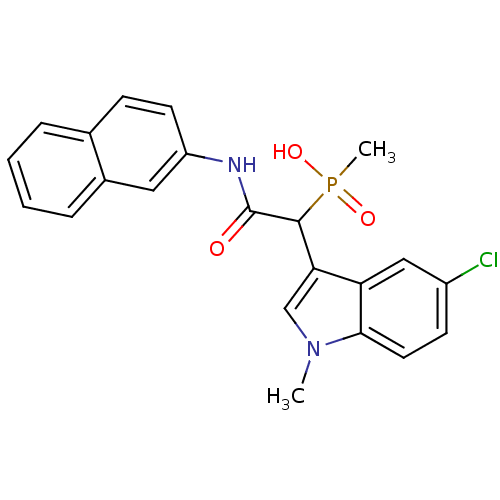
(1-(5-chloro-1-methyl-1H-indol-3-yl)-2-(naphthalen-...)Show SMILES Cn1cc(C(C(=O)Nc2ccc3ccccc3c2)P(C)(O)=O)c2cc(Cl)ccc12 Show InChI InChI=1S/C22H20ClN2O3P/c1-25-13-19(18-12-16(23)8-10-20(18)25)21(29(2,27)28)22(26)24-17-9-7-14-5-3-4-6-15(14)11-17/h3-13,21H,1-2H3,(H,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208243
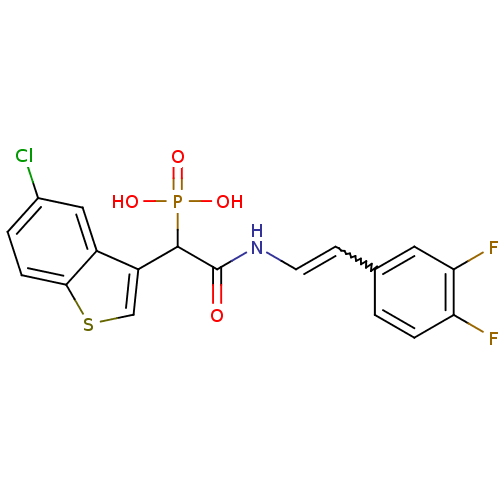
((E)-2-(3,4-difluorostyrylamino)-1-(5-chlorobenzo[b...)Show SMILES OP(O)(=O)C(C(=O)NC=Cc1ccc(F)c(F)c1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C18H13ClF2NO4PS/c19-11-2-4-16-12(8-11)13(9-28-16)17(27(24,25)26)18(23)22-6-5-10-1-3-14(20)15(21)7-10/h1-9,17H,(H,22,23)(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208232
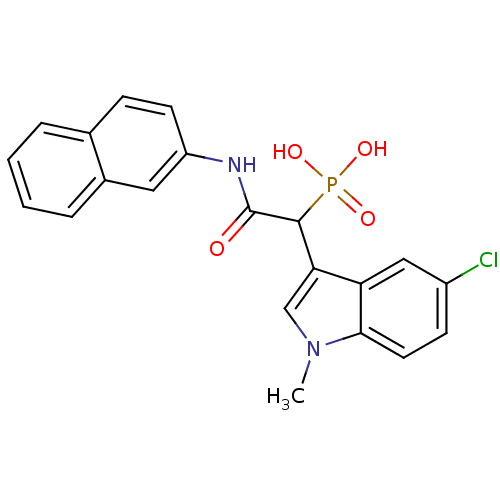
(1-(5-chloro-1-methyl-1H-indol-3-yl)-2-(naphthalen-...)Show SMILES Cn1cc(C(C(=O)Nc2ccc3ccccc3c2)P(O)(O)=O)c2cc(Cl)ccc12 Show InChI InChI=1S/C21H18ClN2O4P/c1-24-12-18(17-11-15(22)7-9-19(17)24)20(29(26,27)28)21(25)23-16-8-6-13-4-2-3-5-14(13)10-16/h2-12,20H,1H3,(H,23,25)(H2,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208244

((E)-2-(4-fluorostyrylamino)-1-(5-chloro-1-methyl-1...)Show SMILES Cn1cc(C(C(=O)NC=Cc2ccc(F)cc2)P(O)(O)=O)c2cc(Cl)ccc12 |w:9.9| Show InChI InChI=1S/C19H17ClFN2O4P/c1-23-11-16(15-10-13(20)4-7-17(15)23)18(28(25,26)27)19(24)22-9-8-12-2-5-14(21)6-3-12/h2-11,18H,1H3,(H,22,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208222

