

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pantetheinase (Homo sapiens (Human)) | BDBM408834 (US10364255, Ex. 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408835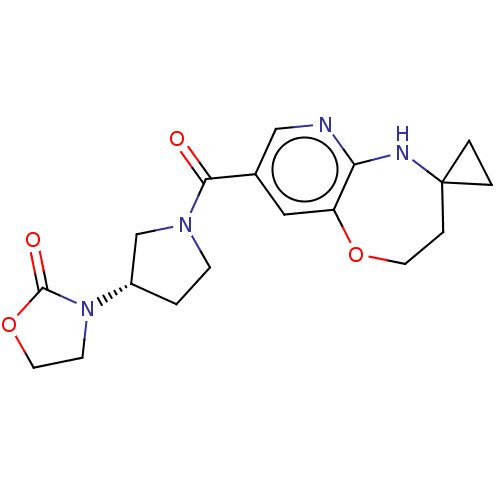 (US10364255, Ex. 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303609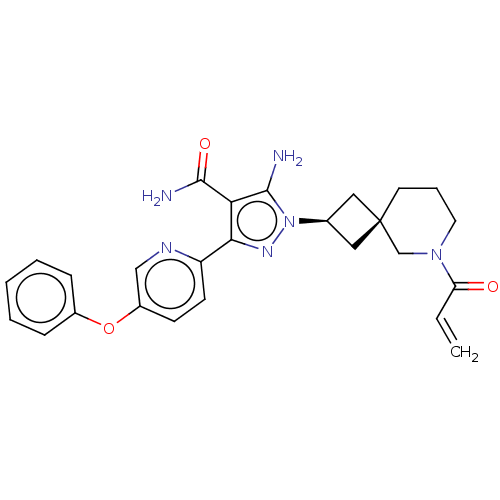 (US10138229, Example 121 | US10875852, Example 121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50508208 (CHEMBL4457209) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... | Bioorg Med Chem Lett 29: 441-448 (2019) Article DOI: 10.1016/j.bmcl.2018.12.024 BindingDB Entry DOI: 10.7270/Q2P55RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50508174 (CHEMBL4464625) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... | Bioorg Med Chem Lett 29: 441-448 (2019) Article DOI: 10.1016/j.bmcl.2018.12.024 BindingDB Entry DOI: 10.7270/Q2P55RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303609 (US10138229, Example 121 | US10875852, Example 121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408845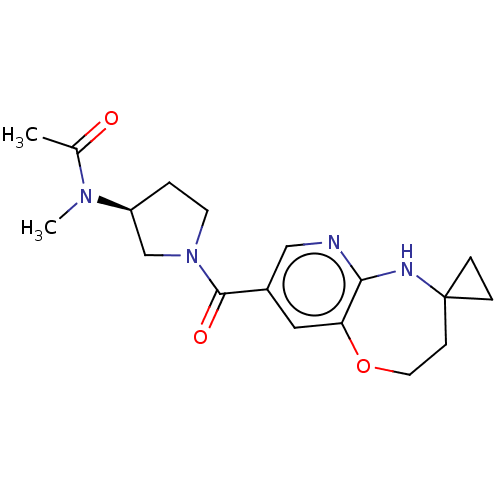 (US10364255, Ex. 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50508174 (CHEMBL4464625) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CCR1 in human THP1 cells assessed as inhibition of chemotaxis after 30 mins by Celltiter-glo reagent based luminescence assay | Bioorg Med Chem