 Found 239 hits with Last Name = 'dixit' and Initial = 'a'
Found 239 hits with Last Name = 'dixit' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24516

((2E,5E)-5-[(4-methoxyphenyl)methylidene]-2-(1,3-th...)Show InChI InChI=1S/C14H11N3O2S2/c1-19-10-4-2-9(3-5-10)8-11-12(18)16-14(21-11)17-13-15-6-7-20-13/h2-8H,1H3,(H,15,16,17,18)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | -28.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24524

((2E,5E)-2-(1,3-benzothiazol-2-ylimino)-5-[(4-metho...)Show SMILES COc1ccc(\C=C2\S\C(NC2=O)=N\c2nc3ccccc3s2)cc1 Show InChI InChI=1S/C18H13N3O2S2/c1-23-12-8-6-11(7-9-12)10-15-16(22)20-18(25-15)21-17-19-13-4-2-3-5-14(13)24-17/h2-10H,1H3,(H,19,20,21,22)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | -28.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24523

((2E,5E)-2-{[4-(adamantan-1-yl)-1,3-thiazol-2-yl]im...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N\c2nc(cs2)C23CC4CC(CC(C4)C2)C3)ccc1O |TLB:21:22:26:20.25.19,THB:21:20:26:22.27.23,23:24:22.21.27:19,23:22:24.25.26:19| Show InChI InChI=1S/C24H25N3O3S2/c1-30-18-7-13(2-3-17(18)28)8-19-21(29)26-23(32-19)27-22-25-20(12-31-22)24-9-14-4-15(10-24)6-16(5-14)11-24/h2-3,7-8,12,14-16,28H,4-6,9-11H2,1H3,(H,25,26,27,29)/b19-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24526
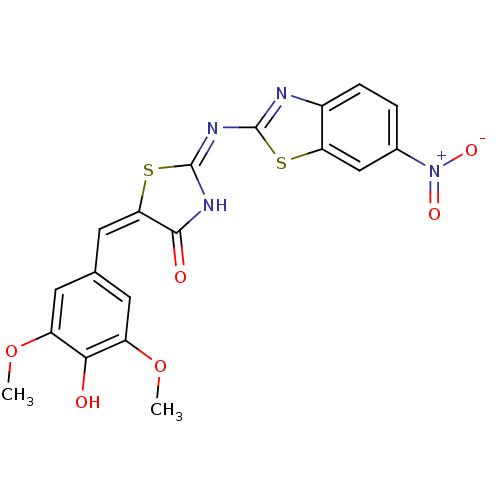
((2E,5E)-5-[(4-hydroxy-3,5-dimethoxyphenyl)methylid...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N\c2nc3ccc(cc3s2)[N+]([O-])=O)cc(OC)c1O Show InChI InChI=1S/C19H14N4O6S2/c1-28-12-5-9(6-13(29-2)16(12)24)7-15-17(25)21-19(31-15)22-18-20-11-4-3-10(23(26)27)8-14(11)30-18/h3-8,24H,1-2H3,(H,20,21,22,25)/b15-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+4 | -27.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24527

((2E,5E)-2-(1,3-benzothiazol-2-ylimino)-5-[(4-nitro...)Show SMILES [O-][N+](=O)c1ccc(\C=C2\S\C(NC2=O)=N\c2nc3ccccc3s2)cc1 Show InChI InChI=1S/C17H10N4O3S2/c22-15-14(9-10-5-7-11(8-6-10)21(23)24)26-17(19-15)20-16-18-12-3-1-2-4-13(12)25-16/h1-9H,(H,18,19,20,22)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62E+4 | -27.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24525

((2E,5E)-2-(1,3-benzothiazol-2-ylimino)-5-[(4-hydro...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N\c2nc3ccccc3s2)cc(OC)c1O Show InChI InChI=1S/C19H15N3O4S2/c1-25-12-7-10(8-13(26-2)16(12)23)9-15-17(24)21-19(28-15)22-18-20-11-5-3-4-6-14(11)27-18/h3-9,23H,1-2H3,(H,20,21,22,24)/b15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.81E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24529
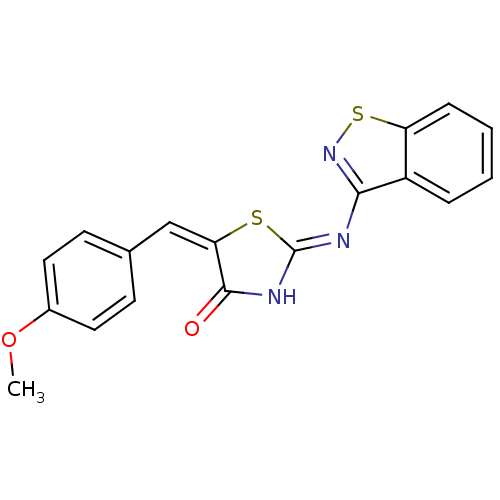
((2Z,5E)-2-(1,2-benzothiazol-3-ylimino)-5-[(4-metho...)Show SMILES COc1ccc(\C=C2\S\C(NC2=O)=N/c2nsc3ccccc23)cc1 Show InChI InChI=1S/C18H13N3O2S2/c1-23-12-8-6-11(7-9-12)10-15-17(22)20-18(24-15)19-16-13-4-2-3-5-14(13)25-21-16/h2-10H,1H3,(H,19,20,21,22)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.28E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24515
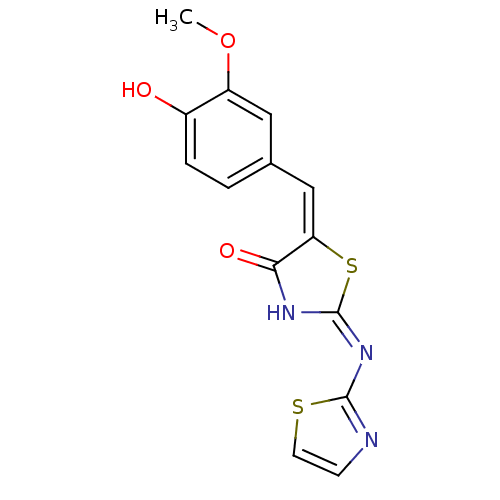
((2E,5E)-5-[(4-hydroxy-3-methoxyphenyl)methylidene]...)Show InChI InChI=1S/C14H11N3O3S2/c1-20-10-6-8(2-3-9(10)18)7-11-12(19)16-14(22-11)17-13-15-4-5-21-13/h2-7,18H,1H3,(H,15,16,17,19)/b11-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.56E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24528