((E)-2-(3-chloro-5-fluorostyrylamino)-1-(5-chlorobe...)Show SMILES CP(O)(=O)C(C(=O)NC=Cc1cc(F)cc(Cl)c1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C19H15Cl2FNO3PS/c1-27(25,26)18(16-10-28-17-3-2-12(20)9-15(16)17)19(24)23-5-4-11-6-13(21)8-14(22)7-11/h2-10,18H,1H3,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208229
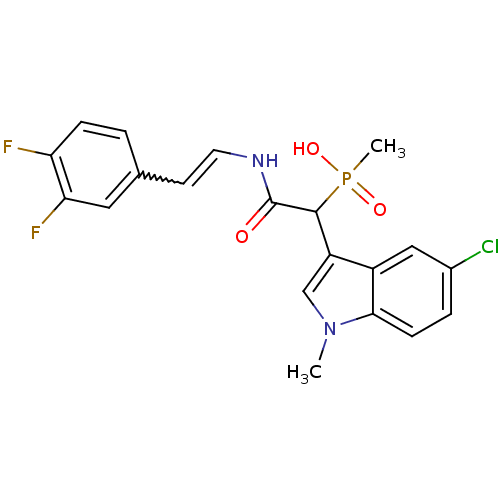
((E)-2-(3,4-difluorostyrylamino)-1-(5-chloro-1-meth...)Show SMILES Cn1cc(C(C(=O)NC=Cc2ccc(F)c(F)c2)P(C)(O)=O)c2cc(Cl)ccc12 |w:9.9| Show InChI InChI=1S/C20H18ClF2N2O3P/c1-25-11-15(14-10-13(21)4-6-18(14)25)19(29(2,27)28)20(26)24-8-7-12-3-5-16(22)17(23)9-12/h3-11,19H,1-2H3,(H,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208228

(1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...)Show SMILES OP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1csc2ccc(Cl)cc12 Show InChI InChI=1S/C20H15ClNO4PS/c21-14-6-8-18-16(10-14)17(11-28-18)19(27(24,25)26)20(23)22-15-7-5-12-3-1-2-4-13(12)9-15/h1-11,19H,(H,22,23)(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM50208241
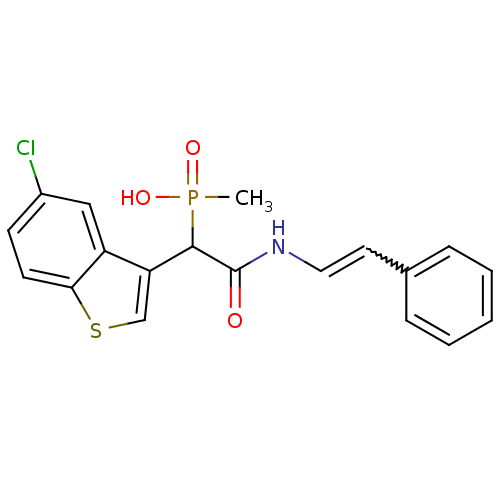
((E)-1-(5-chlorobenzo[b]thiophen-3-yl)-2-oxo-2-(sty...)Show SMILES CP(O)(=O)C(C(=O)NC=Cc1ccccc1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C19H17ClNO3PS/c1-25(23,24)18(16-12-26-17-8-7-14(20)11-15(16)17)19(22)21-10-9-13-5-3-2-4-6-13/h2-12,18H,1H3,(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208242