Lett 29: 441-448 (2019) Article DOI: 10.1016/j.bmcl.2018.12.024 BindingDB Entry DOI: 10.7270/Q2P55RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50508182 (CHEMBL4475581) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... | Bioorg Med Chem Lett 29: 441-448 (2019) Article DOI: 10.1016/j.bmcl.2018.12.024 BindingDB Entry DOI: 10.7270/Q2P55RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408828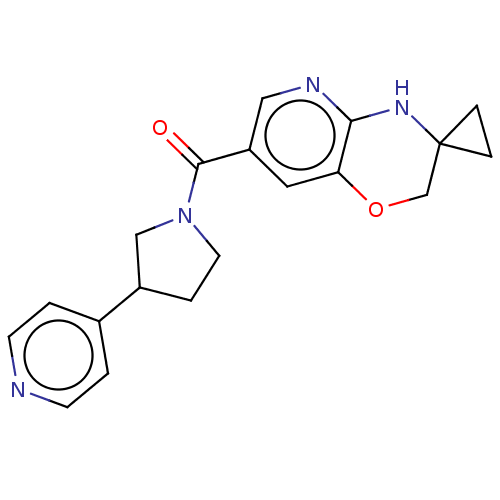 (US10364255, Ex. 20 | US10364255, Ex. 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408829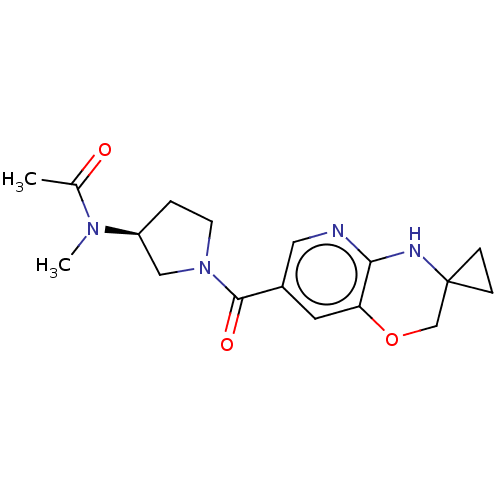 (US10364255, Ex. 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435486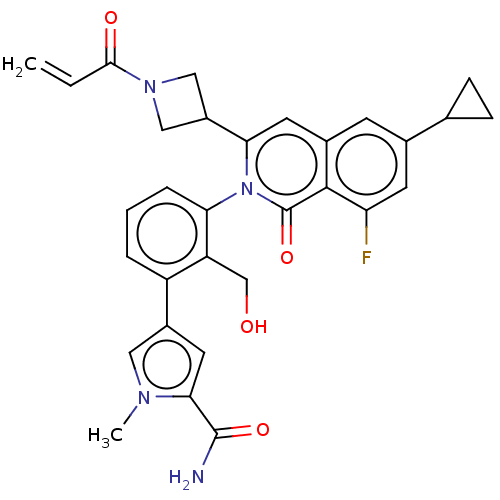 (US10570118, Example 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435467 (US10570118, Example 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408822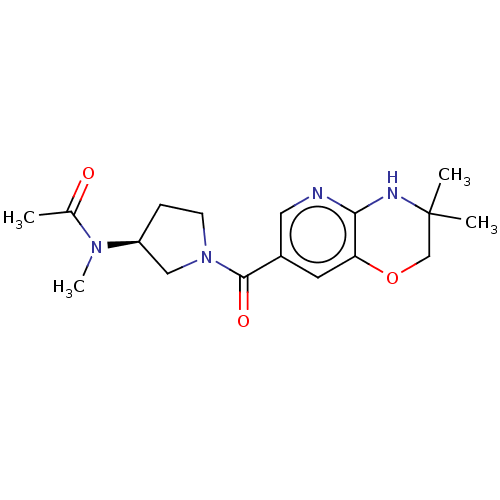 (US10364255, Ex. 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303652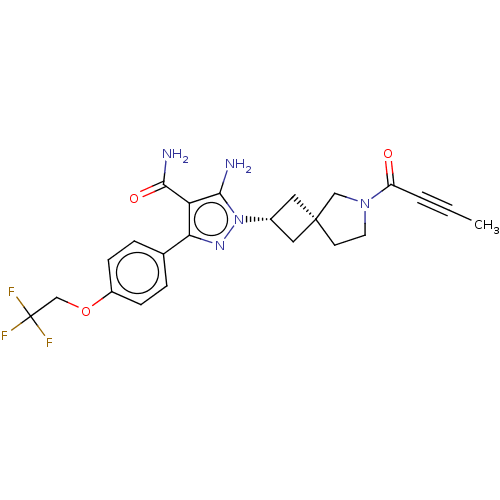 (US10138229, Example 164 | US10875852, Example 164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303606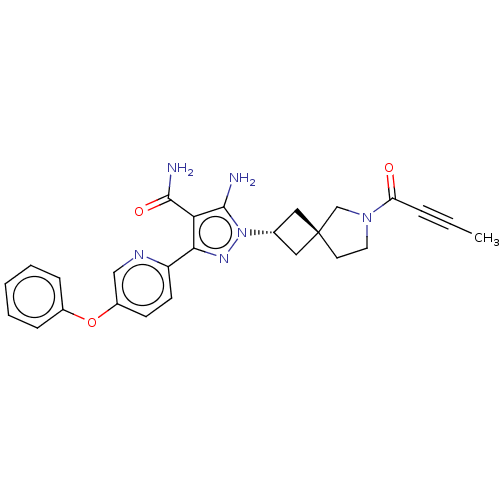 (US10138229, Example 118 | US10138229, Example 119 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303652 (US10138229, Example 164 | US10875852, Example 164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in