

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9911 (3-Phenyl-7-(4-biphenylmethoxy)-2-[(4-pyridylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | -44.0 | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 6571-7 (2005) Article DOI: 10.1016/j.bmc.2005.07.038 BindingDB Entry DOI: 10.7270/Q2PK0DCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9903 (3-Phenyl-7-(beta-naphthylmethoxy)-2-[(4-pyridylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 74 | -42.3 | 90 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 6571-7 (2005) Article DOI: 10.1016/j.bmc.2005.07.038 BindingDB Entry DOI: 10.7270/Q2PK0DCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9902 (3-Phenyl-7-(alpha-naphthylmethoxy)-2-[(4-pyridylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 114 | -41.2 | 112 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 6571-7 (2005) Article DOI: 10.1016/j.bmc.2005.07.038 BindingDB Entry DOI: 10.7270/Q2PK0DCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.41E+3 | -34.7 | 2.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 6571-7 (2005) Article DOI: 10.1016/j.bmc.2005.07.038 BindingDB Entry DOI: 10.7270/Q2PK0DCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451322 (CHEMBL4210026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451338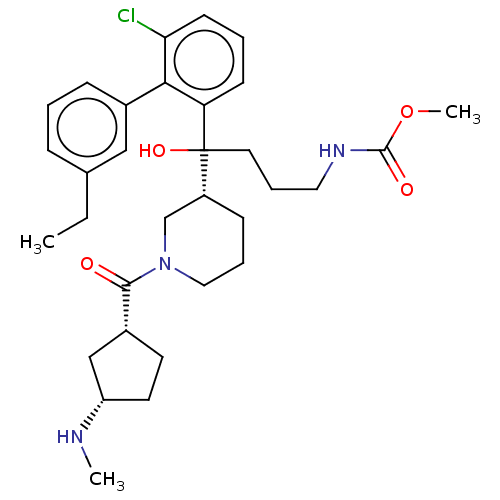 (CHEMBL4207673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451337 (CHEMBL4204601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451325 (CHEMBL4202424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451321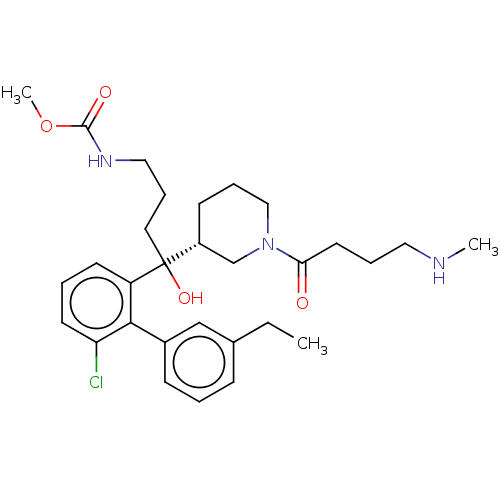 (CHEMBL4211388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458764 (CHEMBL4209125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458775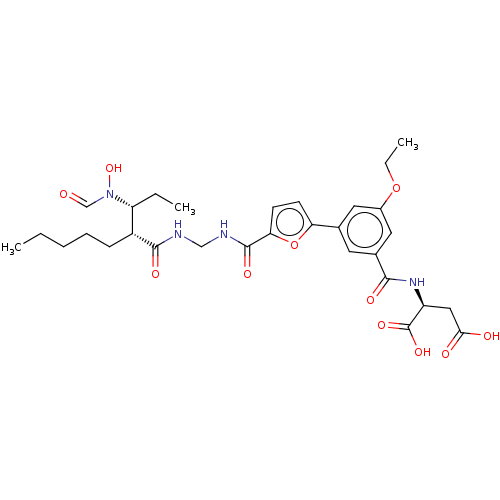 (CHEMBL4218415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of GST-tagged PI4K-alpha (1 to 2044) (unknown origin) using D-myo-phosphatidylinositol as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458774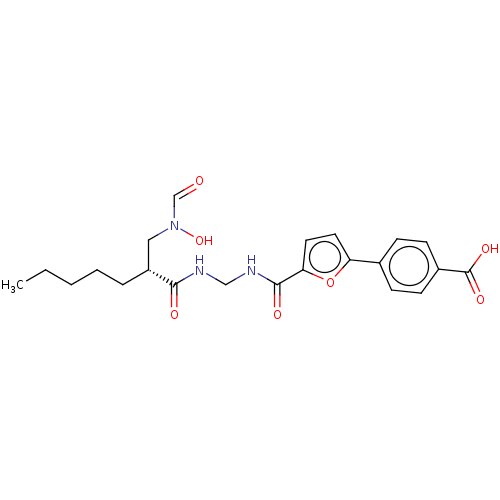 (CHEMBL4202714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458765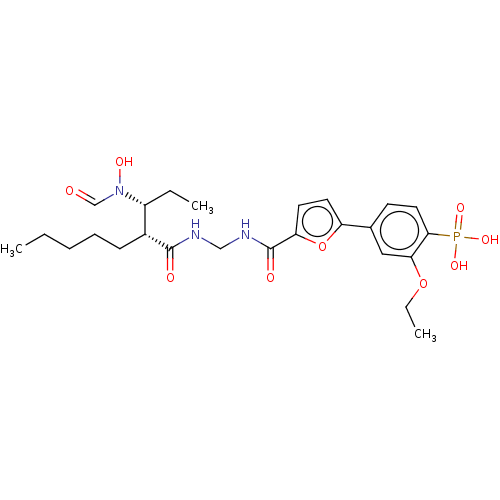 (CHEMBL4205697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458773 (CHEMBL4204567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458763 (CHEMBL4202848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451334 (CHEMBL4213138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451314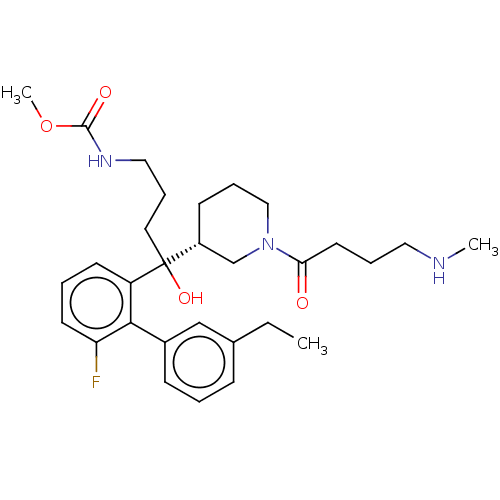 (CHEMBL4207648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451328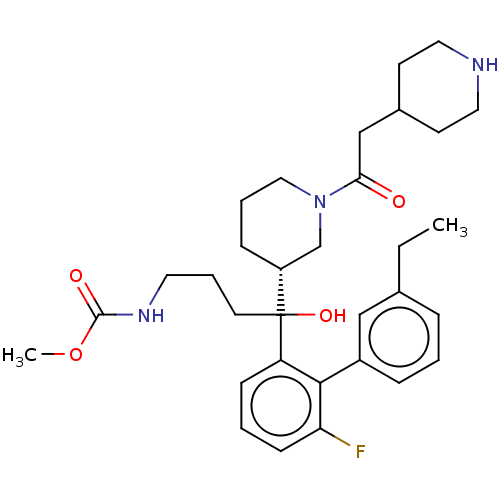 (CHEMBL4211697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451318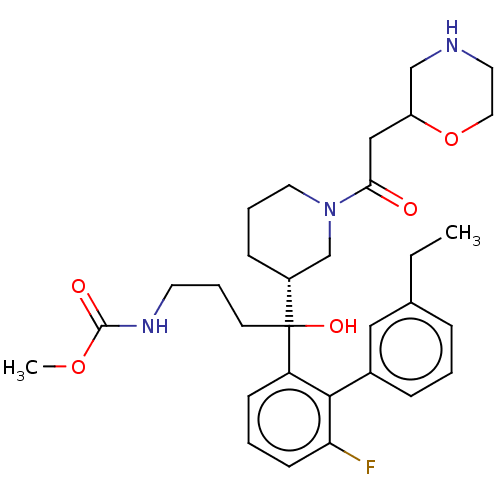 (CHEMBL4218098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451322 (CHEMBL4210026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of renin in human plasma using angiotensinogen as substrate measured for 90 mins by [125I]-angiotensin based radioimmunoassay | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451329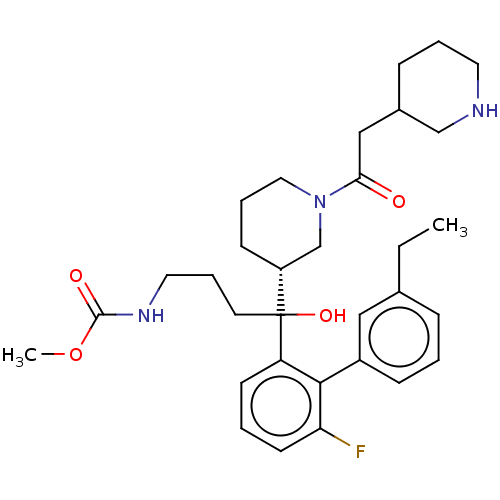 (CHEMBL4212429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451320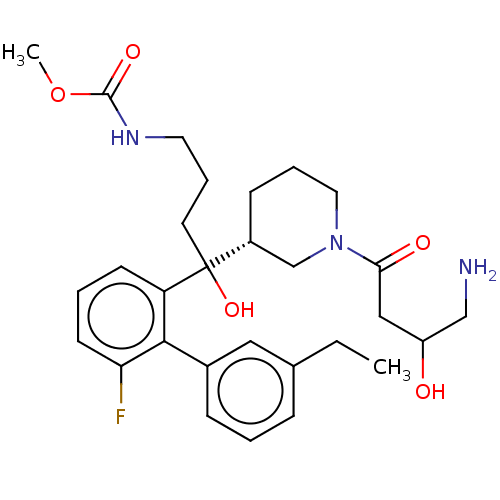 (CHEMBL4216413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451319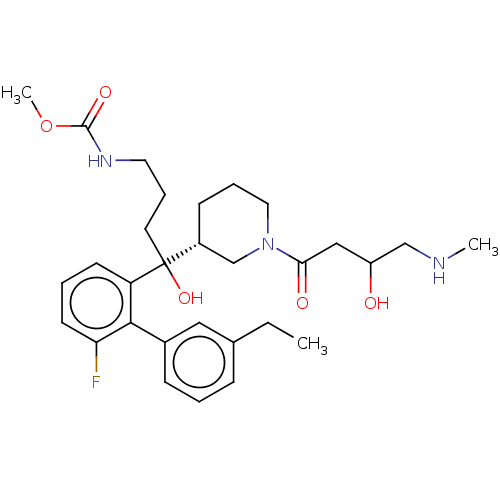 (CHEMBL4212859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451331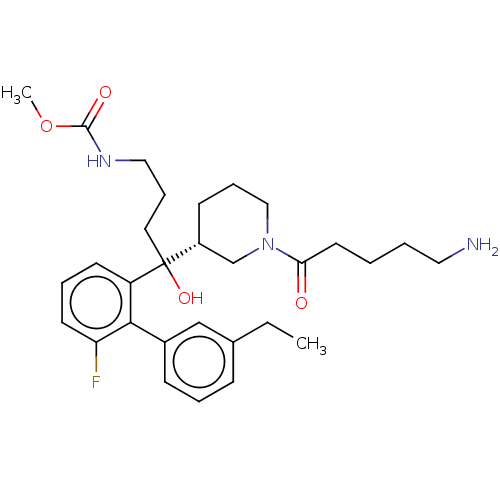 (CHEMBL4217145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451317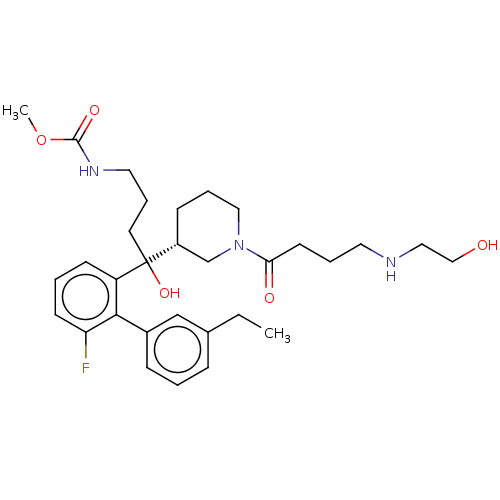 (CHEMBL4202961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451340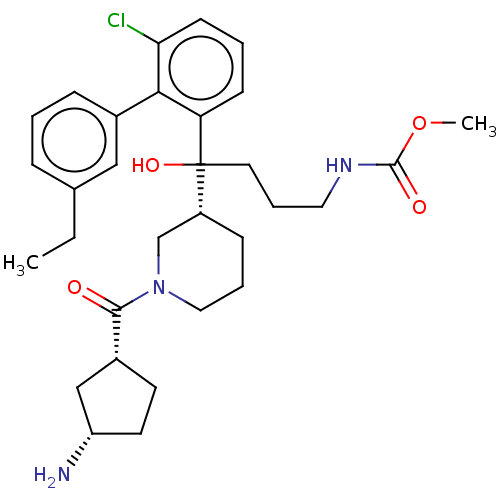 (CHEMBL4214937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451323 (CHEMBL4215302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451312 (CHEMBL4209674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451337 (CHEMBL4204601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of renin in human plasma using angiotensinogen as substrate measured for 90 mins by [125I]-angiotensin based radioimmunoassay | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451338 (CHEMBL4207673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of renin in human plasma using angiotensinogen as substrate measured for 90 mins by [125I]-angiotensin based radioimmunoassay | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451339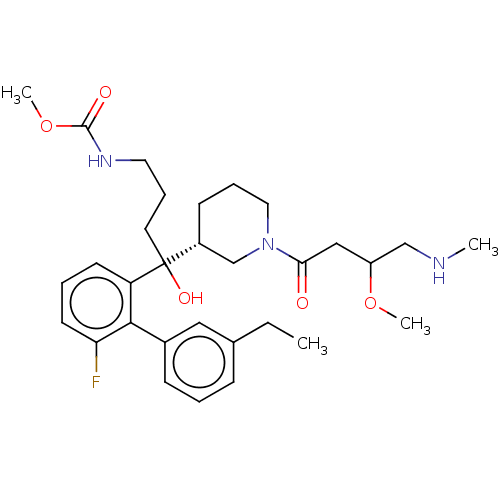 (CHEMBL4210995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50576323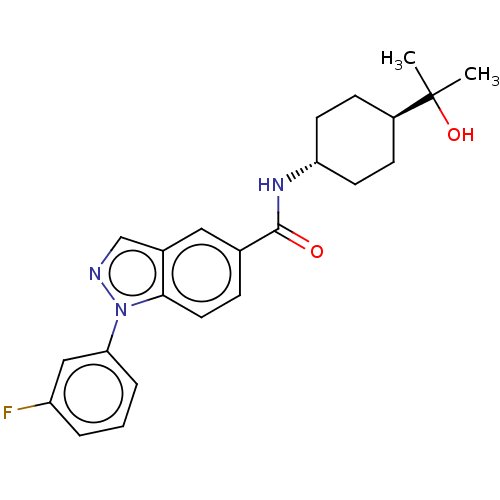 (CHEMBL4866146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451330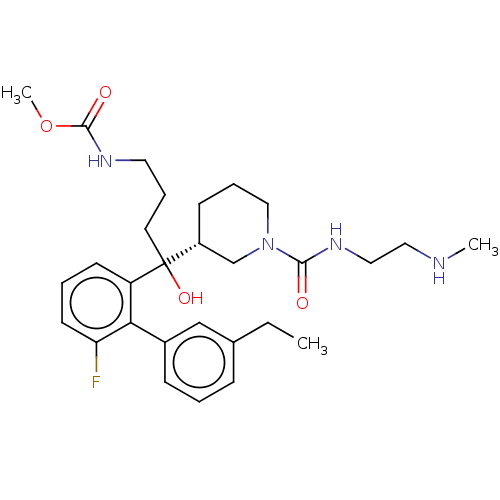 (CHEMBL4217427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451311 (CHEMBL4213141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451326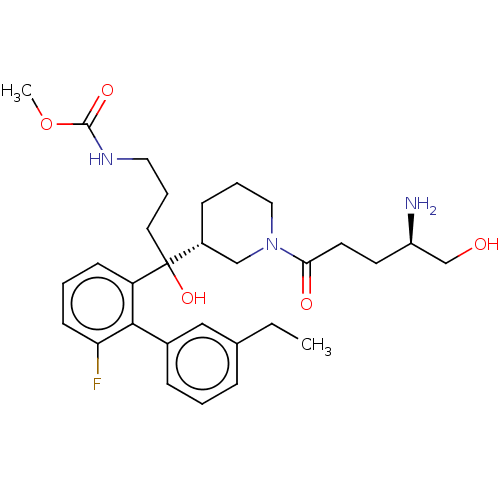 (CHEMBL4215948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50576316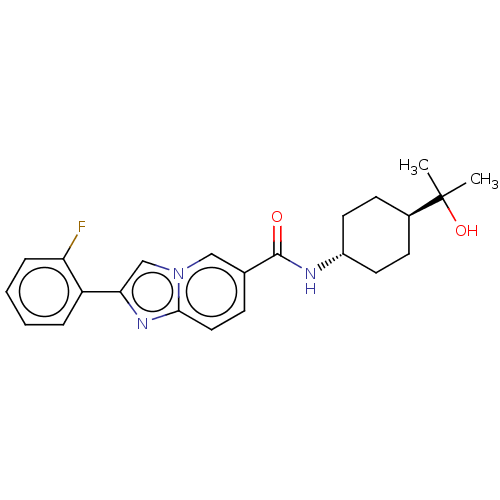 (CHEMBL4870703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458758 (CHEMBL4207907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451340 (CHEMBL4214937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of renin in human plasma using angiotensinogen as substrate measured for 90 mins by [125I]-angiotensin based radioimmunoassay | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50576314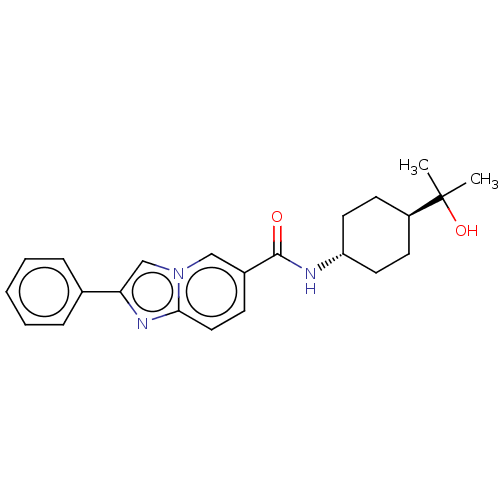 (CHEMBL4864805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50576324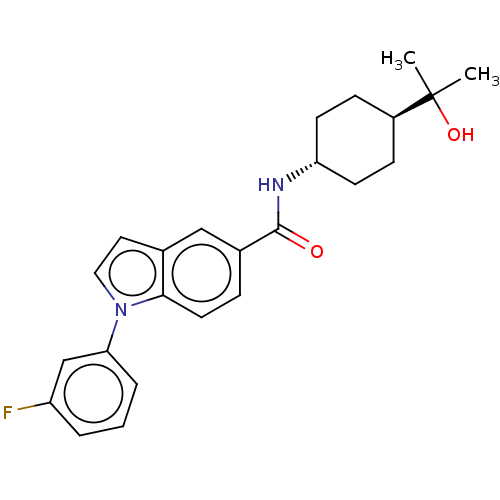 (CHEMBL4868339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50576322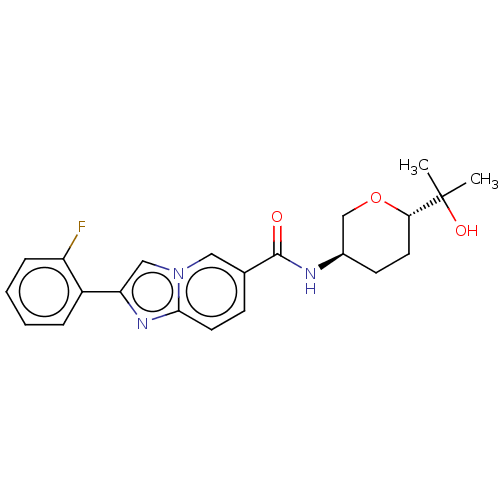 (CHEMBL4859922) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451325 (CHEMBL4202424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of renin in human plasma using angiotensinogen as substrate measured for 90 mins by [125I]-angiotensin based radioimmunoassay | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |