 Found 310 hits with Last Name = 'ding' and Initial = 'n'
Found 310 hits with Last Name = 'ding' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86950
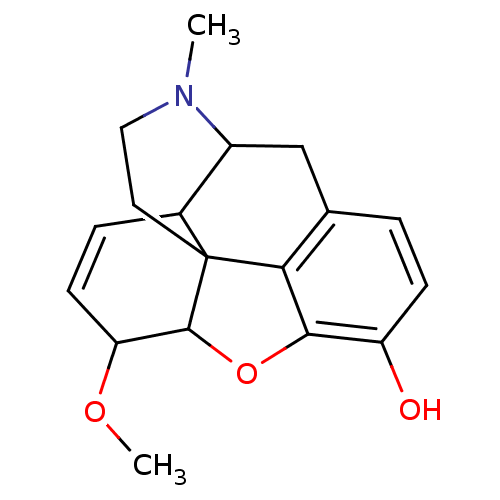
(CAS_5462505 | NSC_5462505 | heterocodeine)Show SMILES COC1C=CC2C3Cc4ccc(O)c5OC1C2(CCN3C)c45 |c:3,TLB:4:5:19.18.17:7.8.21| Show InChI InChI=1S/C18H21NO3/c1-19-8-7-18-11-4-6-14(21-2)17(18)22-16-13(20)5-3-10(15(16)18)9-12(11)19/h3-6,11-12,14,17,20H,7-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86258
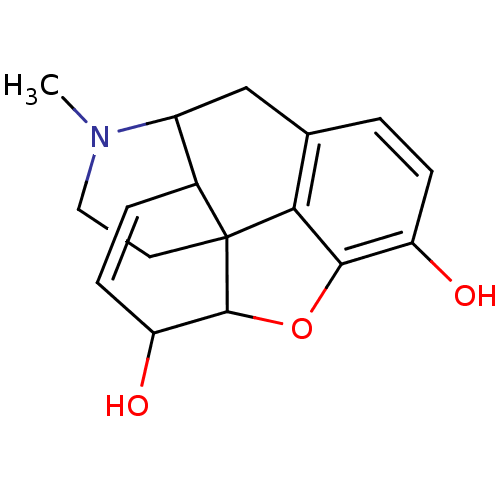
(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86957

(6-desoxymorphine | CAS_44450262 | NSC_44450262)Show SMILES CN1CCC23C4CC=CC2C1Cc1ccc(O)c(O4)c31 |c:7,TLB:8:9:1.2.3:11.12.19| Show InChI InChI=1S/C17H19NO2/c1-18-8-7-17-11-3-2-4-14(17)20-16-13(19)6-5-10(15(16)17)9-12(11)18/h2-3,5-6,11-12,14,19H,4,7-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86957

(6-desoxymorphine | CAS_44450262 | NSC_44450262)Show SMILES CN1CCC23C4CC=CC2C1Cc1ccc(O)c(O4)c31 |c:7,TLB:8:9:1.2.3:11.12.19| Show InChI InChI=1S/C17H19NO2/c1-18-8-7-17-11-3-2-4-14(17)20-16-13(19)6-5-10(15(16)17)9-12(11)18/h2-3,5-6,11-12,14,19H,4,7-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86950

(CAS_5462505 | NSC_5462505 | heterocodeine)Show SMILES COC1C=CC2C3Cc4ccc(O)c5OC1C2(CCN3C)c45 |c:3,TLB:4:5:19.18.17:7.8.21| Show InChI InChI=1S/C18H21NO3/c1-19-8-7-18-11-4-6-14(21-2)17(18)22-16-13(20)5-3-10(15(16)18)9-12(11)19/h3-6,11-12,14,17,20H,7-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86952
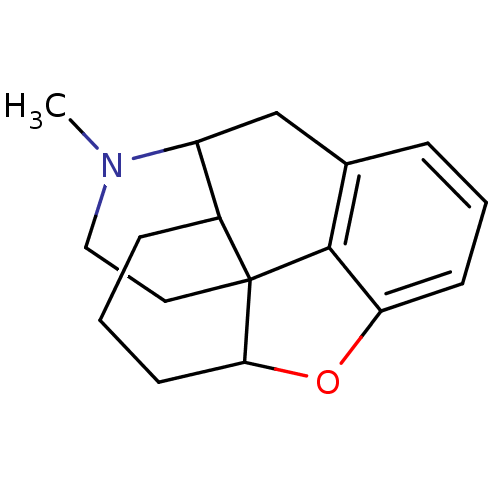
(7,8-dihydro-3,6-didesoxymorphine | CAS_22816300 | ...)Show SMILES CN1CCC23C4CCCC2C1Cc1cccc(O4)c31 |TLB:8:9:1.2.3:11.12.18| Show InChI InChI=1S/C17H21NO/c1-18-9-8-17-12-5-3-7-15(17)19-14-6-2-4-11(16(14)17)10-13(12)18/h2,4,6,12-13,15H,3,5,7-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86957