((E)-2-(3,4-difluorostyrylamino)-1-(5-chlorobenzo[b...)Show SMILES CP(O)(=O)C(C(=O)NC=Cc1ccc(F)c(F)c1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C19H15ClF2NO3PS/c1-27(25,26)18(14-10-28-17-5-3-12(20)9-13(14)17)19(24)23-7-6-11-2-4-15(21)16(22)8-11/h2-10,18H,1H3,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM50208226
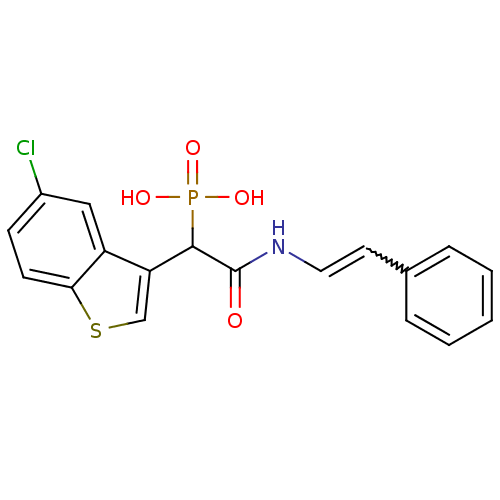
((E)-1-(5-chlorobenzo[b]thiophen-3-yl)-2-oxo-2-(sty...)Show SMILES OP(O)(=O)C(C(=O)NC=Cc1ccccc1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C18H15ClNO4PS/c19-13-6-7-16-14(10-13)15(11-26-16)17(25(22,23)24)18(21)20-9-8-12-4-2-1-3-5-12/h1-11,17H,(H,20,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208239

((E)-2-(4-fluorostyrylamino)-1-(5-chlorobenzo[b]thi...)Show SMILES OP(O)(=O)C(C(=O)NC=Cc1ccc(F)cc1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C18H14ClFNO4PS/c19-12-3-6-16-14(9-12)15(10-27-16)17(26(23,24)25)18(22)21-8-7-11-1-4-13(20)5-2-11/h1-10,17H,(H,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208221
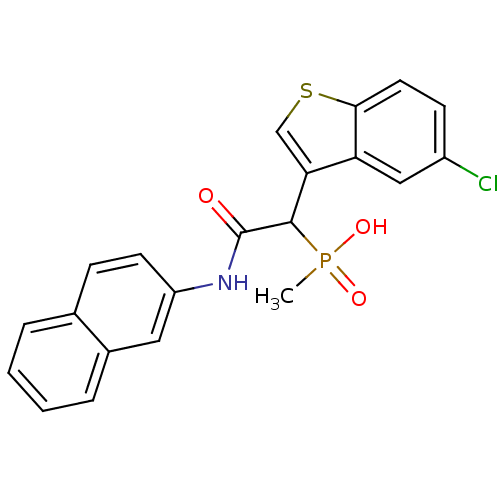
(1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...)Show SMILES CP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1csc2ccc(Cl)cc12 Show InChI InChI=1S/C21H17ClNO3PS/c1-27(25,26)20(18-12-28-19-9-7-15(22)11-17(18)19)21(24)23-16-8-6-13-4-2-3-5-14(13)10-16/h2-12,20H,1H3,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208227
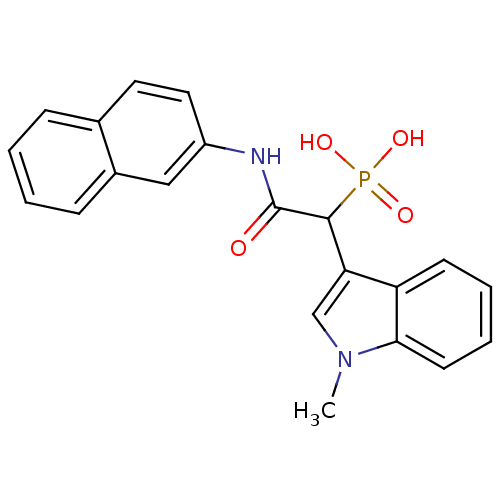
