

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110961 (US8614206, 518) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206677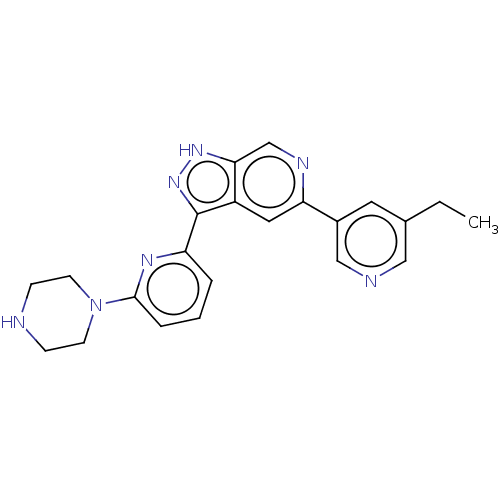 (US9260425, 438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM227170 (US9328106, 118) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505052 (CHEMBL3623150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505054 (CHEMBL4455188) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475328 (CHEMBL414782) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131278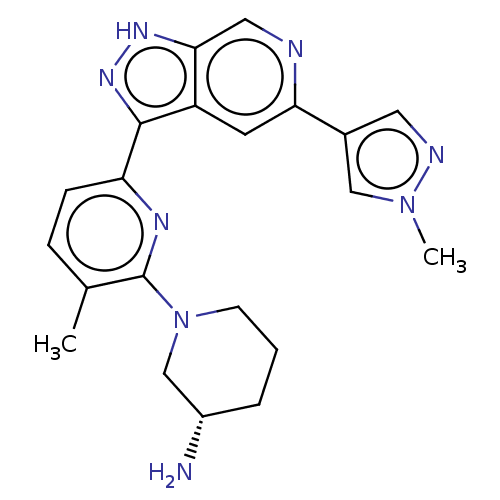 (CHEMBL3634760 | US9260425, 433) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227170 (US9328106, 118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505059 (CHEMBL4459538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505051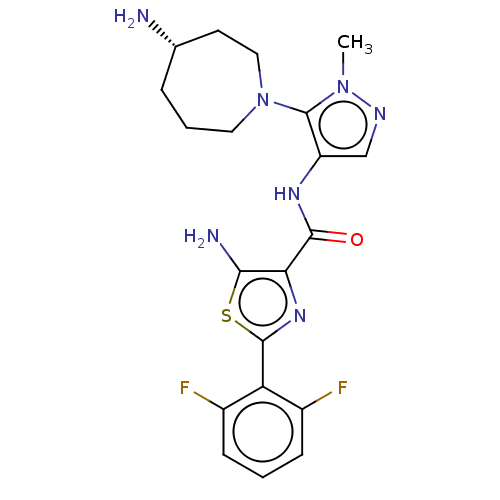 (CHEMBL4437940) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475327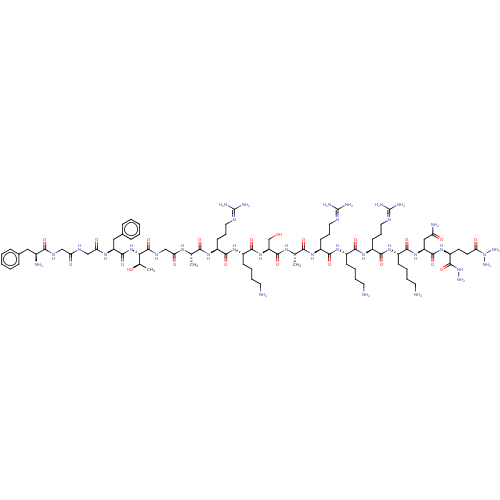 (CHEMBL410653) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50049912 (7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase enzyme from Candida albicans | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206495 (US9260425, 245 | US9260425, 340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248955 (US9434725, 186) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206562 (US9260425, 315) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00806 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206618 (US9260425, 376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM110700 (US8614206, 120 | US8614206, 125 | US8614206, 400) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248989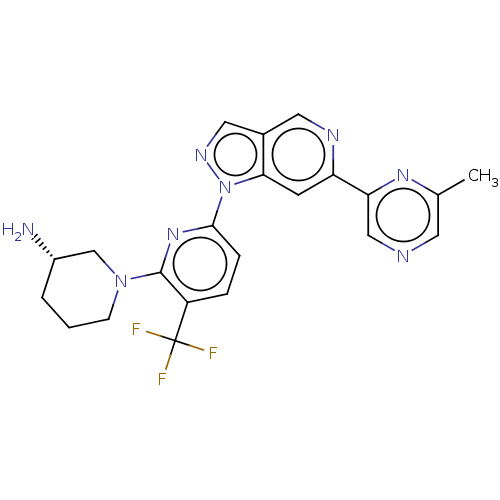 (US9434725, 220) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00900 | -63.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