(6-desoxymorphine | CAS_44450262 | NSC_44450262)Show SMILES CN1CCC23C4CC=CC2C1Cc1ccc(O)c(O4)c31 |c:7,TLB:8:9:1.2.3:11.12.19| Show InChI InChI=1S/C17H19NO2/c1-18-8-7-17-11-3-2-4-14(17)20-16-13(19)6-5-10(15(16)17)9-12(11)18/h2-3,5-6,11-12,14,19H,4,7-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86258

(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 65.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86950

(CAS_5462505 | NSC_5462505 | heterocodeine)Show SMILES COC1C=CC2C3Cc4ccc(O)c5OC1C2(CCN3C)c45 |c:3,TLB:4:5:19.18.17:7.8.21| Show InChI InChI=1S/C18H21NO3/c1-19-8-7-18-11-4-6-14(21-2)17(18)22-16-13(20)5-3-10(15(16)18)9-12(11)19/h3-6,11-12,14,17,20H,7-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86258

(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86955
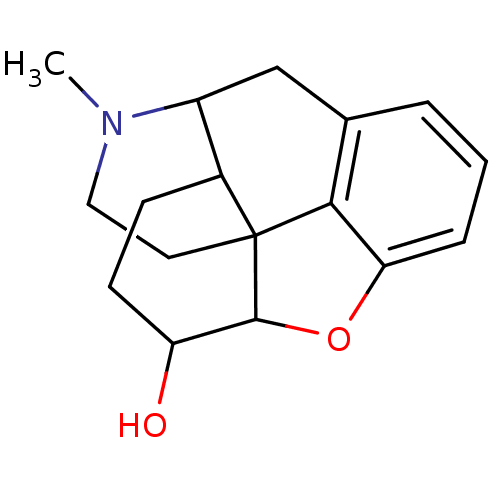
(7,8-dihydro-3-desoxymorphine | CAS_16039356 | NSC_...)Show SMILES CN1CCC23C4Oc5cccc(CC1C2CCC4O)c35 |TLB:15:14:1.2.3:12.11.19| Show InChI InChI=1S/C17H21NO2/c1-18-8-7-17-11-5-6-13(19)16(17)20-14-4-2-3-10(15(14)17)9-12(11)18/h2-4,11-13,16,19H,5-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86952

