 Found 1622 hits with Last Name = 'ding' and Initial = 'p'
Found 1622 hits with Last Name = 'ding' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM93624
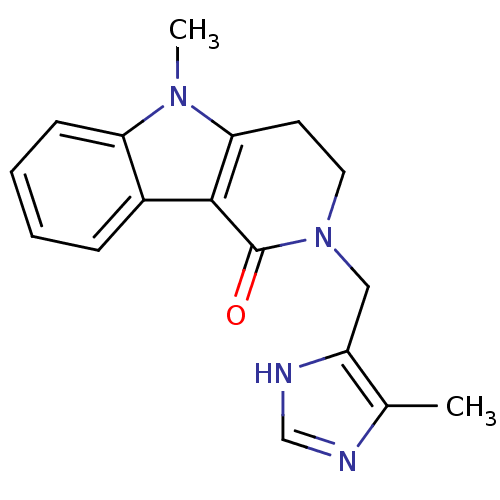
(5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4...)Show InChI InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329746

(CHEMBL1271731 | endo-2-((3S,5S)-3,5-dimethylmorpho...)Show SMILES C[C@H]1COC[C@H](C)N1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:29:28:23.24.25:27.20.21| Show InChI InChI=1S/C23H32N4O3/c1-14-12-29-13-15(2)27(14)23-25-21-19(8-5-9-20(21)30-23)22(28)24-16-10-17-6-4-7-18(11-16)26(17)3/h5,8-9,14-18H,4,6-7,10-13H2,1-3H3,(H,24,28)/t14-,15-,17+,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329748
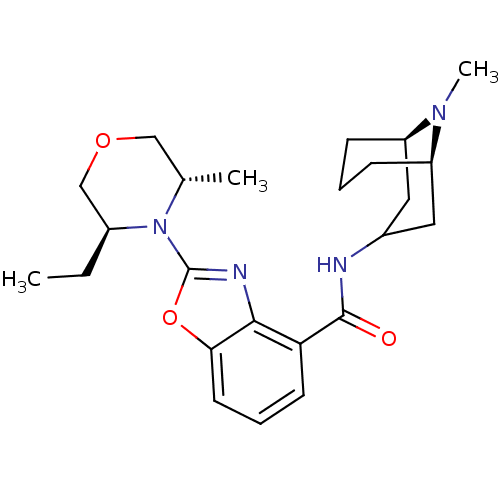
(CHEMBL1271791 | endo-2-((3S,5S)-3-ethyl-5-methylmo...)Show SMILES CC[C@H]1COC[C@H](C)N1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:30:29:24.25.26:28.21.22| Show InChI InChI=1S/C24H34N4O3/c1-4-17-14-30-13-15(2)28(17)24-26-22-20(9-6-10-21(22)31-24)23(29)25-16-11-18-7-5-8-19(12-16)27(18)3/h6,9-10,15-19H,4-5,7-8,11-14H2,1-3H3,(H,25,29)/t15-,17-,18+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50551956
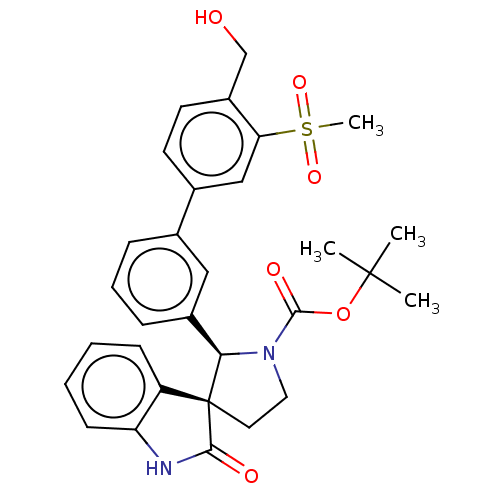
(CHEMBL4756418)Show SMILES CC(C)(C)OC(=O)N1CC[C@@]2([C@H]1c1cccc(c1)-c1ccc(CO)c(c1)S(C)(=O)=O)C(=O)Nc1ccccc21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of hyodeoxycholicacid-based fluorescent tracer from recombinant human LXRbeta LBD (215 to 461 residues) expressed in Escherichia coli BL... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112240
BindingDB Entry DOI: 10.7270/Q2MG7T4D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50011270

(2-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-1...)Show SMILES OC(=O)C(CCc1ccccc1)NC1Cc2ccccc2[C@H]2CCC[C@H](N2C1=O)C(O)=O Show InChI InChI=1S/C25H28N2O5/c28-23-20(26-19(24(29)30)14-13-16-7-2-1-3-8-16)15-17-9-4-5-10-18(17)21-11-6-12-22(25(31)32)27(21)23/h1-5,7-10,19-22,26H,6,11-15H2,(H,29,30)(H,31,32)/t19?,20?,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Calgary
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory potency against angiotensin I converting enzyme. |
J Med Chem 34: 511-7 (1991)
BindingDB Entry DOI: 10.7270/Q2JS9R1G |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177015

(CHEMBL3814206 | US10144715, Compound 19-1)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(CO)c(n1)C(F)(F)F)c1ccc(CO)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H27F3N4O4S/c1-13(2)17-10-27(16-5-4-14(11-29)18(8-16)33(3,31)32)6-7-28(17)20-25-9-15(12-30)19(26-20)21(22,23)24/h4-5,8-9,13,17,29-30H,6-7,10-12H2,1-3H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... |
Eur J Med Chem 178: 458-467 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.011
BindingDB Entry DOI: 10.7270/Q2BC42WH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329745
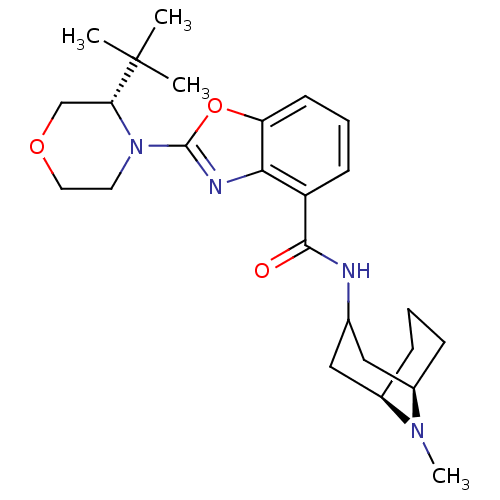
(CHEMBL1271730 | endo-2-((S)-3-tert-butylmorpholino...)Show SMILES CN1[C@@H]2CCC[C@@H]1CC(C2)NC(=O)c1cccc2oc(nc12)N1CCOC[C@@H]1C(C)(C)C |r,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C25H36N4O3/c1-25(2,3)21-15-31-12-11-29(21)24-27-22-19(9-6-10-20(22)32-24)23(30)26-16-13-17-7-5-8-18(14-16)28(17)4/h6,9-10,16-18,21H,5,7-8,11-15H2,1-4H3,(H,26,30)/t17-,18-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19992