(1-(1-methyl-1H-indol-3-yl)-2-(naphthalen-2-ylamino...)Show SMILES Cn1cc(C(C(=O)Nc2ccc3ccccc3c2)P(O)(O)=O)c2ccccc12 Show InChI InChI=1S/C21H19N2O4P/c1-23-13-18(17-8-4-5-9-19(17)23)20(28(25,26)27)21(24)22-16-11-10-14-6-2-3-7-15(14)12-16/h2-13,20H,1H3,(H,22,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208234
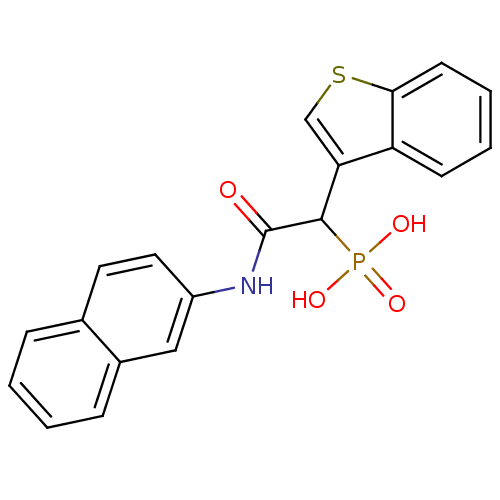
(1-(benzo[b]thiophen-3-yl)-2-(naphthalen-2-ylamino)...)Show SMILES OP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1csc2ccccc12 Show InChI InChI=1S/C20H16NO4PS/c22-20(21-15-10-9-13-5-1-2-6-14(13)11-15)19(26(23,24)25)17-12-27-18-8-4-3-7-16(17)18/h1-12,19H,(H,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208223
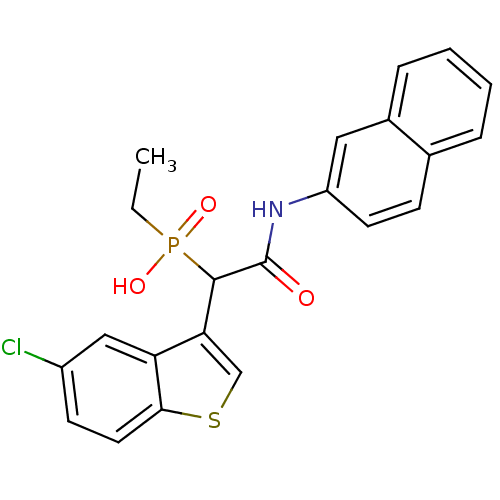
(1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...)Show SMILES CCP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1csc2ccc(Cl)cc12 Show InChI InChI=1S/C22H19ClNO3PS/c1-2-28(26,27)21(19-13-29-20-10-8-16(23)12-18(19)20)22(25)24-17-9-7-14-5-3-4-6-15(14)11-17/h3-13,21H,2H2,1H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208235
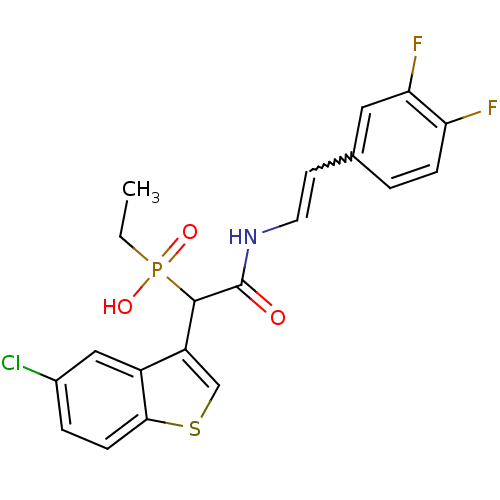
((E)-2-(3,4-difluorostyrylamino)-1-(5-chlorobenzo[b...)Show SMILES CCP(O)(=O)C(C(=O)NC=Cc1ccc(F)c(F)c1)c1csc2ccc(Cl)cc12 |w:10.10| Show InChI InChI=1S/C20H17ClF2NO3PS/c1-2-28(26,27)19(15-11-29-18-6-4-13(21)10-14(15)18)20(25)24-8-7-12-3-5-16(22)17(23)9-12/h3-11,19H,2H2,1H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208237
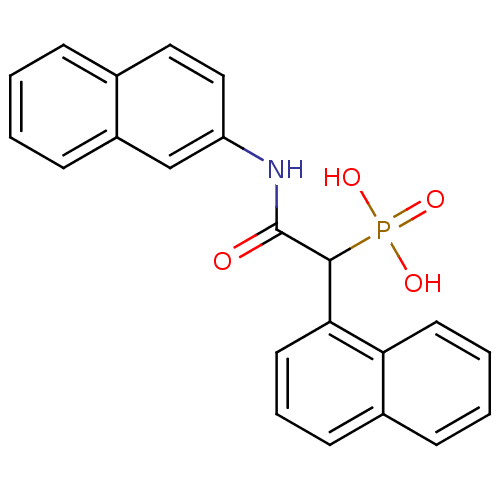