(7,8-dihydro-3,6-didesoxymorphine | CAS_22816300 | ...)Show SMILES CN1CCC23C4CCCC2C1Cc1cccc(O4)c31 |TLB:8:9:1.2.3:11.12.18| Show InChI InChI=1S/C17H21NO/c1-18-9-8-17-12-5-3-7-15(17)19-14-6-2-4-11(16(14)17)10-13(12)18/h2,4,6,12-13,15H,3,5,7-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86954
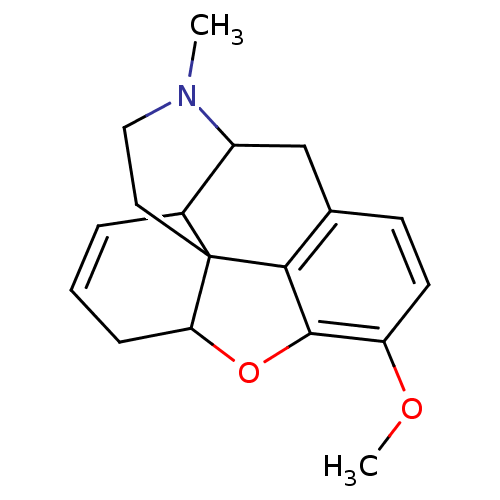
(6-desoxycodeine | CAS_5492830 | NSC_5492830)Show SMILES COc1ccc2CC3C4C=CCC5Oc1c2C45CCN3C |c:9,THB:9:8:19.18.17:6.5.15| Show InChI InChI=1S/C18H21NO2/c1-19-9-8-18-12-4-3-5-15(18)21-17-14(20-2)7-6-11(16(17)18)10-13(12)19/h3-4,6-7,12-13,15H,5,8-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86953
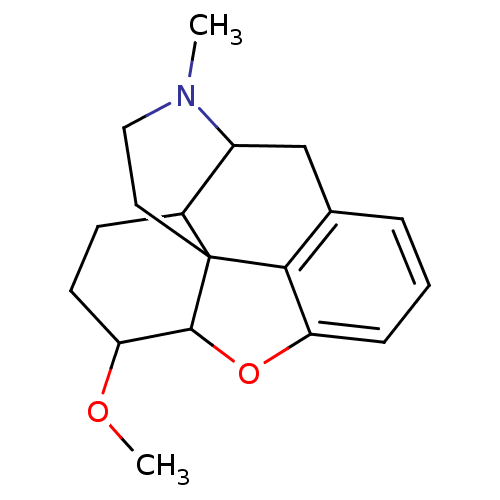
(7,8-dihydro-3-desoxyheterocodeine | CAS_24828463 |...)Show SMILES COC1CCC2C3Cc4cccc5OC1C2(CCN3C)c45 |TLB:4:5:18.17.16:7.8.20| Show InChI InChI=1S/C18H23NO2/c1-19-9-8-18-12-6-7-15(20-2)17(18)21-14-5-3-4-11(16(14)18)10-13(12)19/h3-5,12-13,15,17H,6-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 524 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86952

(7,8-dihydro-3,6-didesoxymorphine | CAS_22816300 | ...)Show SMILES CN1CCC23C4CCCC2C1Cc1cccc(O4)c31 |TLB:8:9:1.2.3:11.12.18| Show InChI InChI=1S/C17H21NO/c1-18-9-8-17-12-5-3-7-15(17)19-14-6-2-4-11(16(14)17)10-13(12)18/h2,4,6,12-13,15H,3,5,7-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM82266
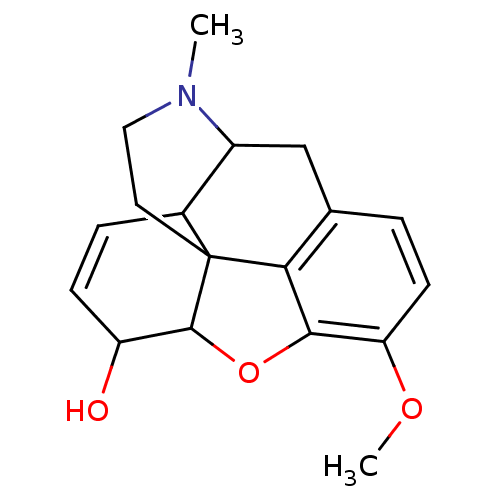
(CAS_29485-83-4 | CODEINE SULFATE | Codeine | NSC_2...)Show SMILES COc1ccc2CC3C4C=CC(O)C5Oc1c2C45CCN3C |c:9,THB:9:8:20.19.18:6.5.16| Show InChI InChI=1S/C18H21NO3/c1-19-8-7-18-11-4-5-13(20)17(18)22-16-14(21-2)6-3-10(15(16)18)9-12(11)19/h3-6,11-13,17,20H,7-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 727 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86956

(CAS_5492619 | NSC_5492619 | codeine-6-methyl ether)Show SMILES COC1C=CC2C3Cc4ccc(OC)c5OC1C2(CCN3C)c45 |c:3,TLB:4:5:20.19.18:7.8.22| Show InChI InChI=1S/C19H23NO3/c1-20-9-8-19-12-5-7-15(22-3)18(19)23-17-14(21-2)6-4-11(16(17)19)10-13(12)20/h4-7,12-13,15,18H,8-10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86954