(2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...)Show SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1 Show InChI InChI=1S/C33H31ClF3NO3/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... |
Eur J Med Chem 178: 458-467 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.011
BindingDB Entry DOI: 10.7270/Q2BC42WH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19992

(2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...)Show SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1 Show InChI InChI=1S/C33H31ClF3NO3/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of hyodeoxycholicacid-based fluorescent tracer from recombinant human LXRbeta LBD (215 to 461 residues) expressed in Escherichia coli BL... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112240
BindingDB Entry DOI: 10.7270/Q2MG7T4D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329724

(CHEMBL1271960 | endo-2-((2S,6S)-2,6-dimethylpipera...)Show SMILES C[C@H]1CNC[C@H](C)N1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:29:28:23.24.25:27.20.21| Show InChI InChI=1S/C23H33N5O2/c1-14-12-24-13-15(2)28(14)23-26-21-19(8-5-9-20(21)30-23)22(29)25-16-10-17-6-4-7-18(11-16)27(17)3/h5,8-9,14-18,24H,4,6-7,10-13H2,1-3H3,(H,25,29)/t14-,15-,17+,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM85330

(CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...)Show InChI InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329744
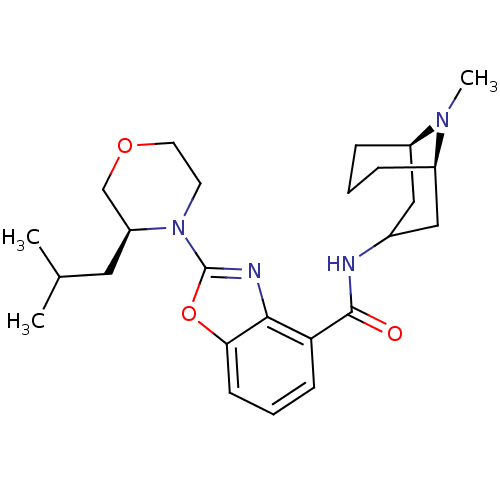
(CHEMBL1271678 | endo-2-((S)-3-isobutylmorpholino)-...)Show SMILES CC(C)C[C@H]1COCCN1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:31:30:25.26.27:29.22.23| Show InChI InChI=1S/C25H36N4O3/c1-16(2)12-20-15-31-11-10-29(20)25-27-23-21(8-5-9-22(23)32-25)24(30)26-17-13-18-6-4-7-19(14-17)28(18)3/h5,8-9,16-20H,4,6-7,10-15H2,1-3H3,(H,26,30)/t18-,19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329742
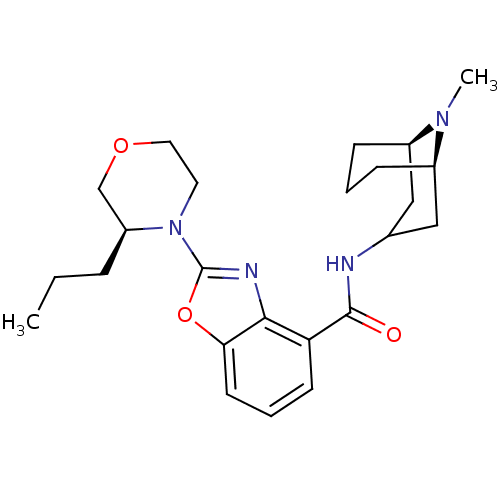
(CHEMBL1271629 | endo-N-((1R,5R)-9-methyl-9-azabicy...)Show SMILES CCC[C@H]1COCCN1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:30:29:24.25.26:28.21.22| Show InChI InChI=1S/C24H34N4O3/c1-3-6-19-15-30-12-11-28(19)24-26-22-20(9-5-10-21(22)31-24)23(29)25-16-13-17-7-4-8-18(14-16)27(17)2/h5,9-10,16-19H,3-4,6-8,11-15H2,1-2H3,(H,25,29)/t17-,18-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24198
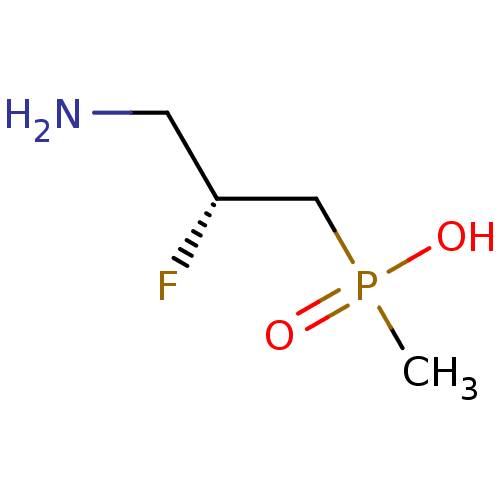
(3-aminopropylphosphinic derivative, (R)-8 | [(2R)-...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8)/t4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | -47.3 | n/a | n/a | 14 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329741

(CHEMBL1271628 | endo-2-((S)-3-ethylmorpholino)-N-(...)Show SMILES CC[C@H]1COCCN1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:29:28:23.24.25:27.20.21| Show InChI InChI=1S/C23H32N4O3/c1-3-16-14-29-11-10-27(16)23-25-21-19(8-5-9-20(21)30-23)22(28)24-15-12-17-6-4-7-18(13-15)26(17)2/h5,8-9,15-18H,3-4,6-7,10-14H2,1-2H3,(H,24,28)/t16-,17+,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329730