((2E,5E)-2-(1,3-benzothiazol-2-ylimino)-5-[(3-nitro...)Show SMILES [O-][N+](=O)c1cccc(\C=C2\S\C(NC2=O)=N\c2nc3ccccc3s2)c1 Show InChI InChI=1S/C17H10N4O3S2/c22-15-14(9-10-4-3-5-11(8-10)21(23)24)26-17(19-15)20-16-18-12-6-1-2-7-13(12)25-16/h1-9H,(H,18,19,20,22)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.87E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24521
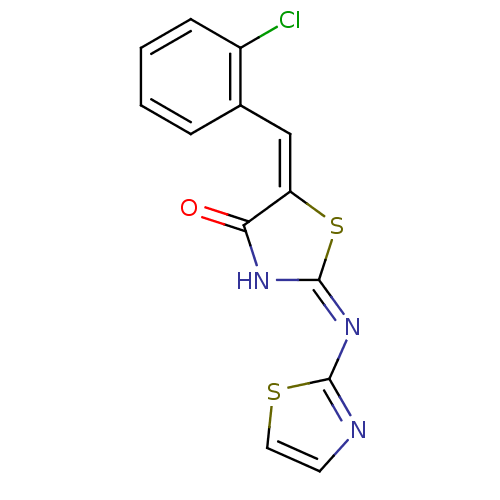
((2E,5E)-5-[(2-chlorophenyl)methylidene]-2-(1,3-thi...)Show InChI InChI=1S/C13H8ClN3OS2/c14-9-4-2-1-3-8(9)7-10-11(18)16-13(20-10)17-12-15-5-6-19-12/h1-7H,(H,15,16,17,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.23E+4 | -25.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24519
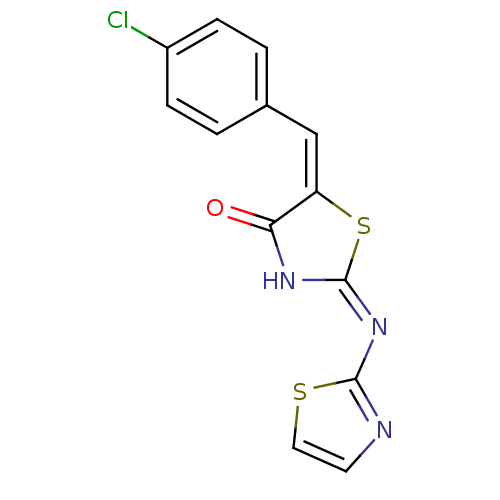
((2E,5E)-5-[(4-chlorophenyl)methylidene]-2-(1,3-thi...)Show InChI InChI=1S/C13H8ClN3OS2/c14-9-3-1-8(2-4-9)7-10-11(18)16-13(20-10)17-12-15-5-6-19-12/h1-7H,(H,15,16,17,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.45E+4 | -24.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24530

((2Z,5E)-2-(1,2-benzothiazol-3-ylimino)-5-[(4-hydro...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N/c2nsc3ccccc23)ccc1O Show InChI InChI=1S/C18H13N3O3S2/c1-24-13-8-10(6-7-12(13)22)9-15-17(23)20-18(25-15)19-16-11-4-2-3-5-14(11)26-21-16/h2-9,22H,1H3,(H,19,20,21,23)/b15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.08E+4 | -23.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24522

((2E,5E)-5-[(4-hydroxy-3-methoxyphenyl)methylidene]...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N\c2nc(cs2)-c2ccccc2)ccc1O Show InChI InChI=1S/C20H15N3O3S2/c1-26-16-9-12(7-8-15(16)24)10-17-18(25)22-20(28-17)23-19-21-14(11-27-19)13-5-3-2-4-6-13/h2-11,24H,1H3,(H,21,22,23,25)/b17-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+5 | -22.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24513
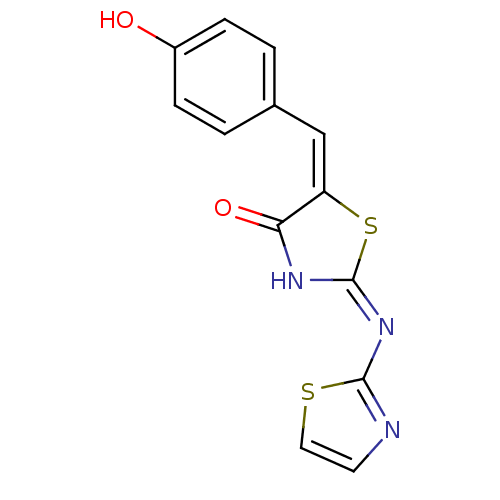
((2E,5E)-5-[(4-hydroxyphenyl)methylidene]-2-(1,3-th...)Show InChI InChI=1S/C13H9N3O2S2/c17-9-3-1-8(2-4-9)7-10-11(18)15-13(20-10)16-12-14-5-6-19-12/h1-7,17H,(H,14,15,16,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.88E+5 | -19.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24517