(6-desoxycodeine | CAS_5492830 | NSC_5492830)Show SMILES COc1ccc2CC3C4C=CCC5Oc1c2C45CCN3C |c:9,THB:9:8:19.18.17:6.5.15| Show InChI InChI=1S/C18H21NO2/c1-19-9-8-18-12-4-3-5-15(18)21-17-14(20-2)7-6-11(16(17)18)10-13(12)19/h3-4,6-7,12-13,15H,5,8-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86956

(CAS_5492619 | NSC_5492619 | codeine-6-methyl ether)Show SMILES COC1C=CC2C3Cc4ccc(OC)c5OC1C2(CCN3C)c45 |c:3,TLB:4:5:20.19.18:7.8.22| Show InChI InChI=1S/C19H23NO3/c1-20-9-8-19-12-5-7-15(22-3)18(19)23-17-14(21-2)6-4-11(16(17)19)10-13(12)20/h4-7,12-13,15,18H,8-10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86955

(7,8-dihydro-3-desoxymorphine | CAS_16039356 | NSC_...)Show SMILES CN1CCC23C4Oc5cccc(CC1C2CCC4O)c35 |TLB:15:14:1.2.3:12.11.19| Show InChI InChI=1S/C17H21NO2/c1-18-8-7-17-11-5-6-13(19)16(17)20-14-4-2-3-10(15(14)17)9-12(11)18/h2-4,11-13,16,19H,5-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86955

(7,8-dihydro-3-desoxymorphine | CAS_16039356 | NSC_...)Show SMILES CN1CCC23C4Oc5cccc(CC1C2CCC4O)c35 |TLB:15:14:1.2.3:12.11.19| Show InChI InChI=1S/C17H21NO2/c1-18-8-7-17-11-5-6-13(19)16(17)20-14-4-2-3-10(15(14)17)9-12(11)18/h2-4,11-13,16,19H,5-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86956

(CAS_5492619 | NSC_5492619 | codeine-6-methyl ether)Show SMILES COC1C=CC2C3Cc4ccc(OC)c5OC1C2(CCN3C)c45 |c:3,TLB:4:5:20.19.18:7.8.22| Show InChI InChI=1S/C19H23NO3/c1-20-9-8-19-12-5-7-15(22-3)18(19)23-17-14(21-2)6-4-11(16(17)19)10-13(12)20/h4-7,12-13,15,18H,8-10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86953

(7,8-dihydro-3-desoxyheterocodeine | CAS_24828463 |...)Show SMILES COC1CCC2C3Cc4cccc5OC1C2(CCN3C)c45 |TLB:4:5:18.17.16:7.8.20| Show InChI InChI=1S/C18H23NO2/c1-19-9-8-18-12-6-7-15(20-2)17(18)21-14-5-3-4-11(16(14)18)10-13(12)19/h3-5,12-13,15,17H,6-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86953

(7,8-dihydro-3-desoxyheterocodeine | CAS_24828463 |...)Show SMILES COC1CCC2C3Cc4cccc5OC1C2(CCN3C)c45 |TLB:4:5:18.17.16:7.8.20| Show InChI InChI=1S/C18H23NO2/c1-19-9-8-18-12-6-7-15(20-2)17(18)21-14-5-3-4-11(16(14)18)10-13(12)19/h3-5,12-13,15,17H,6-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82266

(CAS_29485-83-4 | CODEINE SULFATE | Codeine | NSC_2...)Show SMILES COc1ccc2CC3C4C=CC(O)C5Oc1c2C45CCN3C |c:9,THB:9:8:20.19.18:6.5.16| Show InChI InChI=1S/C18H21NO3/c1-19-8-7-18-11-4-5-13(20)17(18)22-16-14(21-2)6-3-10(15(16)18)9-12(11)19/h3-6,11-13,17,20H,7-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM82266