((S)-2-amino-6-chloro-N-(quinuclidin-3-yl)benzo[d]o...)Show SMILES Nc1nc2c(cc(Cl)cc2o1)C(=O)N[C@@H]1CN2CCC1CC2 |r,wD:14.15,TLB:13:14:18.17:20.21,(4.69,-4.73,;3.15,-4.73,;2.24,-3.48,;.76,-3.96,;-.57,-3.2,;-1.9,-3.97,;-1.9,-5.51,;-3.24,-6.28,;-.57,-6.28,;.76,-5.51,;2.24,-5.99,;-.58,-1.66,;-1.91,-.89,;.76,-.88,;.75,.66,;.56,2.04,;2.02,2.68,;3.38,2.08,;3.65,.68,;2.28,1.32,;2.54,3.21,;2.09,4.32,)| Show InChI InChI=1S/C15H17ClN4O2/c16-9-5-10(13-12(6-9)22-15(17)19-13)14(21)18-11-7-20-3-1-8(11)2-4-20/h5-6,8,11H,1-4,7H2,(H2,17,19)(H,18,21)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329743

(CHEMBL1271677 | endo-2-((S)-3-isopropylmorpholino)...)Show SMILES CC(C)[C@H]1COCCN1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:30:29:24.25.26:28.21.22| Show InChI InChI=1S/C24H34N4O3/c1-15(2)20-14-30-11-10-28(20)24-26-22-19(8-5-9-21(22)31-24)23(29)25-16-12-17-6-4-7-18(13-16)27(17)3/h5,8-9,15-18,20H,4,6-7,10-14H2,1-3H3,(H,25,29)/t17-,18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24195
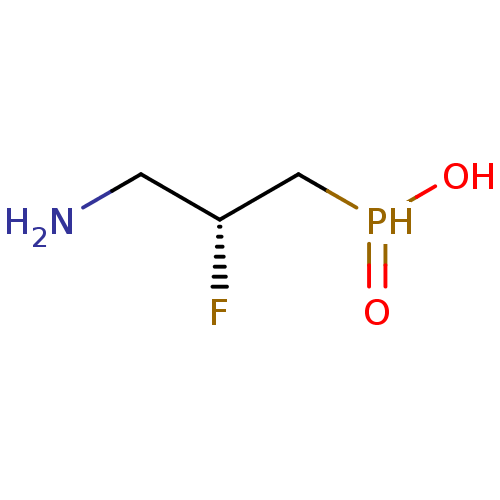
(3-aminopropylphosphinic derivative, (R)-7 | AZD335...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7)/t3-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | -46.9 | n/a | n/a | 8.64 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329747

(2-((3S,5S)-3,5-dimethylmorpholino)-N-((S)-quinucli...)Show SMILES C[C@H]1COC[C@H](C)N1c1nc2c(cccc2o1)C(=O)N[C@@H]1CN2CCC1CC2 |r,wU:1.0,wD:20.22,5.5,TLB:19:20:24.23:26.27,(46.08,-23.8,;46.85,-25.13,;48.39,-25.12,;49.17,-26.46,;48.39,-27.8,;46.86,-27.8,;46.08,-29.13,;46.09,-26.46,;44.55,-26.46,;43.64,-25.21,;42.16,-25.69,;40.83,-24.93,;39.5,-25.7,;39.5,-27.24,;40.83,-28.01,;42.17,-27.24,;43.64,-27.72,;40.82,-23.39,;39.49,-22.62,;42.16,-22.61,;42.15,-21.07,;41.96,-19.69,;43.43,-19.05,;44.78,-19.65,;45.05,-21.05,;43.69,-20.41,;43.94,-18.52,;43.5,-17.41,)| Show InChI InChI=1S/C21H28N4O3/c1-13-11-27-12-14(2)25(13)21-23-19-16(4-3-5-18(19)28-21)20(26)22-17-10-24-8-6-15(17)7-9-24/h3-5,13-15,17H,6-12H2,1-2H3,(H,22,26)/t13-,14-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50551946

(CHEMBL4764328)Show SMILES CC(C)(C)CC(=O)N1CC[C@@]2([C@H]1c1cccc(c1)-c1ccc(CO)c(c1)S(C)(=O)=O)C(=O)Nc1ccccc21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of hyodeoxycholicacid-based fluorescent tracer from recombinant human LXRbeta LBD (215 to 461 residues) expressed in Escherichia coli BL... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112240
BindingDB Entry DOI: 10.7270/Q2MG7T4D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329728

(CHEMBL1272073 | endo-2-cyclopropyl-N-((1R,5R)-9-me...)Show SMILES CN1[C@@H]2CCC[C@@H]1CC(C2)NC(=O)c1cccc2oc(nc12)C1CC1 |r,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C20H25N3O2/c1-23-14-4-2-5-15(23)11-13(10-14)21-19(24)16-6-3-7-17-18(16)22-20(25-17)12-8-9-12/h3,6-7,12-15H,2,4-5,8-11H2,1H3,(H,21,24)/t14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24193
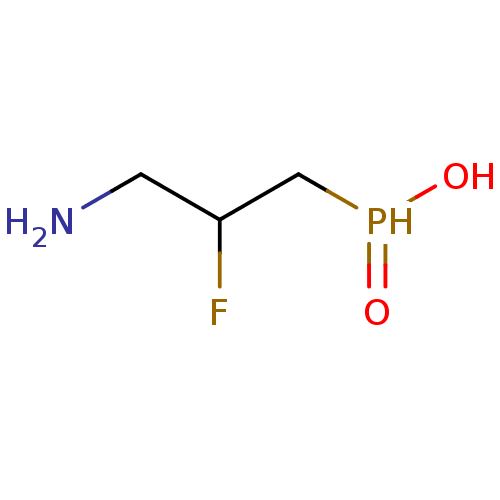
((3-amino-2-fluoropropyl)phosphinic acid | 3-aminop...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | -45.2 | n/a | n/a | 15 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329754