((2E,5E)-5-[(4-nitrophenyl)methylidene]-2-(1,3-thia...)Show SMILES [O-][N+](=O)c1ccc(\C=C2\S\C(NC2=O)=N\c2nccs2)cc1 Show InChI InChI=1S/C13H8N4O3S2/c18-11-10(7-8-1-3-9(4-2-8)17(19)20)22-13(15-11)16-12-14-5-6-21-12/h1-7H,(H,14,15,16,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.74E+5 | -19.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24518
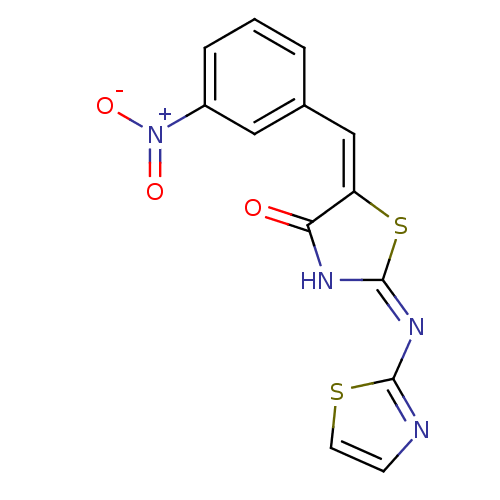
((2E,5E)-5-[(3-nitrophenyl)methylidene]-2-(1,3-thia...)Show SMILES [O-][N+](=O)c1cccc(\C=C2\S\C(NC2=O)=N\c2nccs2)c1 Show InChI InChI=1S/C13H8N4O3S2/c18-11-10(7-8-2-1-3-9(6-8)17(19)20)22-13(15-11)16-12-14-4-5-21-12/h1-7H,(H,14,15,16,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.48E+5 | -18.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
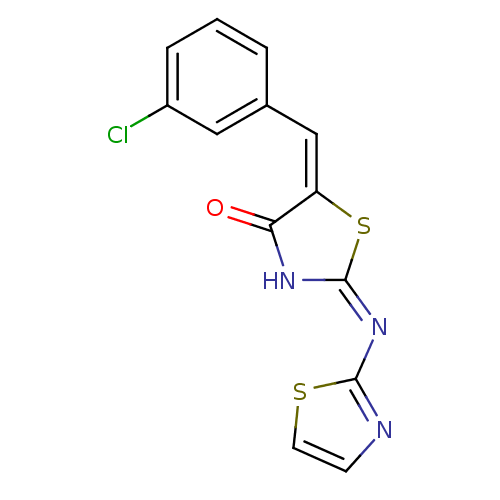
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24520
((2E,5E)-5-[(3-chlorophenyl)methylidene]-2-(1,3-thi...)Show InChI InChI=1S/C13H8ClN3OS2/c14-9-3-1-2-8(6-9)7-10-11(18)16-13(20-10)17-12-15-4-5-19-12/h1-7H,(H,15,16,17,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+6 | -16.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.11E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418564
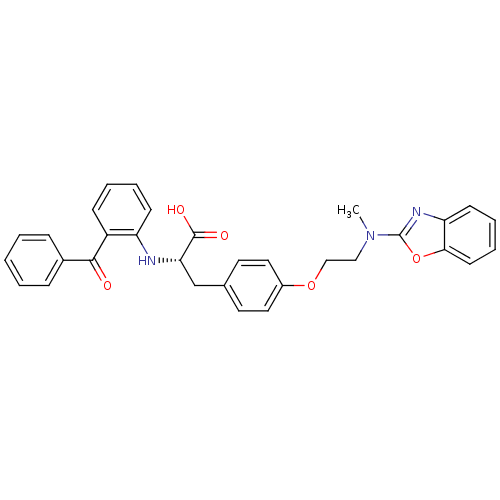
(CHEMBL423026)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H29N3O5/c1-35(32-34-27-13-7-8-14-29(27)40-32)19-20-39-24-17-15-22(16-18-24)21-28(31(37)38)33-26-12-6-5-11-25(26)30(36)23-9-3-2-4-10-23/h2-18,28,33H,19-21H2,1H3,(H,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085048
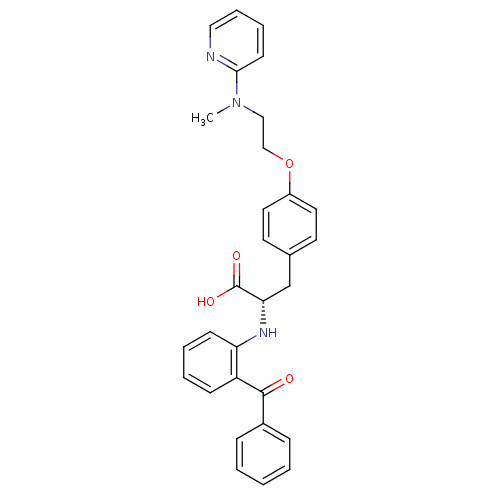
((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1ccccn1 Show InChI InChI=1S/C30H29N3O4/c1-33(28-13-7-8-18-31-28)19-20-37-24-16-14-22(15-17-24)21-27(30(35)36)32-26-12-6-5-11-25(26)29(34)23-9-3-2-4-10-23/h2-18,27,32H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.13E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418576