(CAS_29485-83-4 | CODEINE SULFATE | Codeine | NSC_2...)Show SMILES COc1ccc2CC3C4C=CC(O)C5Oc1c2C45CCN3C |c:9,THB:9:8:20.19.18:6.5.16| Show InChI InChI=1S/C18H21NO3/c1-19-8-7-18-11-4-5-13(20)17(18)22-16-14(21-2)6-3-10(15(16)18)9-12(11)19/h3-6,11-13,17,20H,7-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by PDSP Ki Database
| |
J Med Chem 51: 2316-20 (2008)
Article DOI: 10.1021/jm701457j
BindingDB Entry DOI: 10.7270/Q2G15ZFM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255355
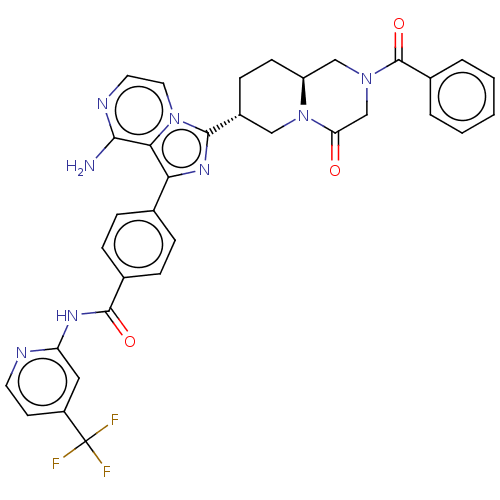
(US9481682, 105)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CN(CC(=O)N2C1)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C34H29F3N8O3/c35-34(36,37)24-12-13-39-26(16-24)41-32(47)21-8-6-20(7-9-21)28-29-30(38)40-14-15-44(29)31(42-28)23-10-11-25-18-43(19-27(46)45(25)17-23)33(48)22-4-2-1-3-5-22/h1-9,12-16,23,25H,10-11,17-19H2,(H2,38,40)(H,39,41,47)/t23-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255335

(US9481682, 85)Show SMILES COc1cc(ccc1-c1nc([C@@H]2CC[C@H]3CN(CC(=O)N3C2)C(=O)c2ccncc2)n2ccnc(N)c12)C(=O)Nc1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C34H30F3N9O4/c1-50-25-14-20(32(48)42-26-15-22(8-11-40-26)34(35,36)37)3-5-24(25)28-29-30(38)41-12-13-45(29)31(43-28)21-2-4-23-17-44(18-27(47)46(23)16-21)33(49)19-6-9-39-10-7-19/h3,5-15,21,23H,2,4,16-18H2,1H3,(H2,38,41)(H,40,42,48)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594234

(CHEMBL5200336)Show SMILES Cc1cc(C(N)=O)c2[nH]c3cc(ccc3c2c1-c1cccc(c1O)-n1c(=O)[nH]c2c(F)cccc2c1=O)C(C)(C)O |(3.12,1.54,;1.79,2.31,;1.79,3.85,;.47,4.61,;.47,6.15,;1.8,6.92,;-.87,6.92,;-.86,3.85,;-2.33,4.32,;-3.23,3.08,;-4.77,2.91,;-5.39,1.5,;-4.49,.27,;-2.95,.43,;-2.33,1.83,;-.86,2.31,;.47,1.55,;.47,.01,;-.87,-.77,;-.86,-2.31,;.47,-3.07,;1.8,-2.3,;1.8,-.76,;3.14,.01,;3.13,-3.07,;3.13,-4.61,;1.8,-5.38,;4.47,-5.38,;5.8,-4.61,;7.14,-5.38,;7.14,-6.92,;8.47,-4.6,;8.47,-3.07,;7.13,-2.3,;5.8,-3.07,;4.47,-2.3,;4.47,-.76,;-6.93,1.5,;-7.7,.17,;-7.7,2.84,;-8.47,1.5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594232