((S)-2-(diethylamino)-N-(quinuclidin-3-yl)benzo[d]o...)Show SMILES CCN(CC)c1nc2c(cccc2o1)C(=O)N[C@@H]1CN2CCC1CC2 |r,wD:17.18,TLB:16:17:21.20:23.24,(.57,-1.9,;-.97,-1.9,;-1.74,-3.24,;-.97,-4.57,;.57,-4.56,;-3.28,-3.24,;-4.19,-1.99,;-5.66,-2.47,;-7,-1.7,;-8.32,-2.47,;-8.33,-4.02,;-6.99,-4.79,;-5.66,-4.02,;-4.19,-4.49,;-7,-.16,;-8.34,.6,;-5.67,.61,;-5.67,2.15,;-5.87,3.53,;-4.4,4.18,;-3.05,3.57,;-2.77,2.17,;-4.14,2.81,;-3.88,4.71,;-4.33,5.81,)| Show InChI InChI=1S/C19H26N4O2/c1-3-23(4-2)19-21-17-14(6-5-7-16(17)25-19)18(24)20-15-12-22-10-8-13(15)9-11-22/h5-7,13,15H,3-4,8-12H2,1-2H3,(H,20,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329739

(CHEMBL1271570 | endo-N-(8-methyl-8-azabicyclo[3.2....)Show SMILES C[C@H]1COCCN1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:28:27:22.23.24:26.19.20| Show InChI InChI=1S/C22H30N4O3/c1-14-13-28-10-9-26(14)22-24-20-18(7-4-8-19(20)29-22)21(27)23-15-11-16-5-3-6-17(12-15)25(16)2/h4,7-8,14-17H,3,5-6,9-13H2,1-2H3,(H,23,27)/t14-,16+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329729
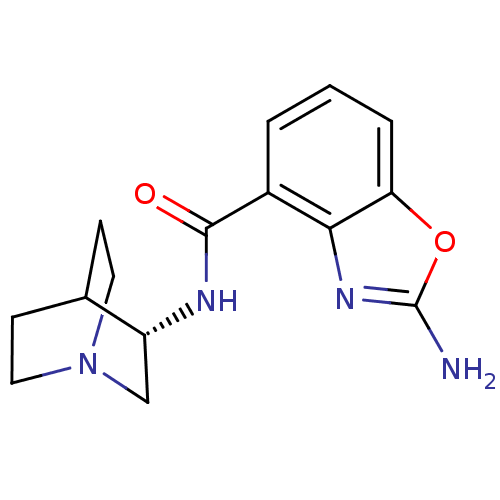
((S)-2-amino-N-(quinuclidin-3-yl)benzo[d]oxazole-4-...)Show SMILES Nc1nc2c(cccc2o1)C(=O)N[C@@H]1CN2CCC1CC2 |r,wD:13.14,TLB:12:13:17.16:19.20,(3.57,-5.44,;2.04,-5.44,;1.13,-4.19,;-.35,-4.67,;-1.68,-3.9,;-3.01,-4.67,;-3.01,-6.22,;-1.68,-6.99,;-.35,-6.22,;1.13,-6.7,;-1.69,-2.36,;-3.02,-1.6,;-.36,-1.59,;-.36,-.05,;-.56,1.33,;.91,1.98,;2.26,1.37,;2.54,-.03,;1.17,.61,;1.43,2.51,;.98,3.61,)| Show InChI InChI=1S/C15H18N4O2/c16-15-18-13-10(2-1-3-12(13)21-15)14(20)17-11-8-19-6-4-9(11)5-7-19/h1-3,9,11H,4-8H2,(H2,16,18)(H,17,20)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24196
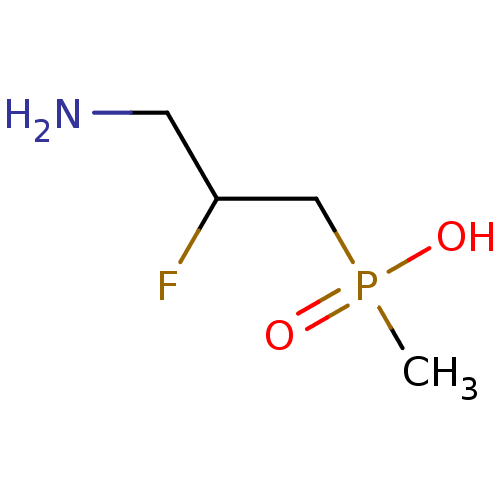
((3-amino-2-fluoropropyl)(methyl)phosphinic acid | ...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | -44.4 | n/a | n/a | 23 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM26066
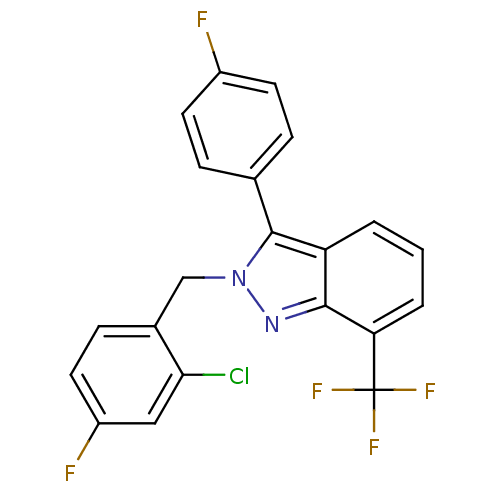
(2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...)Show SMILES Fc1ccc(cc1)-c1n(Cc2ccc(F)cc2Cl)nc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C21H12ClF5N2/c22-18-10-15(24)9-6-13(18)11-29-20(12-4-7-14(23)8-5-12)16-2-1-3-17(19(16)28-29)21(25,26)27/h1-10H,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of hyodeoxycholicacid-based fluorescent tracer from recombinant human LXRbeta LBD (215 to 461 residues) expressed in Escherichia coli BL... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112240
BindingDB Entry DOI: 10.7270/Q2MG7T4D |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM26066

