

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collagenase 3 (Homo sapiens (Human)) | BDBM50259948 (CHEMBL4096462) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay Curated by ChEMBL | Assay Description Inhibition of human MMP13 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate by spectrophotometric analysis | J Med Chem 60: 403-414 (2017) Article DOI: 10.1021/acs.jmedchem.6b01420 BindingDB Entry DOI: 10.7270/Q24X5B7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Rattus norvegicus) | BDBM50530230 (CHEMBL4445882) | MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of rat CPA3 using AAFP as substrate preincubated for 45 mins measured at 30 sec intervals for 15 mins by UV/vis-spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Rattus norvegicus) | BDBM50530230 (CHEMBL4445882) | MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of rat CPA3 using AAFP as substrate preincubated for 45 mins measured at 30 sec intervals for 15 mins by UV/vis-spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530229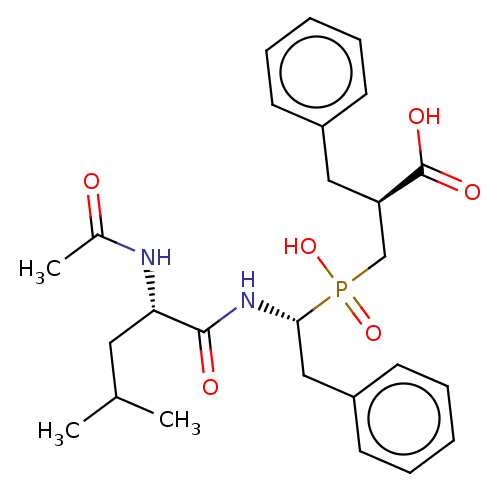 (CHEMBL4460840) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530229 (CHEMBL4460840) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50082556 ((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-2 | J Med Chem 47: 325-36 (2004) Article DOI: 10.1021/jm0308491 BindingDB Entry DOI: 10.7270/Q27080V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530230 (CHEMBL4445882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530230 (CHEMBL4445882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530235 (CHEMBL4436298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530235 (CHEMBL4436298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530231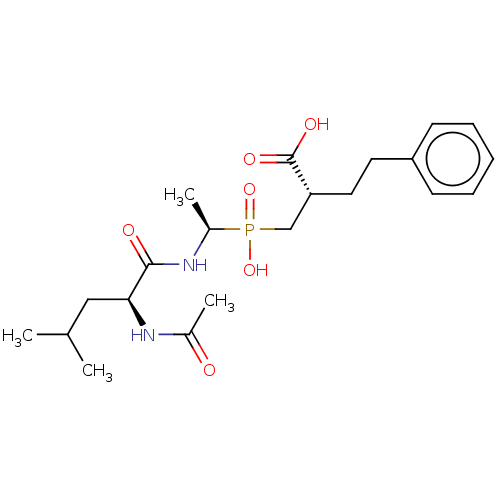 (CHEMBL4528407) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530231 (CHEMBL4528407) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530230 (CHEMBL4445882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530230 (CHEMBL4445882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530228 (CHEMBL4483485) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530228 (CHEMBL4483485) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50530229 (CHEMBL4460840) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPB1 using AAFA as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50530229 (CHEMBL4460840) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPB1 using AAFA as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530227 (CHEMBL4454162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530227 (CHEMBL4454162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21464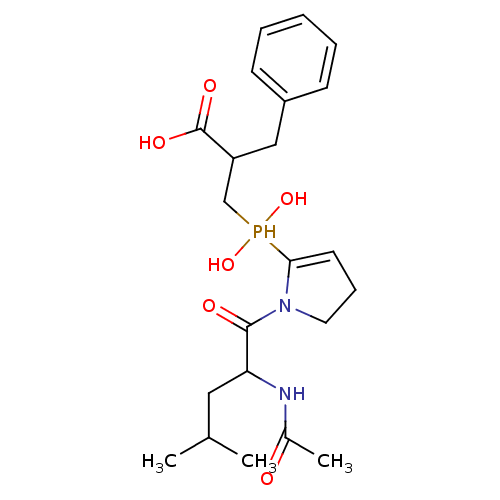 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530231 (CHEMBL4528407) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530231 (CHEMBL4528407) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530227 (CHEMBL4454162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530227 (CHEMBL4454162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530228 (CHEMBL4483485) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530228 (CHEMBL4483485) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM92441 (RXP470, 1 | RXP470, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat à l'Energie Atomique | Assay Description Enzyme assay using matrix metalloproteinases. | J Biol Chem 285: 35900-9 (2010) Article DOI: 10.1074/jbc.M110.139634 BindingDB Entry DOI: 10.7270/Q25X27HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A2 (Homo sapiens) | BDBM50530235 (CHEMBL4436298) | PDB Reactome pathway UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA2 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A2 (Homo sapiens) | BDBM50530235 (CHEMBL4436298) | PDB Reactome pathway UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA2 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM92441 (RXP470, 1 | RXP470, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique | Assay Description Enzyme assay using human matrix metalloproteases or ADAMTS. | J Biol Chem 287: 26647-56 (2012) Article DOI: 10.1074/jbc.M112.380782 BindingDB Entry DOI: 10.7270/Q2H993SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50265078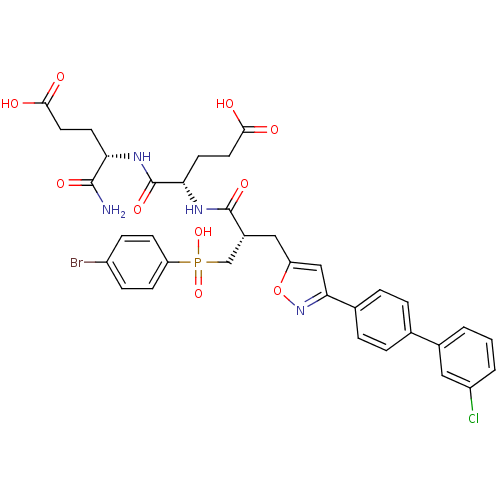 ((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
CEA Curated by ChEMBL | Assay Description Inhibition of human MMP12 catalytic domain (98 to 266) at pH 6.8 by isothermal titration calorimetry | J Med Chem 56: 1149-59 (2013) Article DOI: 10.1021/jm301574d BindingDB Entry DOI: 10.7270/Q24F1S24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530230 (CHEMBL4445882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate assessed as apparent first-order rate constant measured at pre-steady state by measuring Koff/Kon ra... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530230 (CHEMBL4445882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate assessed as apparent first-order rate constant measured at pre-steady state by measuring Koff/Kon ra... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50265078 ((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA Curated by ChEMBL | Assay Description Binding affinity to human MMP12 catalytic domain (98 to 266) by isothermal titration calorimetry | J Med Chem 56: 1149-59 (2013) Article DOI: 10.1021/jm301574d BindingDB Entry DOI: 10.7270/Q24F1S24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50426080 (CHEMBL2316257) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA Curated by ChEMBL | Assay Description Binding affinity to human MMP12 catalytic domain (98 to 266) by isothermal titration calorimetry | J Med Chem 56: 1149-59 (2013) Article DOI: 10.1021/jm301574d BindingDB Entry DOI: 10.7270/Q24F1S24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530237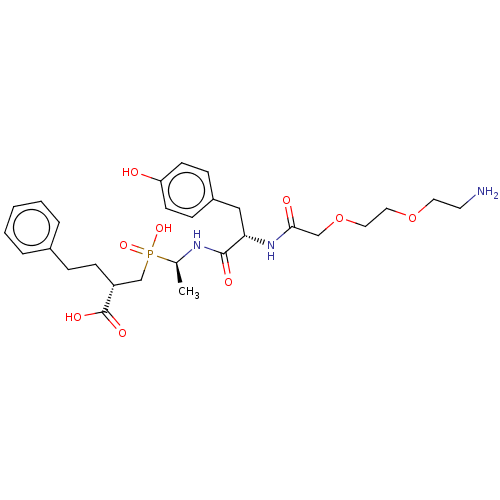 (CHEMBL4513310) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM120258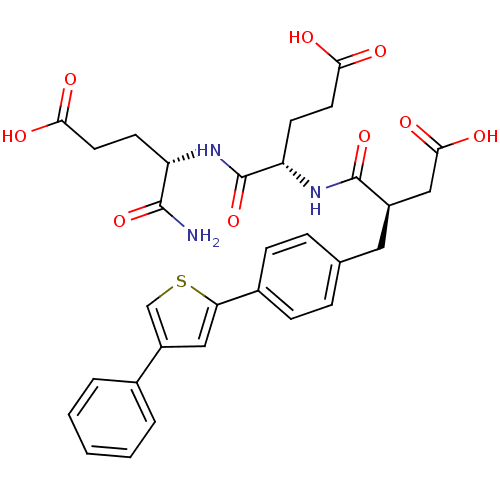 (US8691753, 95 bis) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530237 (CHEMBL4513310) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21471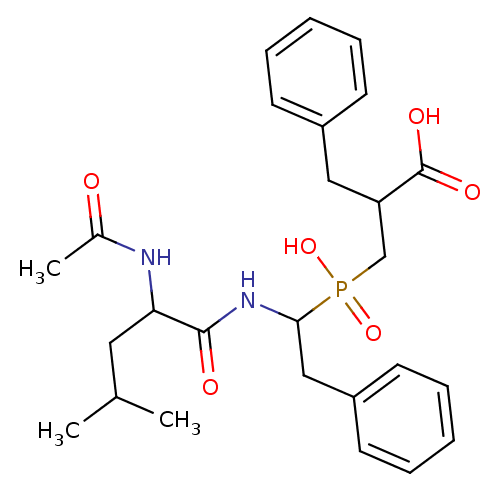 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanamido)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A4 (Homo sapiens) | BDBM50530229 (CHEMBL4460840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA4 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A4 (Homo sapiens) | BDBM50530229 (CHEMBL4460840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA4 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303321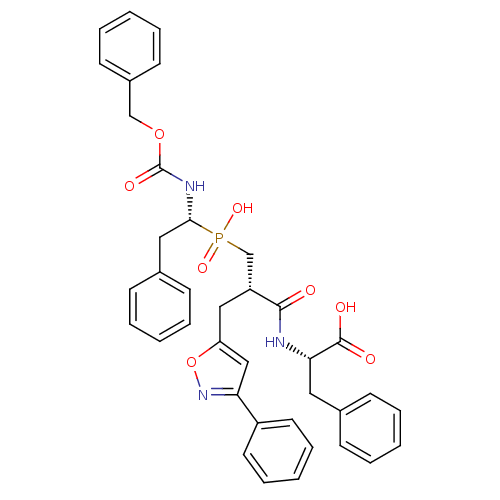 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE C-terminal domain | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21474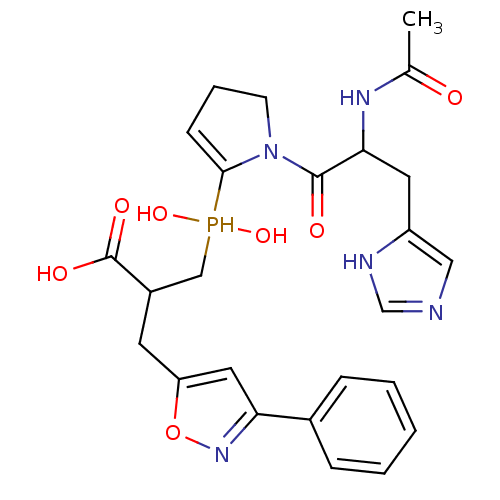 (3-({1-[2-acetamido-3-(1H-imidazol-4-yl)propanoyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303319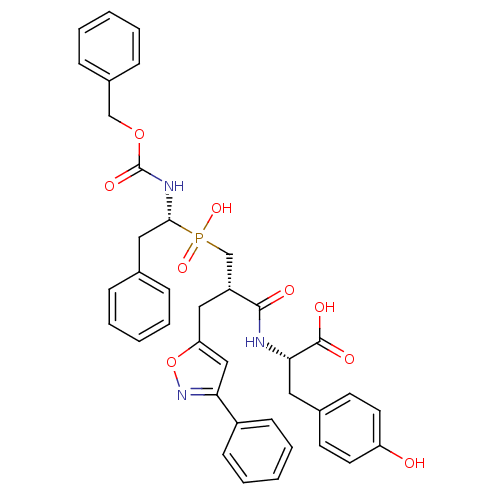 ((S)-2-[(S)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE C-terminal domain | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530231 (CHEMBL4528407) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate assessed as apparent first-order rate constant measured at pre-steady state by measuring Koff/Kon ra... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530231 (CHEMBL4528407) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate assessed as apparent first-order rate constant measured at pre-steady state by measuring Koff/Kon ra... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21464 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50078937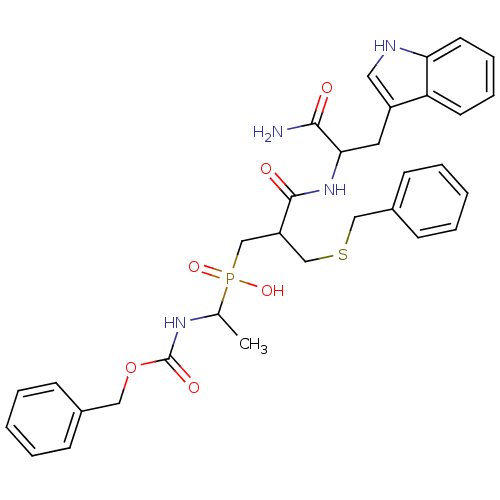 ((1-Benzyloxycarbonylamino-ethyl)-{3-benzylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-8 | J Med Chem 42: 2610-20 (1999) Article DOI: 10.1021/jm9900164 BindingDB Entry DOI: 10.7270/Q22B8X71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50530227 (CHEMBL4454162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of human CPA1 using AAFP as substrate assessed as apparent first-order rate constant measured at pre-steady state by measuring Koff/Kon ra... | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1601 total ) | Next | Last >> |