HFF assessed as inhibition of IL-1-induced IL-6 production after 24 hrs | Bioorg Med Chem Lett 23: 6640-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.052 BindingDB Entry DOI: 10.7270/Q2DN46G5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303606 (US10138229, Example 118 | US10138229, Example 119 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435452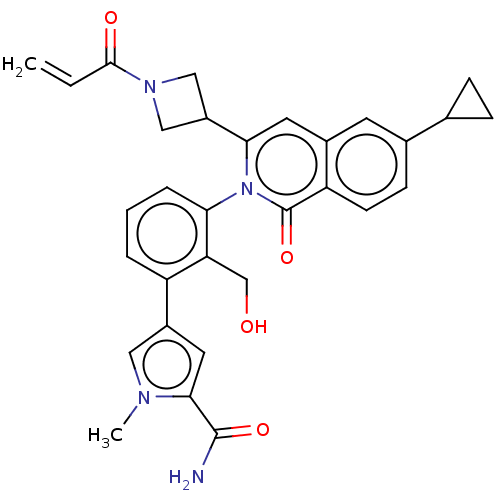 (US10570118, Example 42 | US10570118, Example 43 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303642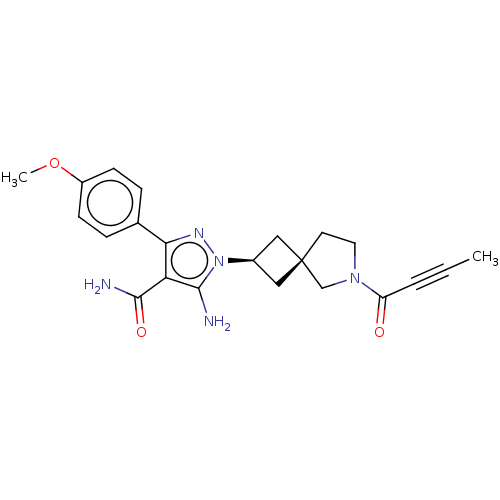 (US10138229, Example 154 | US10875852, Example 154) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408824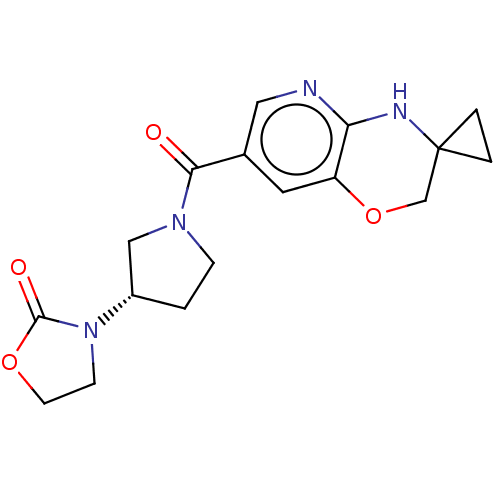 (US10364255, Ex. 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408833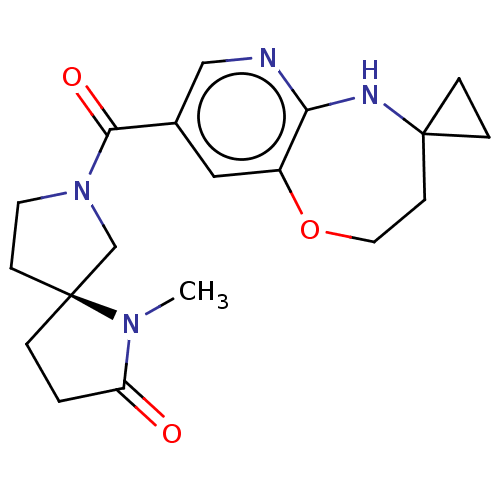 (US10364255, Ex. 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303642 (US10138229, Example 154 | US10875852, Example 154) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303639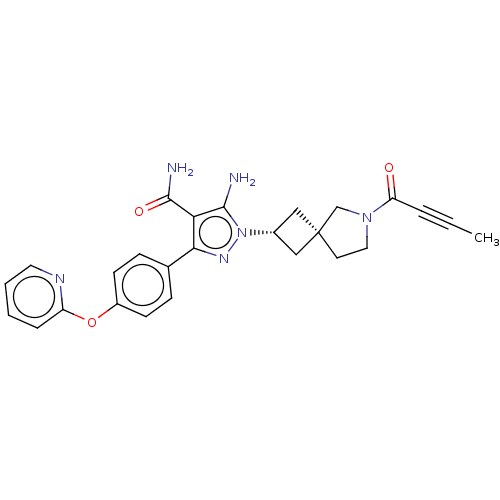 (US10138229, Example 151 | US10875852, Example 151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303586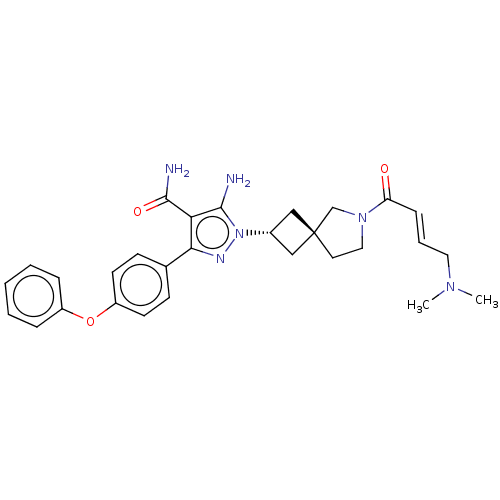 (US10138229, Example 98 | US10875852, Example 98) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303639 (US10138229, Example 151 | US10875852, Example 