 Found 94 hits with Last Name = 'dixon' and Initial = 'k'
Found 94 hits with Last Name = 'dixon' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749
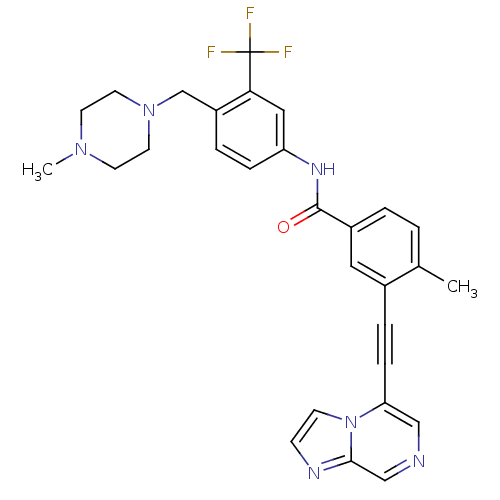
(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748
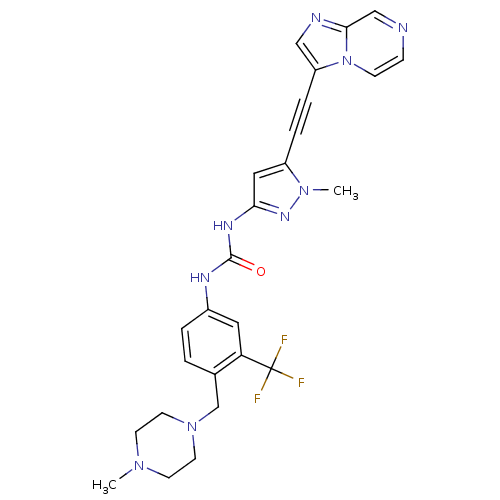
(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404
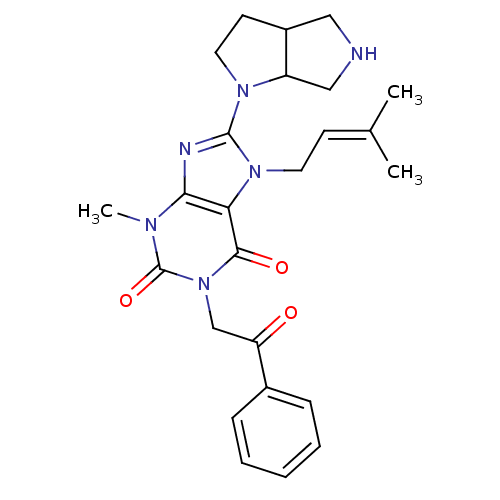
(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427747
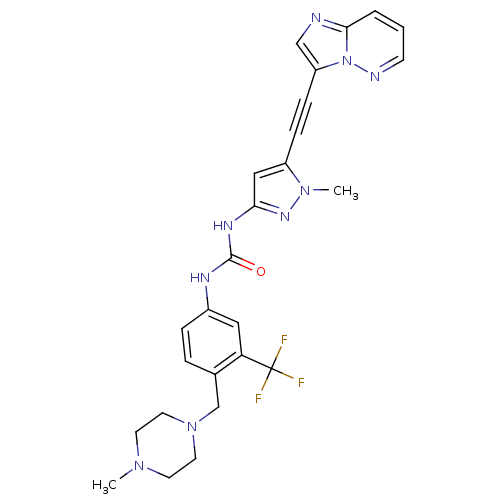
(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427750
(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427747

(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437395
(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427742
(CHEMBL2324930)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)C#Cc2cnc3cccnn23)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-9-24(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)35-28(39)22-6-7-23(26(17-22)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437395

(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427745
(CHEMBL2324927)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5ccccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C27H27F3N8O/c1-35-11-13-37(14-12-35)18-19-6-7-20(15-23(19)27(28,29)30)32-26(39)33-24-16-21(36(2)34-24)8-9-22-17-31-25-5-3-4-10-38(22)25/h3-7,10,15-17H,11-14,18H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427747