(2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...)Show SMILES Fc1ccc(cc1)-c1n(Cc2ccc(F)cc2Cl)nc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C21H12ClF5N2/c22-18-10-15(24)9-6-13(18)11-29-20(12-4-7-14(23)8-5-12)16-2-1-3-17(19(16)28-29)21(25,26)27/h1-10H,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... |
Eur J Med Chem 178: 458-467 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.011
BindingDB Entry DOI: 10.7270/Q2BC42WH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329726
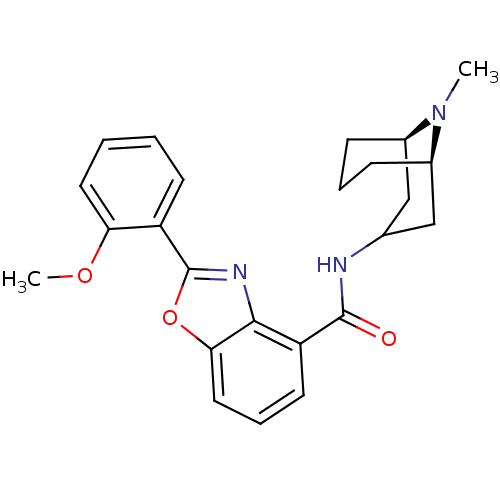
(CHEMBL1272017 | endo-2-(2-methoxyphenyl)-N-((1R,5R...)Show SMILES COc1ccccc1-c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:29:28:23.24.25:27.20.21| Show InChI InChI=1S/C24H27N3O3/c1-27-16-7-5-8-17(27)14-15(13-16)25-23(28)19-10-6-12-21-22(19)26-24(30-21)18-9-3-4-11-20(18)29-2/h3-4,6,9-12,15-17H,5,7-8,13-14H2,1-2H3,(H,25,28)/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24184
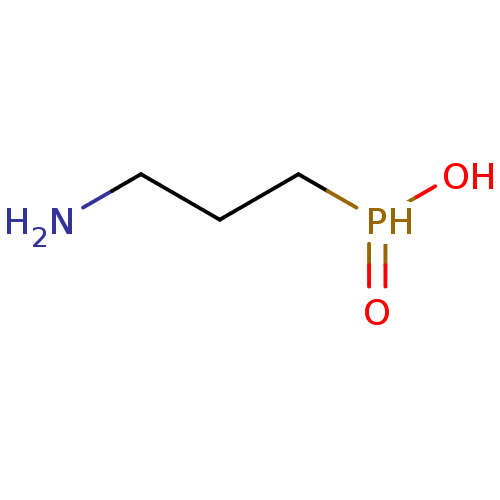
((3-aminopropyl)phosphinic acid | 3-aminopropylphos...)Show InChI InChI=1S/C3H10NO2P/c4-2-1-3-7(5)6/h7H,1-4H2,(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | -44.2 | n/a | n/a | 19 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329731
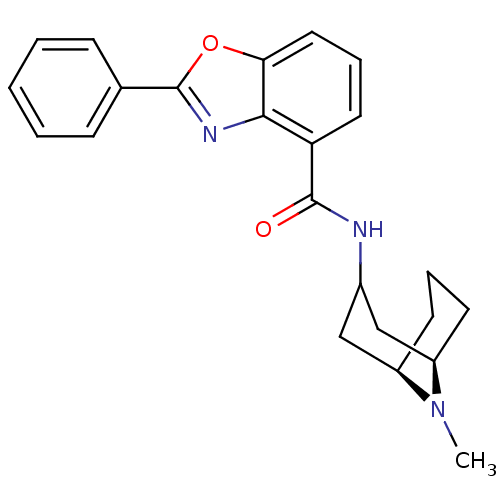
(CHEMBL1272174 | endo-N-((1R,5R)-9-methyl-9-azabicy...)Show SMILES CN1[C@@H]2CCC[C@@H]1CC(C2)NC(=O)c1cccc2oc(nc12)-c1ccccc1 |r,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C23H25N3O2/c1-26-17-9-5-10-18(26)14-16(13-17)24-22(27)19-11-6-12-20-21(19)25-23(28-20)15-7-3-2-4-8-15/h2-4,6-8,11-12,16-18H,5,9-10,13-14H2,1H3,(H,24,27)/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329732
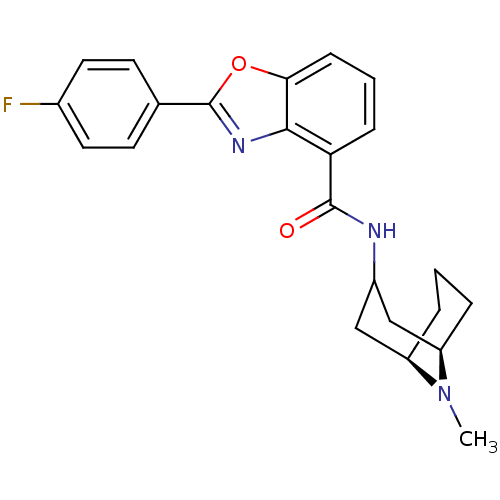
(CHEMBL1272175 | endo-2-(4-fluorophenyl)-N-((1R,5R)...)Show SMILES CN1[C@@H]2CCC[C@@H]1CC(C2)NC(=O)c1cccc2oc(nc12)-c1ccc(F)cc1 |r,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C23H24FN3O2/c1-27-17-4-2-5-18(27)13-16(12-17)25-22(28)19-6-3-7-20-21(19)26-23(29-20)14-8-10-15(24)11-9-14/h3,6-11,16-18H,2,4-5,12-13H2,1H3,(H,25,28)/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329751
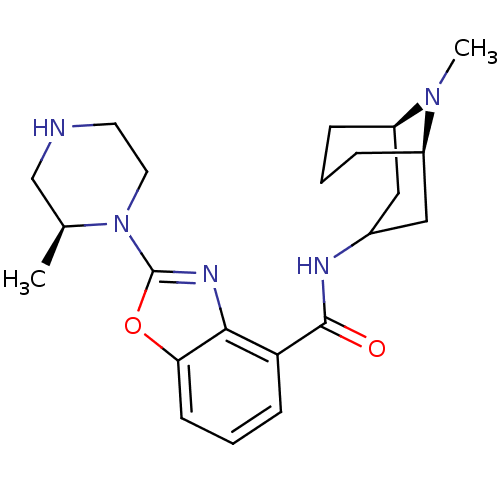
(CHEMBL1271904 | endo-N-((1R,5R)-9-methyl-9-azabicy...)Show SMILES C[C@H]1CNCCN1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:28:27:22.23.24:26.19.20| Show InChI InChI=1S/C22H31N5O2/c1-14-13-23-9-10-27(14)22-25-20-18(7-4-8-19(20)29-22)21(28)24-15-11-16-5-3-6-17(12-15)26(16)2/h4,7-8,14-17,23H,3,5-6,9-13H2,1-2H3,(H,24,28)/t14-,16+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329733