(CHEMBL1785077)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2Oc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C31H29N3O5/c1-34(31-33-26-12-6-8-14-29(26)39-31)19-20-37-23-17-15-22(16-18-23)21-27(30(35)36)32-25-11-5-7-13-28(25)38-24-9-3-2-4-10-24/h2-18,27,32H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418568
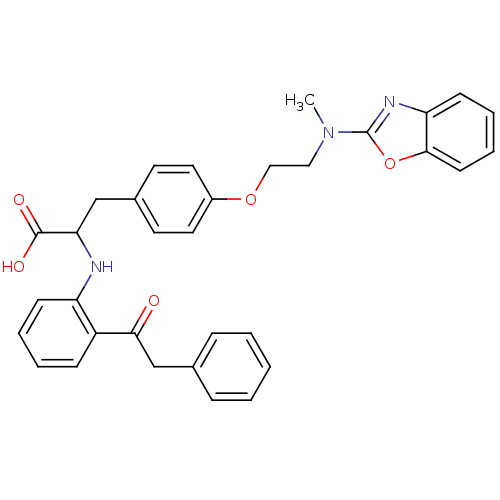
(CHEMBL146822)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)Cc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H31N3O5/c1-36(33-35-28-13-7-8-14-31(28)41-33)19-20-40-25-17-15-24(16-18-25)21-29(32(38)39)34-27-12-6-5-11-26(27)30(37)22-23-9-3-2-4-10-23/h2-18,29,34H,19-22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418575
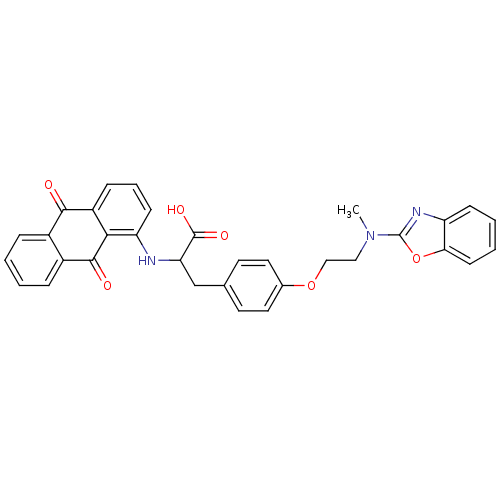
(CHEMBL358137)Show SMILES CN(CCOc1ccc(CC(Nc2cccc3C(=O)c4ccccc4C(=O)c23)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H27N3O6/c1-36(33-35-25-10-4-5-12-28(25)42-33)17-18-41-21-15-13-20(14-16-21)19-27(32(39)40)34-26-11-6-9-24-29(26)31(38)23-8-3-2-7-22(23)30(24)37/h2-16,27,34H,17-19H2,1H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418563
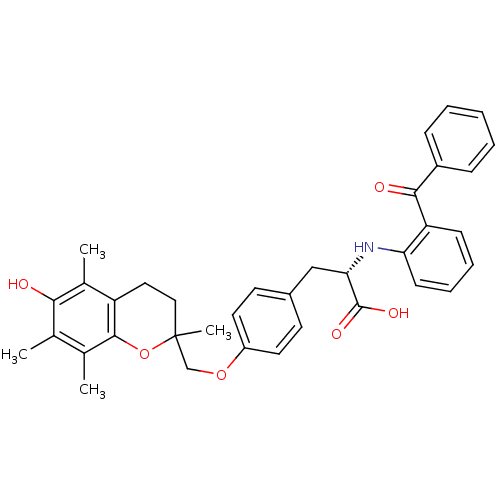
(CHEMBL148301)Show SMILES Cc1c(C)c2OC(C)(COc3ccc(C[C@H](Nc4ccccc4C(=O)c4ccccc4)C(O)=O)cc3)CCc2c(C)c1O Show InChI InChI=1S/C36H37NO6/c1-22-23(2)34-28(24(3)32(22)38)18-19-36(4,43-34)21-42-27-16-14-25(15-17-27)20-31(35(40)41)37-30-13-9-8-12-29(30)33(39)26-10-6-5-7-11-26/h5-17,31,37-38H,18-21H2,1-4H3,(H,40,41)/t31-,36?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418571
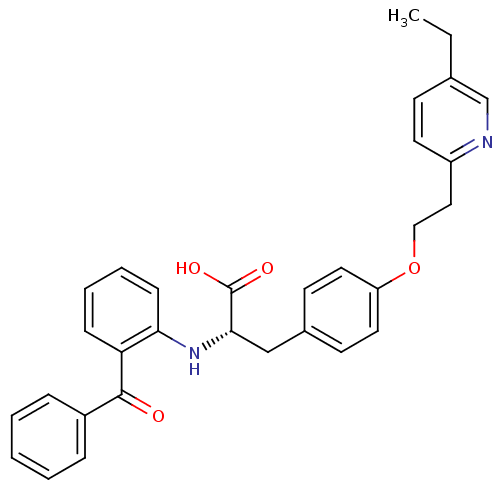
(CHEMBL346219)Show SMILES CCc1ccc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)nc1 Show InChI InChI=1S/C31H30N2O4/c1-2-22-12-15-25(32-21-22)18-19-37-26-16-13-23(14-17-26)20-29(31(35)36)33-28-11-7-6-10-27(28)30(34)24-8-4-3-5-9-24/h3-17,21,29,33H,2,18-20H2,1H3,(H,35,36)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.22E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418557
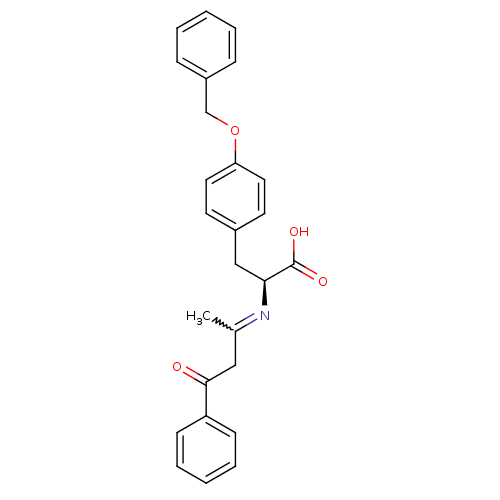
(CHEMBL1785028)Show SMILES CC(CC(=O)c1ccccc1)=N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(O)=O |r,w:1.0| Show InChI InChI=1S/C26H25NO4/c1-19(16-25(28)22-10-6-3-7-11-22)27-24(26(29)30)17-20-12-14-23(15-13-20)31-18-21-8-4-2-5-9-21/h2-15,24H,16-18H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418565