(CHEMBL5171706)Show SMILES OC[C@H]1CCC[C@H](Nc2ncnc3[nH]cc(C(=O)c4ccc(Oc5ccccc5)cc4Cl)c23)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50020476
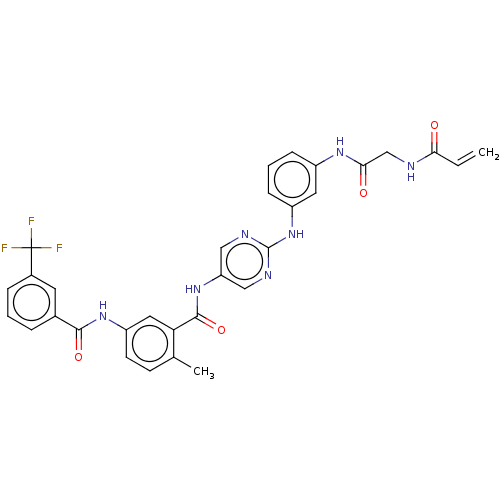
(CHEMBL3290148)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(NC(=O)CNC(=O)C=C)c2)nc1 Show InChI InChI=1S/C31H26F3N7O4/c1-3-26(42)35-17-27(43)38-21-8-5-9-22(13-21)41-30-36-15-24(16-37-30)40-29(45)25-14-23(11-10-18(25)2)39-28(44)19-6-4-7-20(12-19)31(32,33)34/h3-16H,1,17H2,2H3,(H,35,42)(H,38,43)(H,39,44)(H,40,45)(H,36,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302107
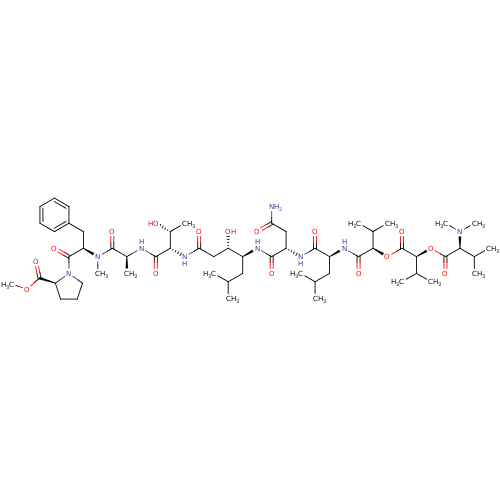
(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E using MCA-KPILFFRLK(Dnp)-D-R-NH2 as substrate incubated for 10 to 15 mins prior to substrate addition measured every 5 min ... |
Bioorg Med Chem 20: 4774-80 (2012)
Article DOI: 10.1016/j.bmc.2012.05.077
BindingDB Entry DOI: 10.7270/Q2JH3N8B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594232

(CHEMBL5171706)Show SMILES OC[C@H]1CCC[C@H](Nc2ncnc3[nH]cc(C(=O)c4ccc(Oc5ccccc5)cc4Cl)c23)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50020449
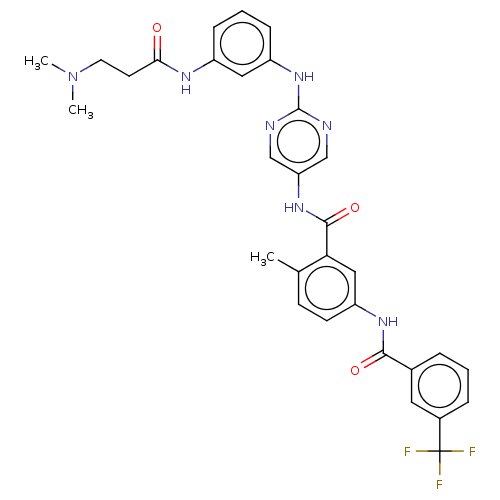
(CHEMBL3290125)Show SMILES CN(C)CCC(=O)Nc1cccc(Nc2ncc(NC(=O)c3cc(NC(=O)c4cccc(c4)C(F)(F)F)ccc3C)cn2)c1 Show InChI InChI=1S/C31H30F3N7O3/c1-19-10-11-24(38-28(43)20-6-4-7-21(14-20)31(32,33)34)16-26(19)29(44)39-25-17-35-30(36-18-25)40-23-9-5-8-22(15-23)37-27(42)12-13-41(2)3/h4-11,14-18H,12-13H2,1-3H3,(H,37,42)(H,38,43)(H,39,44)(H,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594233
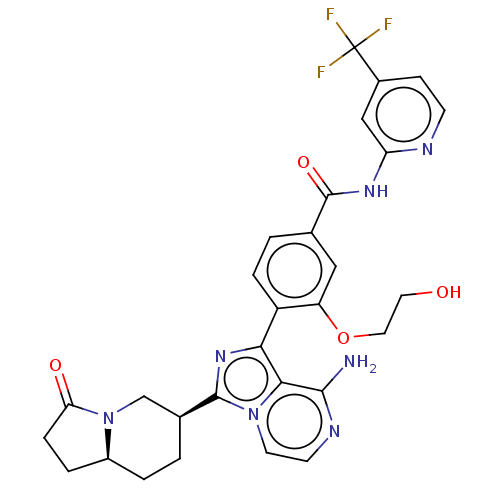
(CHEMBL5175002)Show SMILES [H][C@]12CCC(=O)N1C[C@H](CC2)c1nc(-c2ccc(cc2OCCO)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50020476