(CHEMBL1272230 | endo-2-(4-chlorophenyl)-N-((1R,5R)...)Show SMILES CN1[C@@H]2CCC[C@@H]1CC(C2)NC(=O)c1cccc2oc(nc12)-c1ccc(Cl)cc1 |r,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C23H24ClN3O2/c1-27-17-4-2-5-18(27)13-16(12-17)25-22(28)19-6-3-7-20-21(19)26-23(29-20)14-8-10-15(24)11-9-14/h3,6-11,16-18H,2,4-5,12-13H2,1H3,(H,25,28)/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329756
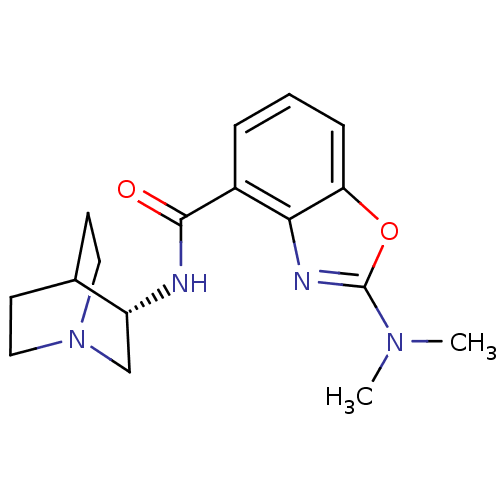
((S)-2-(dimethylamino)-N-(quinuclidin-3-yl)benzo[d]...)Show SMILES CN(C)c1nc2c(cccc2o1)C(=O)N[C@@H]1CN2CCC1CC2 |r,wD:15.16,TLB:14:15:19.18:21.22,(4.16,-5.27,;3.39,-3.94,;4.15,-2.6,;1.85,-3.94,;.94,-2.69,;-.54,-3.17,;-1.87,-2.4,;-3.2,-3.17,;-3.2,-4.72,;-1.87,-5.49,;-.53,-4.72,;.94,-5.19,;-1.88,-.86,;-3.21,-.1,;-.54,-.09,;-.55,1.45,;-.74,2.83,;.73,3.48,;2.08,2.87,;2.35,1.47,;.99,2.11,;1.24,4.01,;.8,5.11,)| Show InChI InChI=1S/C17H22N4O2/c1-20(2)17-19-15-12(4-3-5-14(15)23-17)16(22)18-13-10-21-8-6-11(13)7-9-21/h3-5,11,13H,6-10H2,1-2H3,(H,18,22)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329734
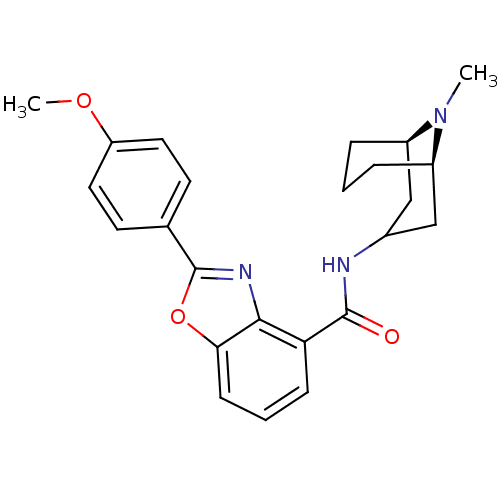
(CHEMBL1272231 | endo-2-(4-methoxyphenyl)-N-((1R,5R...)Show SMILES COc1ccc(cc1)-c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:29:28:23.24.25:27.20.21| Show InChI InChI=1S/C24H27N3O3/c1-27-17-5-3-6-18(27)14-16(13-17)25-23(28)20-7-4-8-21-22(20)26-24(30-21)15-9-11-19(29-2)12-10-15/h4,7-12,16-18H,3,5-6,13-14H2,1-2H3,(H,25,28)/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329727
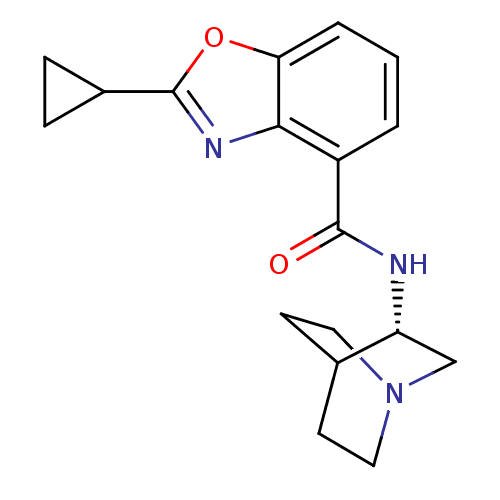
((S)-2-cyclopropyl-N-(quinuclidin-3-yl)benzo[d]oxaz...)Show SMILES O=C(N[C@@H]1CN2CCC1CC2)c1cccc2oc(nc12)C1CC1 |r,wD:3.2,TLB:2:3:7.6:9.10,(3.1,1.5,;4.44,.73,;5.77,1.51,;5.77,3.05,;5.57,4.43,;7.04,5.08,;8.39,4.47,;8.67,3.07,;7.3,3.71,;7.56,5.61,;7.11,6.71,;4.44,-.81,;3.11,-1.58,;3.11,-3.13,;4.45,-3.9,;5.78,-3.12,;7.25,-3.6,;8.17,-2.35,;7.25,-1.09,;5.78,-1.57,;9.7,-2.34,;11.04,-3.11,;11.03,-1.57,)| Show InChI InChI=1S/C18H21N3O2/c22-17(19-14-10-21-8-6-11(14)7-9-21)13-2-1-3-15-16(13)20-18(23-15)12-4-5-12/h1-3,11-12,14H,4-10H2,(H,19,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24185
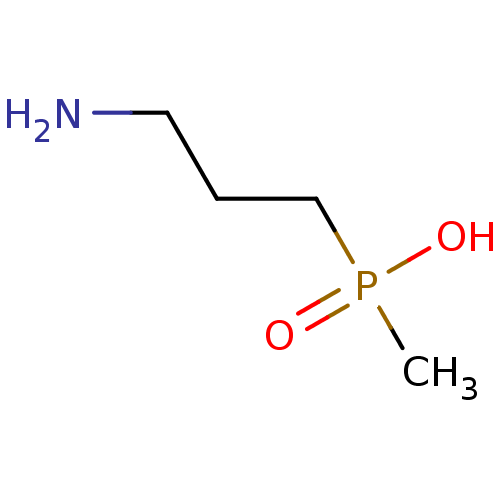
((3-aminopropyl)(methyl)phosphinic acid | 3-Apmpa |...)Show InChI InChI=1S/C4H12NO2P/c1-8(6,7)4-2-3-5/h2-5H2,1H3,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 33 | -42.3 | n/a | n/a | 41 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329749