151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435452 (US10570118, Example 42 | US10570118, Example 43 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435446 (US10570118, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408823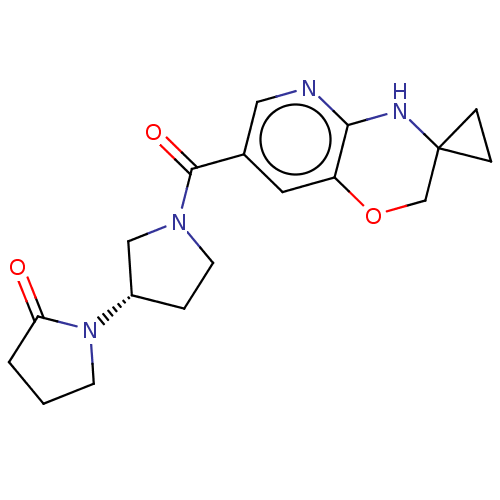 (US10364255, Ex. 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408825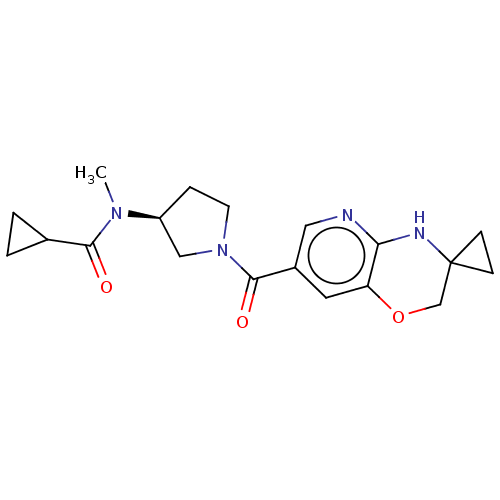 (US10364255, Ex. 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303586 (US10138229, Example 98 | US10875852, Example 98) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435487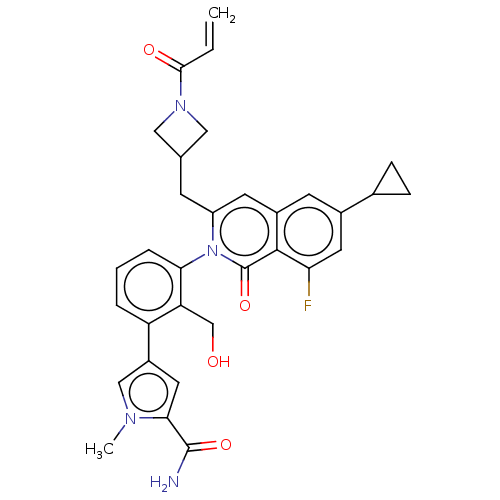 (US10570118, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303528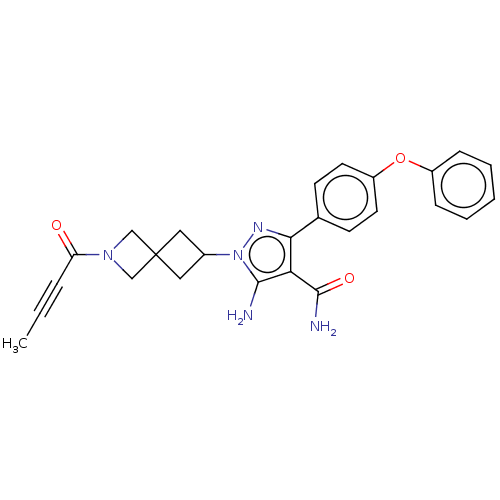 (US10138229, Example 41 | US10875852, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303528 (US10138229, Example 41 | US10875852, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303543 (US10138229, Example 54 | US10266513, Example 127 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303543 (US10138229, Example 54 | US10266513, Example 127 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM143328 (US8940893, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US8940893 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303521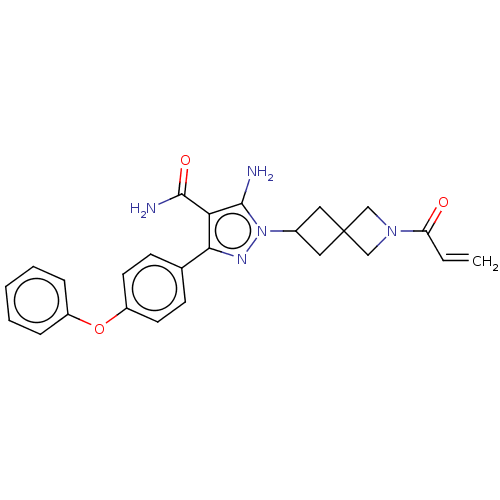 (US10138229, Example 35 | US10875852, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303521 (US10138229, Example 35 | US10875852, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303569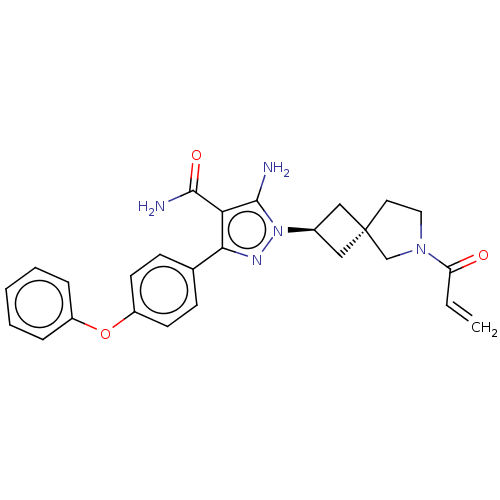 (US10138229, Example 81 | US10875852, Example 81) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303569 (US10138229, Example 81 | US10875852, Example 81) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303579 (US10138229, Example 91 | US10875852, Example 91) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303579 (US10138229, Example 91 | US10875852, Example 91) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303638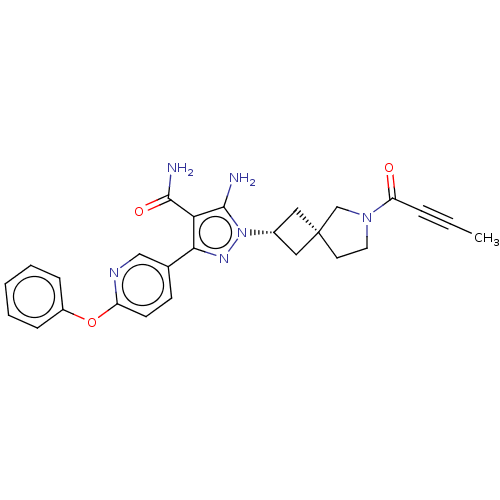 (US10138229, Example 150 | US10875852, Example 150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303588 (US10138229, Example 100 | US10875852, Example 100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303585 (US10138229, Example 97 | US10875852, Example 97) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303638 (US10138229, Example 150 | US10875852, Example 150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303588 (US10138229, Example 100 | US10875852, Example 100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303585 (US10138229, Example 97 | US10875852, Example 97) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) BindingDB Entry DOI: 10.7270/Q2DN484H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 871 total ) | Next | Last >> |