(CHEMBL3290148)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(NC(=O)CNC(=O)C=C)c2)nc1 Show InChI InChI=1S/C31H26F3N7O4/c1-3-26(42)35-17-27(43)38-21-8-5-9-22(13-21)41-30-36-15-24(16-37-30)40-29(45)25-14-23(11-10-18(25)2)39-28(44)19-6-4-7-20(12-19)31(32,33)34/h3-16H,1,17H2,2H3,(H,35,42)(H,38,43)(H,39,44)(H,40,45)(H,36,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Rlk (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255370

(US9481682, 121)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3OC3CC3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CCC(=O)N2C1 |r| Show InChI InChI=1S/C30H28F3N7O3/c31-30(32,33)18-9-10-35-23(14-18)37-29(42)16-2-7-21(22(13-16)43-20-5-6-20)25-26-27(34)36-11-12-39(26)28(38-25)17-1-3-19-4-8-24(41)40(19)15-17/h2,7,9-14,17,19-20H,1,3-6,8,15H2,(H2,34,36)(H,35,37,42)/t17-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50020471
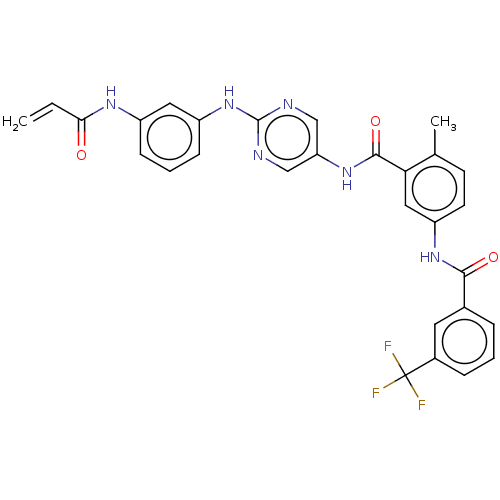
(CHEMBL3290142)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(NC(=O)C=C)c2)nc1 Show InChI InChI=1S/C29H23F3N6O3/c1-3-25(39)35-20-8-5-9-21(13-20)38-28-33-15-23(16-34-28)37-27(41)24-14-22(11-10-17(24)2)36-26(40)18-6-4-7-19(12-18)29(30,31)32/h3-16H,1H2,2H3,(H,35,39)(H,36,40)(H,37,41)(H,33,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Rlk (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50020471

(CHEMBL3290142)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(NC(=O)C=C)c2)nc1 Show InChI InChI=1S/C29H23F3N6O3/c1-3-25(39)35-20-8-5-9-21(13-20)38-28-33-15-23(16-34-28)37-27(41)24-14-22(11-10-17(24)2)36-26(40)18-6-4-7-19(12-18)29(30,31)32/h3-16H,1H2,2H3,(H,35,39)(H,36,40)(H,37,41)(H,33,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594227

(CHEMBL5169992)Show SMILES CN1CCn2nc(Nc3cc(nn4ccnc34)-c3ccnc(N4CCn5c6CCCCc6cc5C4=O)c3CO)cc2C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594229

(CHEMBL5177238)Show SMILES Cc1cc(Nc2cc(cn(C)c2=O)-c2ccnc(c2CO)-n2ncc3cc(cc(F)c3c2=O)C(C)(C)C)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50020471

(CHEMBL3290142)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(NC(=O)C=C)c2)nc1 Show InChI InChI=1S/C29H23F3N6O3/c1-3-25(39)35-20-8-5-9-21(13-20)38-28-33-15-23(16-34-28)37-27(41)24-14-22(11-10-17(24)2)36-26(40)18-6-4-7-19(12-18)29(30,31)32/h3-16H,1H2,2H3,(H,35,39)(H,36,40)(H,37,41)(H,33,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594229