(CHEMBL1271848 | endo-2-((2R,6S)-2,6-dimethylmorpho...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:29:28:23.24.25:27.20.21| Show InChI InChI=1S/C23H32N4O3/c1-14-12-27(13-15(2)29-14)23-25-21-19(8-5-9-20(21)30-23)22(28)24-16-10-17-6-4-7-18(11-16)26(17)3/h5,8-9,14-18H,4,6-7,10-13H2,1-3H3,(H,24,28)/t14-,15+,17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329757
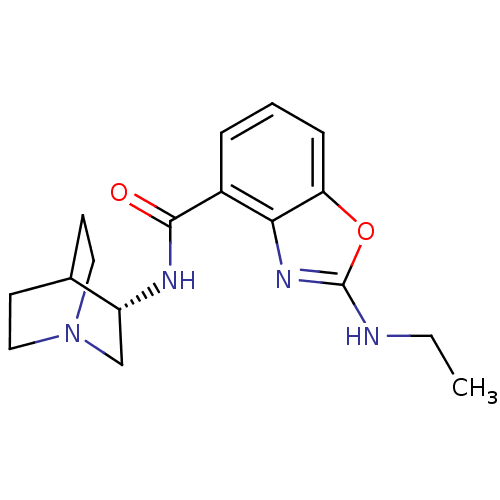
((S)-2-(ethylamino)-N-(quinuclidin-3-yl)benzo[d]oxa...)Show SMILES CCNc1nc2c(cccc2o1)C(=O)N[C@@H]1CN2CCC1CC2 |r,wD:15.16,TLB:14:15:19.18:21.22,(5.36,-2.91,;3.82,-2.91,;3.05,-4.25,;1.52,-4.25,;.6,-3,;-.87,-3.48,;-2.2,-2.71,;-3.53,-3.48,;-3.54,-5.03,;-2.2,-5.8,;-.87,-5.03,;.6,-5.51,;-2.21,-1.17,;-3.55,-.41,;-.88,-.4,;-.88,1.14,;-1.08,2.52,;.39,3.17,;1.74,2.56,;2.02,1.16,;.65,1.8,;.91,3.7,;.46,4.8,)| Show InChI InChI=1S/C17H22N4O2/c1-2-18-17-20-15-12(4-3-5-14(15)23-17)16(22)19-13-10-21-8-6-11(13)7-9-21/h3-5,11,13H,2,6-10H2,1H3,(H,18,20)(H,19,22)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329758
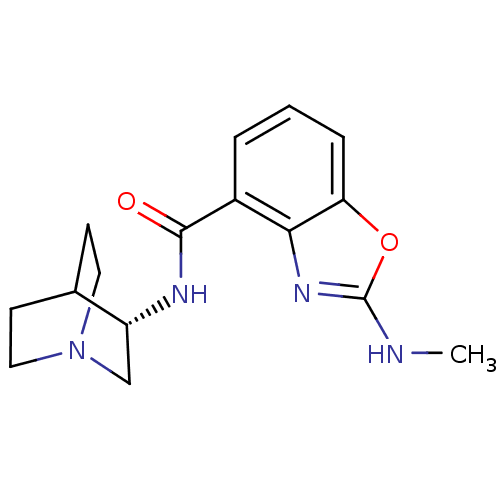
((S)-2-(methylamino)-N-(quinuclidin-3-yl)benzo[d]ox...)Show SMILES CNc1nc2c(cccc2o1)C(=O)N[C@@H]1CN2CCC1CC2 |r,wD:14.15,TLB:13:14:18.17:20.21,(2.65,-3.98,;1.89,-5.31,;.35,-5.32,;-.56,-4.06,;-2.04,-4.54,;-3.37,-3.78,;-4.7,-4.55,;-4.7,-6.09,;-3.37,-6.87,;-2.04,-6.09,;-.56,-6.57,;-3.38,-2.24,;-4.71,-1.47,;-2.04,-1.47,;-2.05,.07,;-2.24,1.45,;-.78,2.1,;.58,1.49,;.85,.09,;-.52,.73,;-.26,2.63,;-.71,3.74,)| Show InChI InChI=1S/C16H20N4O2/c1-17-16-19-14-11(3-2-4-13(14)22-16)15(21)18-12-9-20-7-5-10(12)6-8-20/h2-4,10,12H,5-9H2,1H3,(H,17,19)(H,18,21)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50551953