(CHEMBL1785075)Show SMILES CN(CCOc1ccc(CC(Cc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H30N2O5/c1-35(33-34-29-13-7-8-14-30(29)40-33)19-20-39-27-17-15-23(16-18-27)21-26(32(37)38)22-25-11-5-6-12-28(25)31(36)24-9-3-2-4-10-24/h2-18,26H,19-22H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418566
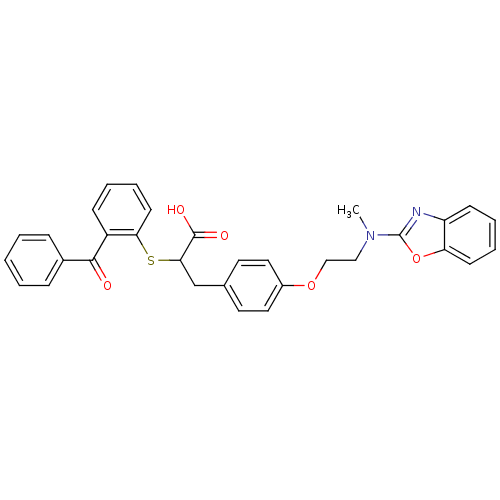
(CHEMBL146322)Show SMILES CN(CCOc1ccc(CC(Sc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H28N2O5S/c1-34(32-33-26-12-6-7-13-27(26)39-32)19-20-38-24-17-15-22(16-18-24)21-29(31(36)37)40-28-14-8-5-11-25(28)30(35)23-9-3-2-4-10-23/h2-18,29H,19-21H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418573
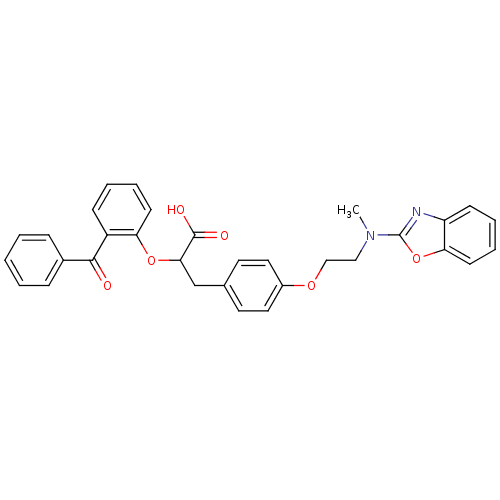
(CHEMBL147694)Show SMILES CN(CCOc1ccc(CC(Oc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H28N2O6/c1-34(32-33-26-12-6-8-14-28(26)40-32)19-20-38-24-17-15-22(16-18-24)21-29(31(36)37)39-27-13-7-5-11-25(27)30(35)23-9-3-2-4-10-23/h2-18,29H,19-21H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.33E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418562
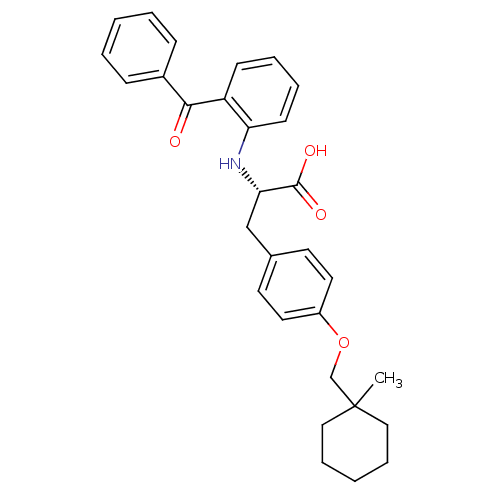
(CHEMBL357479)Show SMILES CC1(COc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)CCCCC1 Show InChI InChI=1S/C30H33NO4/c1-30(18-8-3-9-19-30)21-35-24-16-14-22(15-17-24)20-27(29(33)34)31-26-13-7-6-12-25(26)28(32)23-10-4-2-5-11-23/h2,4-7,10-17,27,31H,3,8-9,18-21H2,1H3,(H,33,34)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.37E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418567
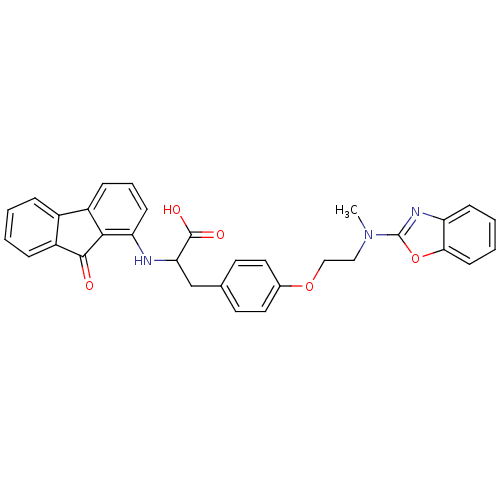
(CHEMBL148060)Show SMILES CN(CCOc1ccc(CC(Nc2cccc3-c4ccccc4C(=O)c23)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H27N3O5/c1-35(32-34-25-10-4-5-12-28(25)40-32)17-18-39-21-15-13-20(14-16-21)19-27(31(37)38)33-26-11-6-9-23-22-7-2-3-8-24(22)30(36)29(23)26/h2-16,27,33H,17-19H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.41E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418569
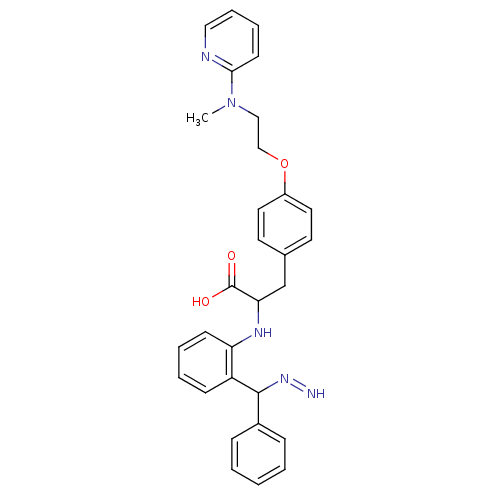
(CHEMBL145829)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(N=N)c2ccccc2)C(O)=O)cc1)c1ccccn1 Show InChI InChI=1S/C30H31N5O3/c1-35(28-13-7-8-18-32-28)19-20-38-24-16-14-22(15-17-24)21-27(30(36)37)33-26-12-6-5-11-25(26)29(34-31)23-9-3-2-4-10-23/h2-18,27,29,31,33H,19-21H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418560
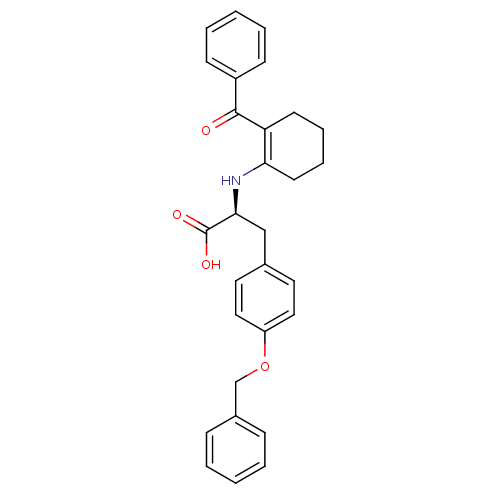
(CHEMBL1785031)Show SMILES OC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC1=C(CCCC1)C(=O)c1ccccc1 |r,t:22| Show InChI InChI=1S/C29H29NO4/c31-28(23-11-5-2-6-12-23)25-13-7-8-14-26(25)30-27(29(32)33)19-21-15-17-24(18-16-21)34-20-22-9-3-1-4-10-22/h1-6,9-12,15-18,27,30H,7-8,13-14,19-20H2,(H,32,33)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.47E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418574