(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427741
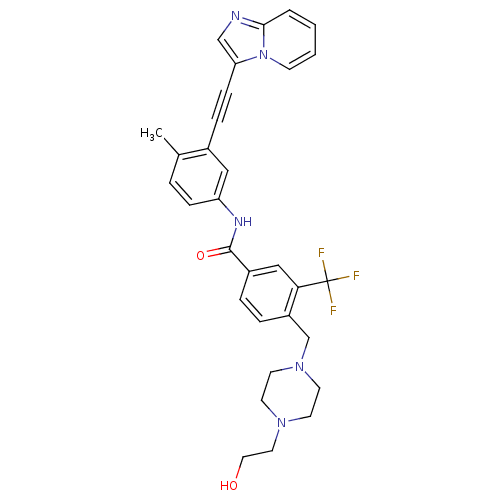
(CHEMBL2324931)Show SMILES Cc1ccc(NC(=O)c2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)cc1C#Cc1cnc2ccccn12 Show InChI InChI=1S/C31H30F3N5O2/c1-22-5-9-26(18-23(22)8-10-27-20-35-29-4-2-3-11-39(27)29)36-30(41)24-6-7-25(28(19-24)31(32,33)34)21-38-14-12-37(13-15-38)16-17-40/h2-7,9,11,18-20,40H,12-17,21H2,1H3,(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427744
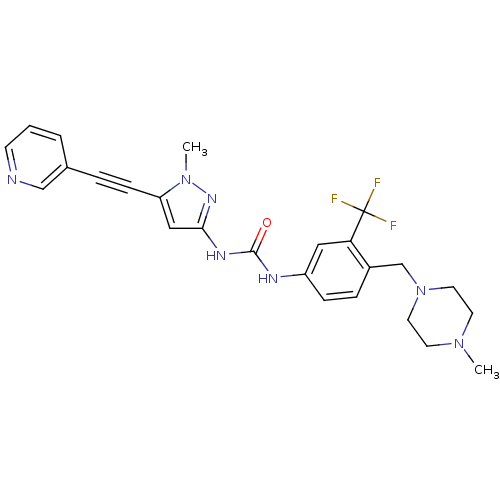
(CHEMBL2324928)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cccnc4)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C25H26F3N7O/c1-33-10-12-35(13-11-33)17-19-6-7-20(14-22(19)25(26,27)28)30-24(36)31-23-15-21(34(2)32-23)8-5-18-4-3-9-29-16-18/h3-4,6-7,9,14-16H,10-13,17H2,1-2H3,(H2,30,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427742

(CHEMBL2324930)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)C#Cc2cnc3cccnn23)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-9-24(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)35-28(39)22-6-7-23(26(17-22)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437396

(CHEMBL2408651)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-13-28-20-21(26-23(28)33-15-18-10-7-12-25-18)27(3)24(32)29(22(20)31)14-19(30)17-8-5-4-6-9-17/h4-6,8-9,11,18,25H,7,10,12-15H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437402
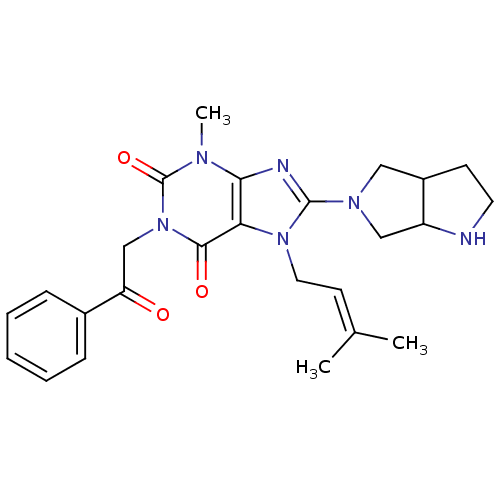
(CHEMBL2408774)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C25H30N6O3/c1-16(2)10-12-30-21-22(27-24(30)29-13-18-9-11-26-19(18)14-29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-8,10,18-19,26H,9,11-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437396