(CHEMBL4764838)Show SMILES COC(=O)c1ccc(cc1S(C)(=O)=O)-c1cccc(c1)[C@H]1N(CC[C@]11C(=O)Nc2ccccc12)C(=O)OC(C)(C)C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of hyodeoxycholicacid-based fluorescent tracer from recombinant human LXRbeta LBD (215 to 461 residues) expressed in Escherichia coli BL... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112240
BindingDB Entry DOI: 10.7270/Q2MG7T4D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329752

(CHEMBL1271905 | endo-2-((2S,6R)-2,6-dimethylpipera...)Show SMILES C[C@H]1CNC[C@@H](C)N1c1nc2c(cccc2o1)C(=O)NC1C[C@H]2CCC[C@H](C1)N2C |r,TLB:29:28:23.24.25:27.20.21| Show InChI InChI=1S/C23H33N5O2/c1-14-12-24-13-15(2)28(14)23-26-21-19(8-5-9-20(21)30-23)22(29)25-16-10-17-6-4-7-18(11-16)27(17)3/h5,8-9,14-18,24H,4,6-7,10-13H2,1-3H3,(H,25,29)/t14-,15+,17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329722

(CHEMBL1271519 | endo-N-((1R,5R)-9-methyl-9-azabicy...)Show SMILES CN1[C@@H]2CCC[C@@H]1CC(C2)NC(=O)c1cccc2oc(nc12)N1CCOCC1 |r,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C21H28N4O3/c1-24-15-4-2-5-16(24)13-14(12-15)22-20(26)17-6-3-7-18-19(17)23-21(28-18)25-8-10-27-11-9-25/h3,6-7,14-16H,2,4-5,8-13H2,1H3,(H,22,26)/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329735

(CHEMBL1272283 | endo-N-((1R,5R)-9-methyl-9-azabicy...)Show SMILES CN1[C@@H]2CCC[C@@H]1CC(C2)NC(=O)c1cccc2nc(oc12)-c1ccccc1 |r,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C23H25N3O2/c1-26-17-9-5-10-18(26)14-16(13-17)24-22(27)19-11-6-12-20-21(19)28-23(25-20)15-7-3-2-4-8-15/h2-4,6-8,11-12,16-18H,5,9-10,13-14H2,1H3,(H,24,27)/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... |
Eur J Med Chem 178: 458-467 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.011
BindingDB Entry DOI: 10.7270/Q2BC42WH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of hyodeoxycholicacid-based fluorescent tracer from recombinant human LXRbeta LBD (215 to 461 residues) expressed in Escherichia coli BL... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112240
BindingDB Entry DOI: 10.7270/Q2MG7T4D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50329725
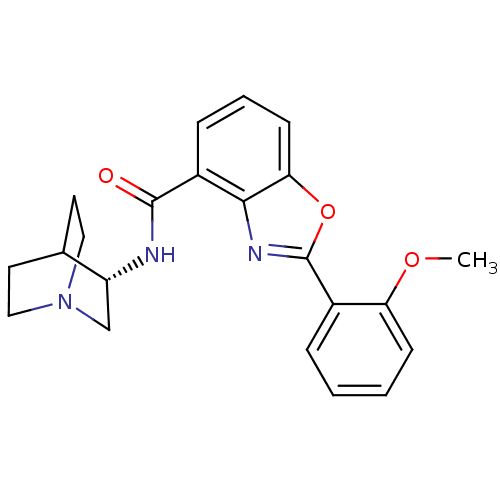
((S)-2-(2-methoxyphenyl)-N-(quinuclidin-8-yl)benzo[...)Show SMILES COc1ccccc1-c1nc2c(cccc2o1)C(=O)N[C@@H]1CN2CCC1CC2 |r,wD:20.22,TLB:19:20:24.23:26.27,(3.15,-.28,;2.38,-1.62,;3.16,-2.95,;4.69,-2.94,;5.47,-4.28,;4.7,-5.62,;3.16,-5.62,;2.39,-4.28,;.85,-4.28,;-.06,-3.03,;-1.53,-3.51,;-2.87,-2.74,;-4.19,-3.52,;-4.2,-5.06,;-2.86,-5.83,;-1.53,-5.06,;-.06,-5.54,;-2.87,-1.2,;-4.21,-.44,;-1.54,-.43,;-1.54,1.11,;-1.74,2.49,;-.27,3.14,;1.08,2.53,;1.36,1.13,;-.01,1.77,;.25,3.66,;-.2,4.77,)| Show InChI InChI=1S/C22H23N3O3/c1-27-18-7-3-2-5-15(18)22-24-20-16(6-4-8-19(20)28-22)21(26)23-17-13-25-11-9-14(17)10-12-25/h2-8,14,17H,9-13H2,1H3,(H,23,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human HT3A receptor |
Bioorg Med Chem Lett 20: 6538-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.038
BindingDB Entry DOI: 10.7270/Q2Z31ZVH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24191

((3-amino-2-oxopropyl)phosphinic acid | (3-amino-2-...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h8H,1-2,4H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | -41.4 | n/a | n/a | 81 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24186
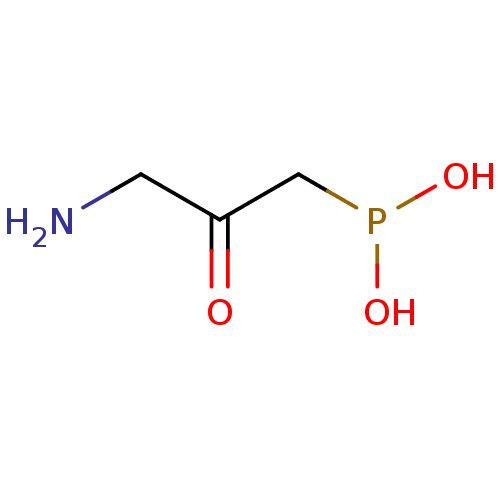
((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h6-7H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | -41.3 | n/a | n/a | 130 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data