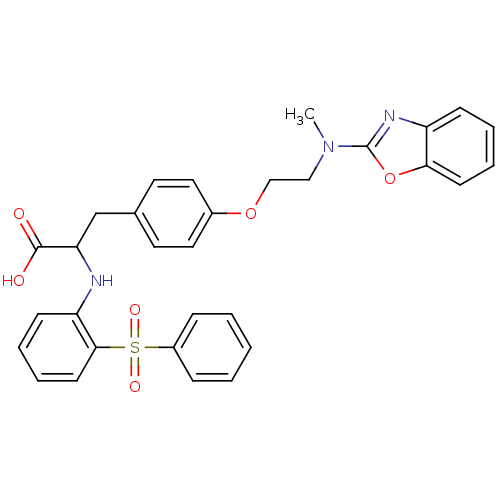
(CHEMBL146123)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2S(=O)(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C31H29N3O6S/c1-34(31-33-25-11-5-7-13-28(25)40-31)19-20-39-23-17-15-22(16-18-23)21-27(30(35)36)32-26-12-6-8-14-29(26)41(37,38)24-9-3-2-4-10-24/h2-18,27,32H,19-21H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.47E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418559
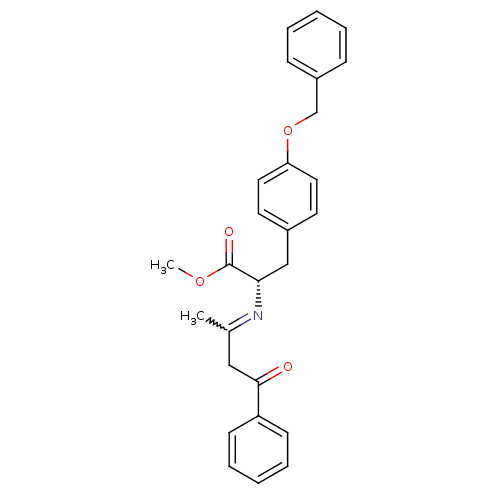
(CHEMBL1785030)Show SMILES COC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)N=C(C)CC(=O)c1ccccc1 |r,w:21.23| Show InChI InChI=1S/C27H27NO4/c1-20(17-26(29)23-11-7-4-8-12-23)28-25(27(30)31-2)18-21-13-15-24(16-14-21)32-19-22-9-5-3-6-10-22/h3-16,25H,17-19H2,1-2H3/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.63E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418572
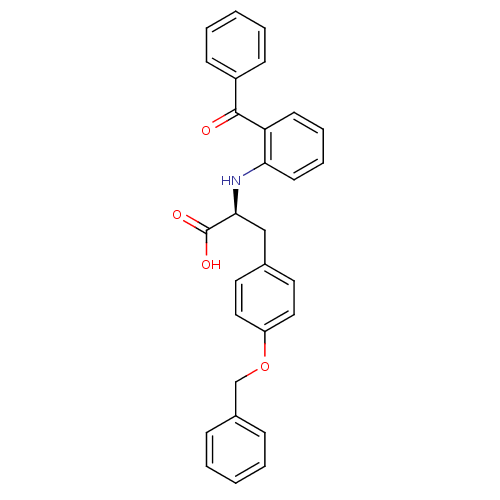
(CHEMBL359377)Show SMILES OC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)Nc1ccccc1C(=O)c1ccccc1 Show InChI InChI=1S/C29H25NO4/c31-28(23-11-5-2-6-12-23)25-13-7-8-14-26(25)30-27(29(32)33)19-21-15-17-24(18-16-21)34-20-22-9-3-1-4-10-22/h1-18,27,30H,19-20H2,(H,32,33)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418561
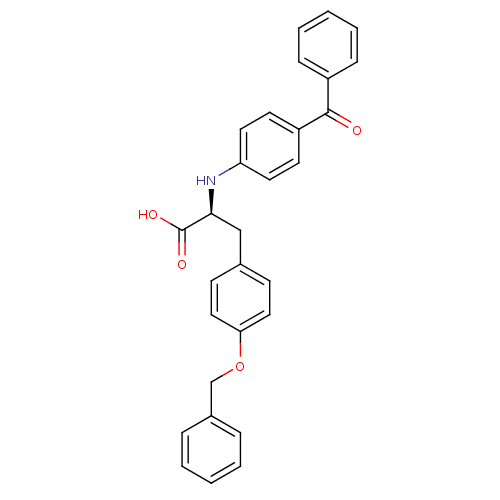
(CHEMBL147042)Show SMILES OC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)Nc1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C29H25NO4/c31-28(23-9-5-2-6-10-23)24-13-15-25(16-14-24)30-27(29(32)33)19-21-11-17-26(18-12-21)34-20-22-7-3-1-4-8-22/h1-18,27,30H,19-20H2,(H,32,33)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.69E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418558
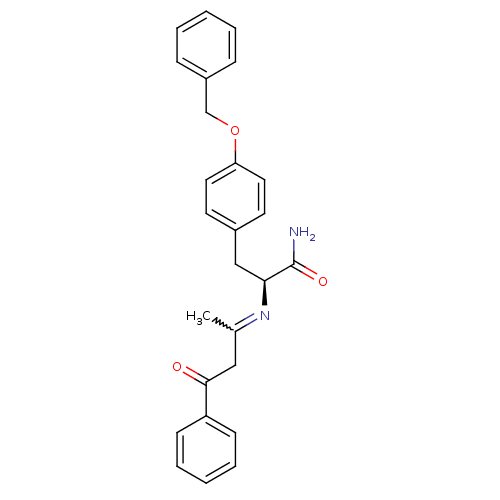
(CHEMBL1785029)Show SMILES CC(CC(=O)c1ccccc1)=N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(N)=O |r,w:1.0| Show InChI InChI=1S/C26H26N2O3/c1-19(16-25(29)22-10-6-3-7-11-22)28-24(26(27)30)17-20-12-14-23(15-13-20)31-18-21-8-4-2-5-9-21/h2-15,24H,16-18H2,1H3,(H2,27,30)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418570
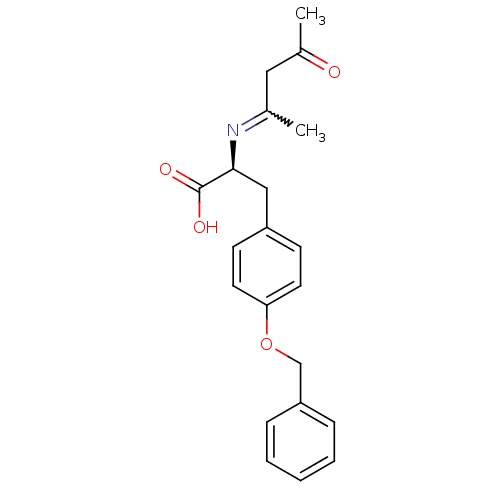
(CHEMBL1785076)Show SMILES CC(=O)CC(C)=N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(O)=O |r,w:4.4| Show InChI InChI=1S/C21H23NO4/c1-15(12-16(2)23)22-20(21(24)25)13-17-8-10-19(11-9-17)26-14-18-6-4-3-5-7-18/h3-11,20H,12-14H2,1-2H3,(H,24,25)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma |
Eur J Med Chem 43: 73-80 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.004
BindingDB Entry DOI: 10.7270/Q2XG9SCP |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426742