(CHEMBL5177238)Show SMILES Cc1cc(Nc2cc(cn(C)c2=O)-c2ccnc(c2CO)-n2ncc3cc(cc(F)c3c2=O)C(C)(C)C)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594227

(CHEMBL5169992)Show SMILES CN1CCn2nc(Nc3cc(nn4ccnc34)-c3ccnc(N4CCn5c6CCCCc6cc5C4=O)c3CO)cc2C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50020476

(CHEMBL3290148)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(NC(=O)CNC(=O)C=C)c2)nc1 Show InChI InChI=1S/C31H26F3N7O4/c1-3-26(42)35-17-27(43)38-21-8-5-9-22(13-21)41-30-36-15-24(16-37-30)40-29(45)25-14-23(11-10-18(25)2)39-28(44)19-6-4-7-20(12-19)31(32,33)34/h3-16H,1,17H2,2H3,(H,35,42)(H,38,43)(H,39,44)(H,40,45)(H,36,37,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of HER4 (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Xinxiang Medical University
Curated by ChEMBL
| Assay Description
Inhibition of TNF-alpha release was determined in LPS-stimulated human whole blood assay |
Eur J Med Chem 140: 392-402 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.038
BindingDB Entry DOI: 10.7270/Q2HH6NK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50020445
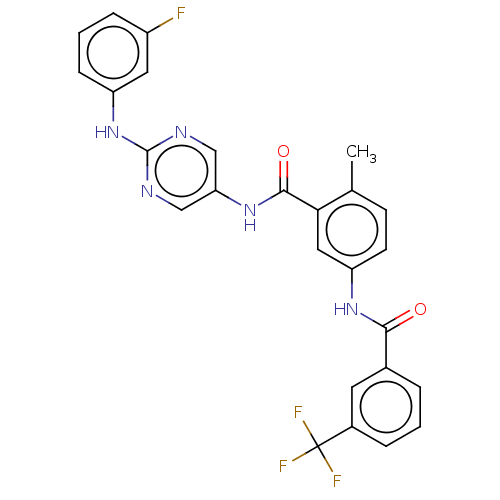
(CHEMBL3290121)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(F)c2)nc1 Show InChI InChI=1S/C26H19F4N5O2/c1-15-8-9-20(33-23(36)16-4-2-5-17(10-16)26(28,29)30)12-22(15)24(37)34-21-13-31-25(32-14-21)35-19-7-3-6-18(27)11-19/h2-14H,1H3,(H,33,36)(H,34,37)(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50020446

(CHEMBL3290122)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2ccc(N)cc2)nc1 Show InChI InChI=1S/C26H21F3N6O2/c1-15-5-8-20(33-23(36)16-3-2-4-17(11-16)26(27,28)29)12-22(15)24(37)34-21-13-31-25(32-14-21)35-19-9-6-18(30)7-10-19/h2-14H,30H2,1H3,(H,33,36)(H,34,37)(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50020444
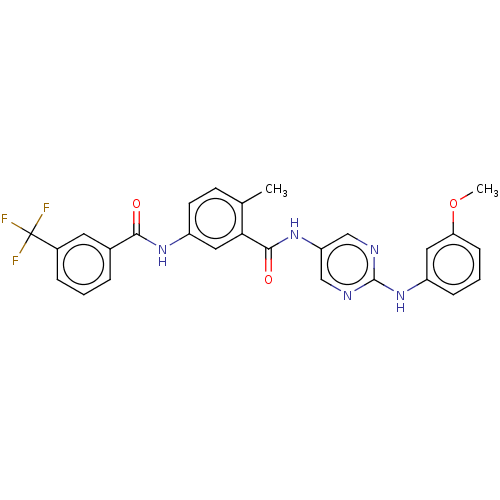
(CHEMBL3290120)Show SMILES COc1cccc(Nc2ncc(NC(=O)c3cc(NC(=O)c4cccc(c4)C(F)(F)F)ccc3C)cn2)c1 Show InChI InChI=1S/C27H22F3N5O3/c1-16-9-10-20(33-24(36)17-5-3-6-18(11-17)27(28,29)30)13-23(16)25(37)34-21-14-31-26(32-15-21)35-19-7-4-8-22(12-19)38-2/h3-15H,1-2H3,(H,33,36)(H,34,37)(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data