(CHEMBL2408651)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-13-28-20-21(26-23(28)33-15-18-10-7-12-25-18)27(3)24(32)29(22(20)31)14-19(30)17-8-5-4-6-9-17/h4-6,8-9,11,18,25H,7,10,12-15H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427741

(CHEMBL2324931)Show SMILES Cc1ccc(NC(=O)c2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)cc1C#Cc1cnc2ccccn12 Show InChI InChI=1S/C31H30F3N5O2/c1-22-5-9-26(18-23(22)8-10-27-20-35-29-4-2-3-11-39(27)29)36-30(41)24-6-7-25(28(19-24)31(32,33)34)21-38-14-12-37(13-15-38)16-17-40/h2-7,9,11,18-20,40H,12-17,21H2,1H3,(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427743
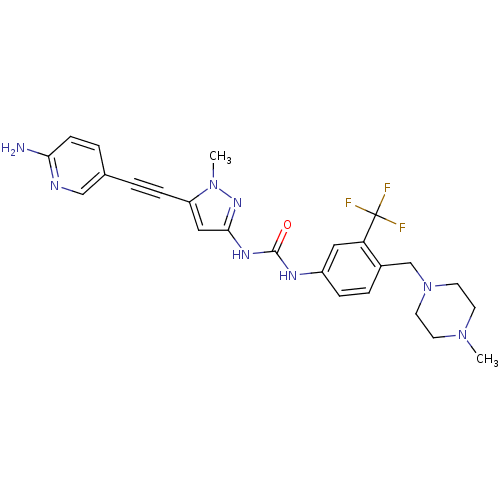
(CHEMBL2324929)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4ccc(N)nc4)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C25H27F3N8O/c1-34-9-11-36(12-10-34)16-18-5-6-19(13-21(18)25(26,27)28)31-24(37)32-23-14-20(35(2)33-23)7-3-17-4-8-22(29)30-15-17/h4-6,8,13-15H,9-12,16H2,1-2H3,(H2,29,30)(H2,31,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50427747

(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427741

(CHEMBL2324931)Show SMILES Cc1ccc(NC(=O)c2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)cc1C#Cc1cnc2ccccn12 Show InChI InChI=1S/C31H30F3N5O2/c1-22-5-9-26(18-23(22)8-10-27-20-35-29-4-2-3-11-39(27)29)36-30(41)24-6-7-25(28(19-24)31(32,33)34)21-38-14-12-37(13-15-38)16-17-40/h2-7,9,11,18-20,40H,12-17,21H2,1H3,(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427747

(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427745

(CHEMBL2324927)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5ccccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C27H27F3N8O/c1-35-11-13-37(14-12-35)18-19-6-7-20(15-23(19)27(28,29)30)32-26(39)33-24-16-21(36(2)34-24)8-9-22-17-31-25-5-3-4-10-38(22)25/h3-7,10,15-17H,11-14,18H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427744

(CHEMBL2324928)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cccnc4)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C25H26F3N7O/c1-33-10-12-35(13-11-33)17-19-6-7-20(14-22(19)25(26,27)28)30-24(36)31-23-15-21(34(2)32-23)8-5-18-4-3-9-29-16-18/h3-4,6-7,9,14-16H,10-13,17H2,1-2H3,(H2,30,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427744

(CHEMBL2324928)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cccnc4)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C25H26F3N7O/c1-33-10-12-35(13-11-33)17-19-6-7-20(14-22(19)25(26,27)28)30-24(36)31-23-15-21(34(2)32-23)8-5-18-4-3-9-29-16-18/h3-4,6-7,9,14-16H,10-13,17H2,1-2H3,(H2,30,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427743

(CHEMBL2324929)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4ccc(N)nc4)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C25H27F3N8O/c1-34-9-11-36(12-10-34)16-18-5-6-19(13-21(18)25(26,27)28)31-24(37)32-23-14-20(35(2)33-23)7-3-17-4-8-22(29)30-15-17/h4-6,8,13-15H,9-12,16H2,1-2H3,(H2,29,30)(H2,31,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427742

(CHEMBL2324930)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)C#Cc2cnc3cccnn23)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-9-24(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)35-28(39)22-6-7-23(26(17-22)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data