(1-(naphthalen-1-yl)-2-(naphthalen-2-ylamino)-2-oxo...)Show SMILES OP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1cccc2ccccc12 Show InChI InChI=1S/C22H18NO4P/c24-22(23-18-13-12-15-6-1-2-8-17(15)14-18)21(28(25,26)27)20-11-5-9-16-7-3-4-10-19(16)20/h1-14,21H,(H,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208238
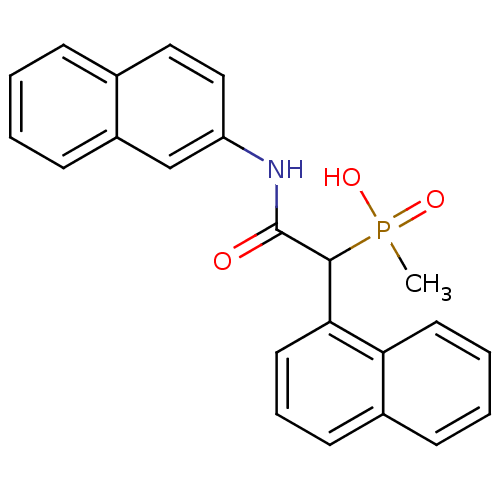
(CHEMBL223624 | methyl(1-(naphthalen-1-yl)-2-(napht...)Show SMILES CP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1cccc2ccccc12 Show InChI InChI=1S/C23H20NO3P/c1-28(26,27)22(21-12-6-10-17-8-4-5-11-20(17)21)23(25)24-19-14-13-16-7-2-3-9-18(16)15-19/h2-15,22H,1H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208220
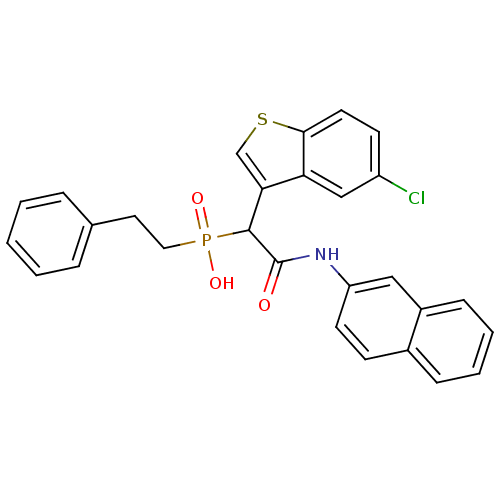
(1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...)Show SMILES OP(=O)(CCc1ccccc1)C(C(=O)Nc1ccc2ccccc2c1)c1csc2ccc(Cl)cc12 Show InChI InChI=1S/C28H23ClNO3PS/c29-22-11-13-26-24(17-22)25(18-35-26)27(34(32,33)15-14-19-6-2-1-3-7-19)28(31)30-23-12-10-20-8-4-5-9-21(20)16-23/h1-13,16-18,27H,14-15H2,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208219
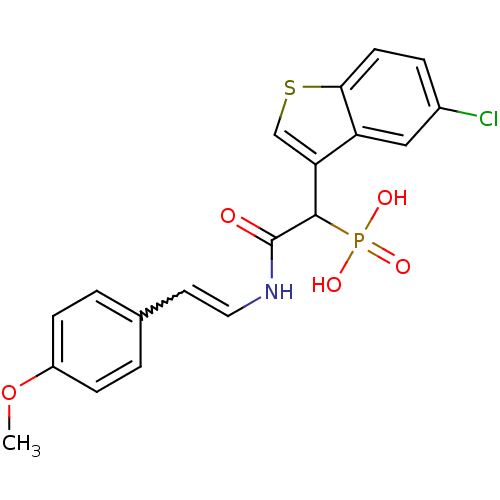
((E)-2-(4-methoxystyrylamino)-1-(5-chlorobenzo[b]th...)Show SMILES COc1ccc(C=CNC(=O)C(c2csc3ccc(Cl)cc23)P(O)(O)=O)cc1 |w:6.5| Show InChI InChI=1S/C19H17ClNO5PS/c1-26-14-5-2-12(3-6-14)8-9-21-19(22)18(27(23,24)25)16-11-28-17-7-4-13(20)10-15(16)17/h2-11,18H,1H3,(H,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208231