(CHEMBL2322167)Show InChI InChI=1S/C16H18N4O2/c1-16(2,3)9-22-13-5-4-10(6-11(13)8-17)12-7-14(21)20-15(18)19-12/h4-7H,9H2,1-3H3,(H3,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426763
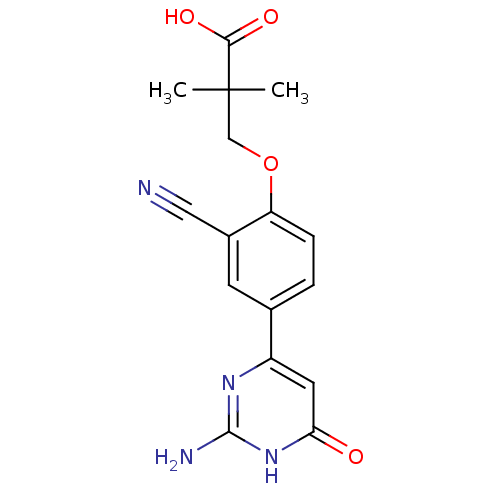
(CHEMBL2322147)Show SMILES CC(C)(COc1ccc(cc1C#N)-c1cc(=O)[nH]c(N)n1)C(O)=O Show InChI InChI=1S/C16H16N4O4/c1-16(2,14(22)23)8-24-12-4-3-9(5-10(12)7-17)11-6-13(21)20-15(18)19-11/h3-6H,8H2,1-2H3,(H,22,23)(H3,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426761