F. HOFFMANN-LA ROCHE AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US9434725 (2016) BindingDB Entry DOI: 10.7270/Q2CF9P1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505052 (CHEMBL3623150) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206724 (US9260425, 486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505050 (CHEMBL4439756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131224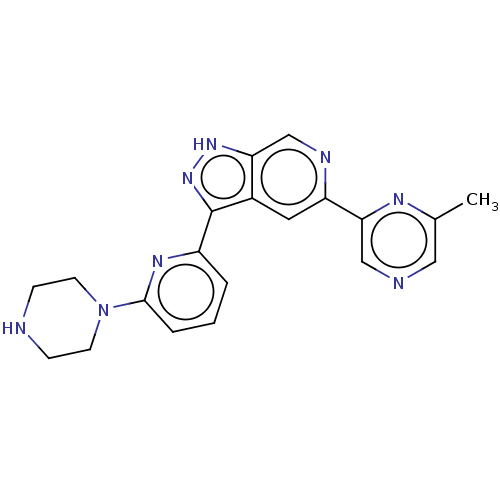 (CHEMBL3634783 | US9260425, 505) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131377 (CHEMBL3634781 | US9260425, 162) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505061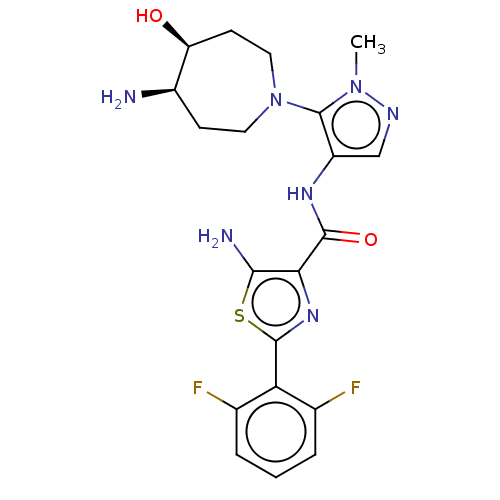 (CHEMBL4453890) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131224 (CHEMBL3634783 | US9260425, 505) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464108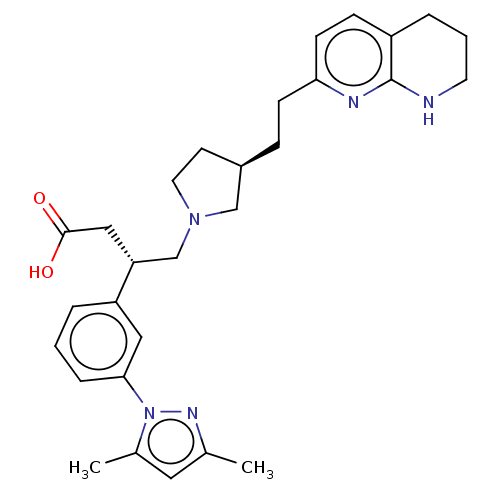 (CHEMBL4241824) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505050 (CHEMBL4439756) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505057 (CHEMBL3676285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505053 (CHEMBL4469964) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474150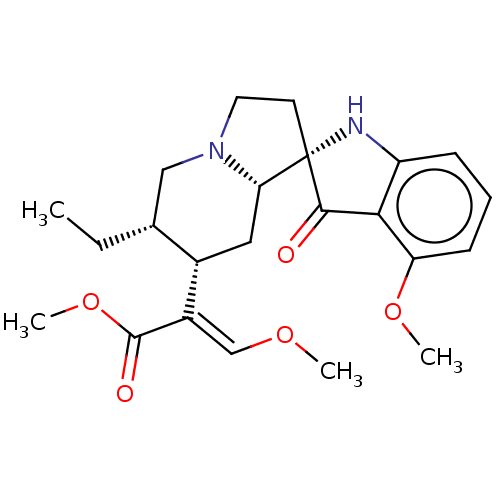 (CHEMBL58362) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor (unknown origin) | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131224 (CHEMBL3634783 | US9260425, 505) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM110961 (US8614206, 518) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206587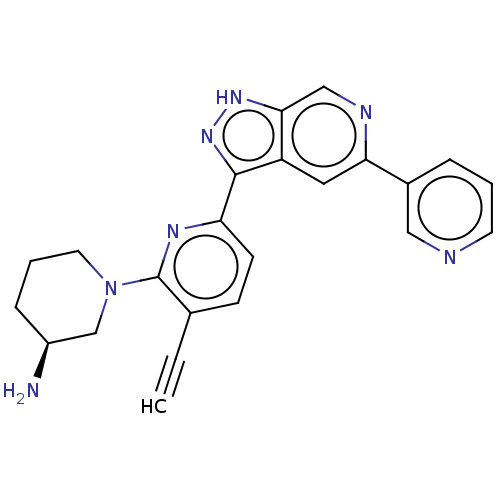 (US9260425, 342) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206555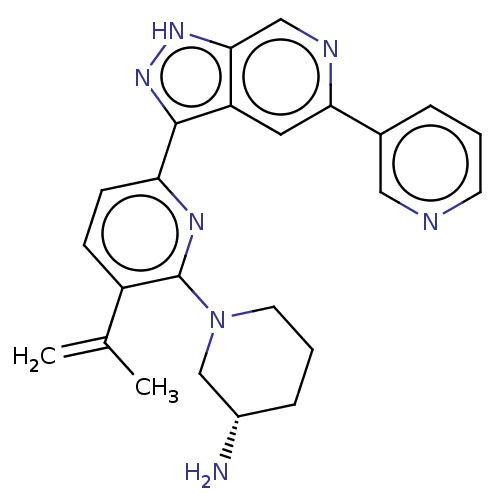 (US9260425, 308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248978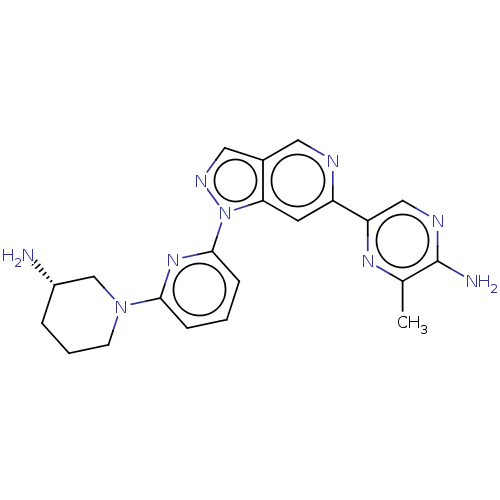 (US9434725, 209) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0110 | -62.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
F. HOFFMANN-LA ROCHE AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US9434725 (2016) BindingDB Entry DOI: 10.7270/Q2CF9P1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248984 (US9434725, 215) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0110 | -62.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
F. HOFFMANN-LA ROCHE AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US9434725 (2016) BindingDB Entry DOI: 10.7270/Q2CF9P1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM248977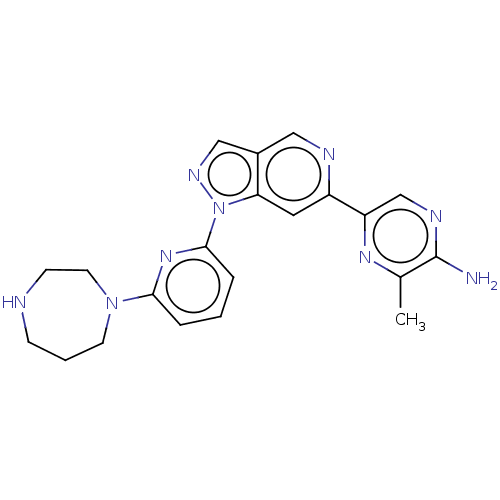 (US9434725, 208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | -62.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
F. HOFFMANN-LA ROCHE AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US9434725 (2016) BindingDB Entry DOI: 10.7270/Q2CF9P1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131278 (CHEMBL3634760 | US9260425, 433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131227 (CHEMBL3634771 | US9260425, 473) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206715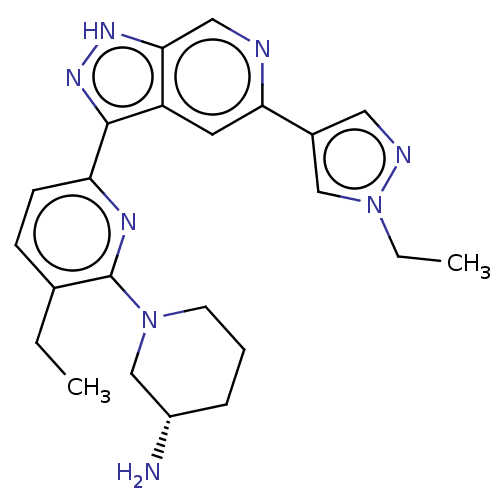 (US9260425, 477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131278 (CHEMBL3634760 | US9260425, 433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131261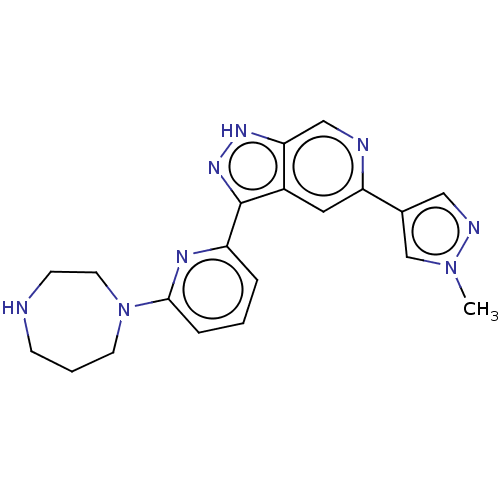 (CHEMBL3634767) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Transcriptional activation of Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248955 (US9434725, 186) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0130 | -62.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
F. HOFFMANN-LA ROCHE AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US9434725 (2016) BindingDB Entry DOI: 10.7270/Q2CF9P1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206727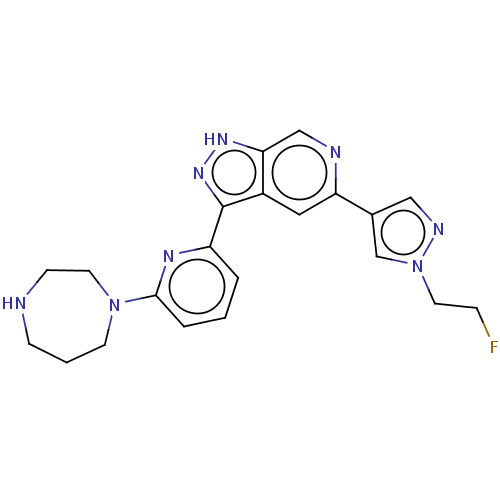 (US9260425, 489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030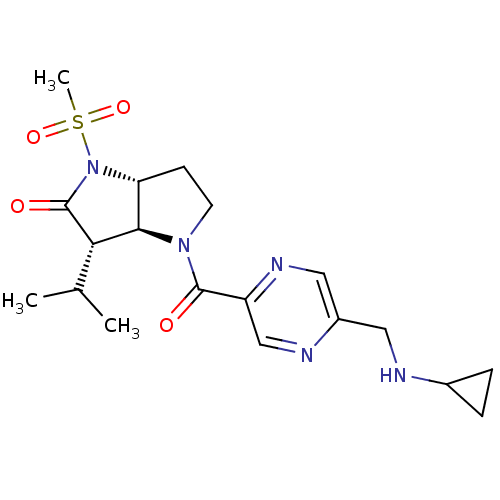 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206651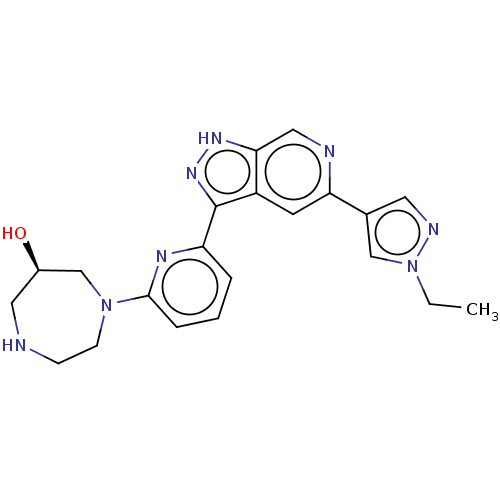 (US9260425, 411) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248977 (US9434725, 208) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
F. HOFFMANN-LA ROCHE AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US9434725 (2016) BindingDB Entry DOI: 10.7270/Q2CF9P1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248974 (US9434725, 205) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
F. HOFFMANN-LA ROCHE AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US9434725 (2016) BindingDB Entry DOI: 10.7270/Q2CF9P1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM248950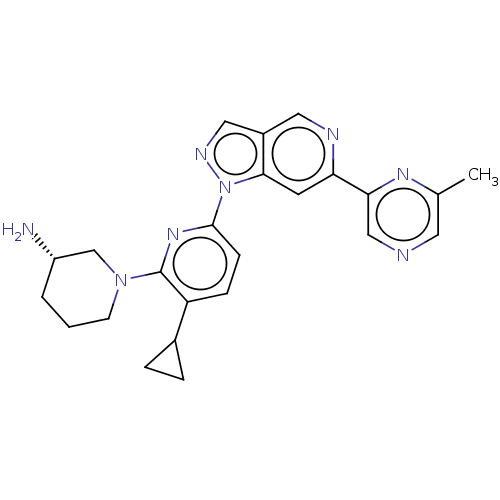 (US9434725, 181) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
F. HOFFMANN-LA ROCHE AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US9434725 (2016) BindingDB Entry DOI: 10.7270/Q2CF9P1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75880 total ) | Next | Last >> |