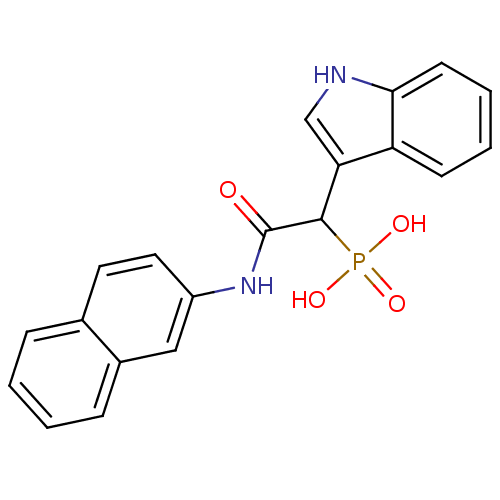
(1-(1H-indol-3-yl)-2-(naphthalen-2-ylamino)-2-oxoet...)Show SMILES OP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H17N2O4P/c23-20(22-15-10-9-13-5-1-2-6-14(13)11-15)19(27(24,25)26)17-12-21-18-8-4-3-7-16(17)18/h1-12,19,21H,(H,22,23)(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208240
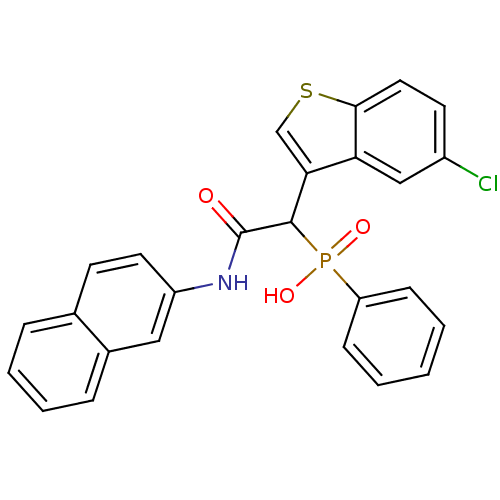
(1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...)Show SMILES OP(=O)(C(C(=O)Nc1ccc2ccccc2c1)c1csc2ccc(Cl)cc12)c1ccccc1 Show InChI InChI=1S/C26H19ClNO3PS/c27-19-11-13-24-22(15-19)23(16-33-24)25(32(30,31)21-8-2-1-3-9-21)26(29)28-20-12-10-17-6-4-5-7-18(17)14-20/h1-16,25H,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208233
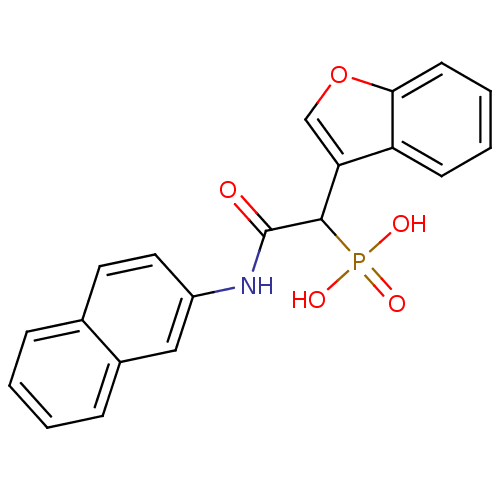
(1-(benzofuran-3-yl)-2-(naphthalen-2-ylamino)-2-oxo...)Show SMILES OP(O)(=O)C(C(=O)Nc1ccc2ccccc2c1)c1coc2ccccc12 Show InChI InChI=1S/C20H16NO5P/c22-20(21-15-10-9-13-5-1-2-6-14(13)11-15)19(27(23,24)25)17-12-26-18-8-4-3-7-16(17)18/h1-12,19H,(H,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208230
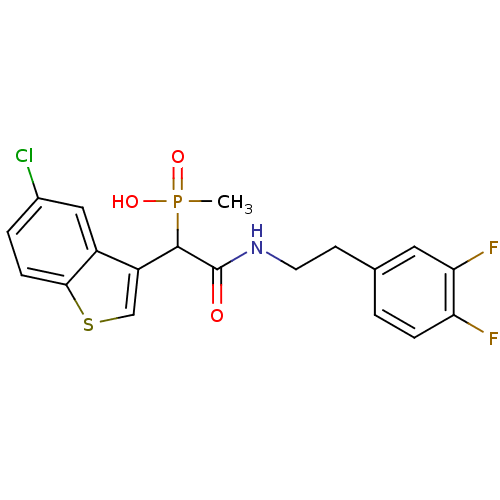
(2-(3,4-difluorophenethylamino)-1-(5-chlorobenzo[b]...)Show SMILES CP(O)(=O)C(C(=O)NCCc1ccc(F)c(F)c1)c1csc2ccc(Cl)cc12 Show InChI InChI=1S/C19H17ClF2NO3PS/c1-27(25,26)18(14-10-28-17-5-3-12(20)9-13(14)17)19(24)23-7-6-11-2-4-15(21)16(22)8-11/h2-5,8-10,18H,6-7H2,1H3,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data