(CHEMBL2322149)Show SMILES Nc1nc(cc(=O)[nH]1)-c1ccc(OCc2ccc(cc2)C(O)=O)c(c1)C#N Show InChI InChI=1S/C19H14N4O4/c20-9-14-7-13(15-8-17(24)23-19(21)22-15)5-6-16(14)27-10-11-1-3-12(4-2-11)18(25)26/h1-8H,10H2,(H,25,26)(H3,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426769

(CHEMBL2322169)Show SMILES Nc1nc(cc(=O)[nH]1)-c1ccc(OCc2ccc(F)cc2)c(c1)C#N Show InChI InChI=1S/C18H13FN4O2/c19-14-4-1-11(2-5-14)10-25-16-6-3-12(7-13(16)9-20)15-8-17(24)23-18(21)22-15/h1-8H,10H2,(H3,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426768

(CHEMBL2322170)Show SMILES Nc1nc(cc(=O)[nH]1)-c1ccc(OCc2ccccc2C(F)(F)F)c(c1)C#N Show InChI InChI=1S/C19H13F3N4O2/c20-19(21,22)14-4-2-1-3-12(14)10-28-16-6-5-11(7-13(16)9-23)15-8-17(27)26-18(24)25-15/h1-8H,10H2,(H3,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426759

(CHEMBL2322151)Show SMILES Nc1nc(cc(=O)[nH]1)-c1ccc(OCC2CCC(CC2)C(O)=O)c(c1)C#N |(13.46,-38.13,;14.8,-37.36,;16.13,-38.13,;17.47,-37.36,;17.46,-35.81,;16.13,-35.05,;16.12,-33.51,;14.8,-35.82,;18.8,-38.13,;20.12,-37.35,;21.46,-38.12,;21.46,-39.66,;22.8,-40.43,;22.8,-41.97,;24.14,-42.74,;24.13,-44.27,;25.46,-45.04,;26.8,-44.27,;26.8,-42.73,;25.46,-41.95,;28.14,-45.04,;28.13,-46.58,;29.47,-44.27,;20.13,-40.44,;18.8,-39.67,;20.14,-41.97,;20.14,-43.51,)| Show InChI InChI=1S/C19H20N4O4/c20-9-14-7-13(15-8-17(24)23-19(21)22-15)5-6-16(14)27-10-11-1-3-12(4-2-11)18(25)26/h5-8,11-12H,1-4,10H2,(H,25,26)(H3,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426760

(CHEMBL2322150)Show SMILES COC(=O)c1ccc(COc2ccc(cc2C#N)-c2cc(=O)[nH]c(N)n2)cc1 Show InChI InChI=1S/C20H16N4O4/c1-27-19(26)13-4-2-12(3-5-13)11-28-17-7-6-14(8-15(17)10-21)16-9-18(25)24-20(22)23-16/h2-9H,11H2,1H3,(H3,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426764

(CHEMBL2322146)Show SMILES Nc1nc(cc(=O)[nH]1)-c1ccc(OCC2CCCCC2)c(c1)C#N Show InChI InChI=1S/C18H20N4O2/c19-10-14-8-13(15-9-17(23)22-18(20)21-15)6-7-16(14)24-11-12-4-2-1-3-5-12/h6-9,12H,1-5,11H2,(H3,20,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50436848

(CHEMBL2403617)Show SMILES CC(C)[C@H](NC(=O)c1ncc(s1)-c1ccc(Nc2nc3ccc(F)cc3s2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H19FN4O3S2/c1-11(2)18(21(29)30)27-19(28)20-24-10-17(31-20)12-3-6-14(7-4-12)25-22-26-15-8-5-13(23)9-16(15)32-22/h3-11,18H,1-2H3,(H,25,26)(H,27,28)(H,29,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... |
Eur J Med Chem 65: 337-47 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.006
BindingDB Entry DOI: 10.7270/Q2QJ7JPW |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426746
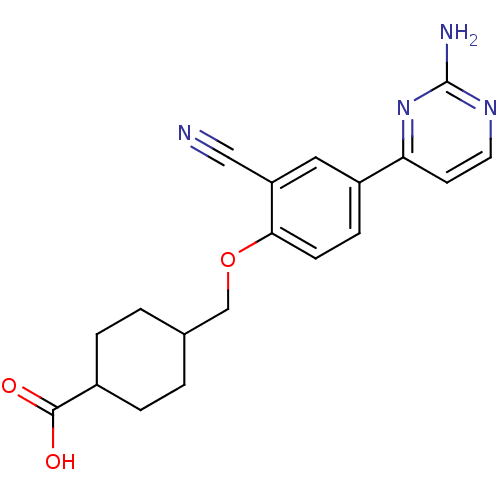
(CHEMBL2322163)Show SMILES Nc1nccc(n1)-c1ccc(OCC2CCC(CC2)C(O)=O)c(c1)C#N |(11.11,-13.05,;12.45,-12.28,;12.45,-10.74,;13.78,-9.97,;15.12,-10.73,;15.12,-12.28,;13.79,-13.05,;16.45,-13.05,;17.78,-12.28,;19.12,-13.04,;19.12,-14.59,;20.46,-15.36,;20.46,-16.9,;21.8,-17.66,;21.8,-19.21,;23.13,-19.97,;24.47,-19.2,;24.46,-17.65,;23.12,-16.89,;25.8,-19.96,;25.8,-21.48,;27.12,-19.19,;17.79,-15.36,;16.45,-14.59,;17.8,-16.9,;17.8,-18.44,)| Show InChI InChI=1S/C19H20N4O3/c20-10-15-9-14(16-7-8-22-19(21)23-16)5-6-17(15)26-11-12-1-3-13(4-2-12)18(24)25/h5-9,12-13H,1-4,11H2,(H,24,25)(H2,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50426744

(CHEMBL2322165)Show InChI InChI=1S/C17H20N4O2/c1-3-11(4-2)10-23-15-6-5-12(7-13(15)9-18)14-8-16(22)21-17(19)20-14/h5-8,11H,3-4,10H2,1-2H3,(H3,19,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 834-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.057
BindingDB Entry DOI: 10.7270/Q28W3FMN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data