

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50005685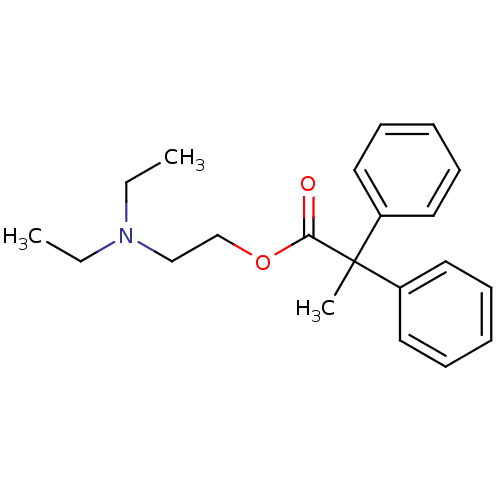 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018230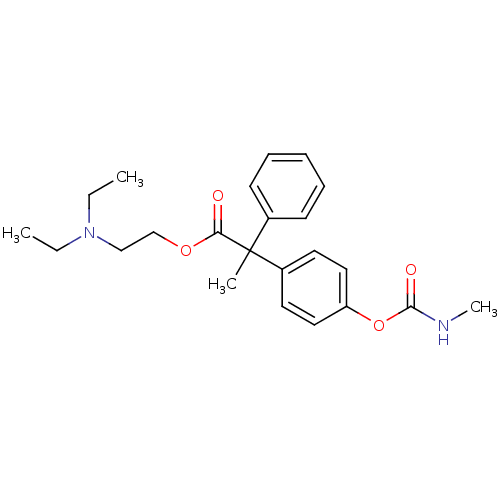 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Binding affinity was determined from the inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 subtype. | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50403547 (ATROPEN | ATROPINE) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity towards Acetylcholinesterase from fetal bovine serum | Bioorg Med Chem Lett 10: 2467-9 (2001) BindingDB Entry DOI: 10.7270/Q2R211X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant for the inhibition of fetal bovine serum acetylcholinesterase was determined.. | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant for the inhibition of fetal bovine serum acetylcholinesterase was determined.. | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50093799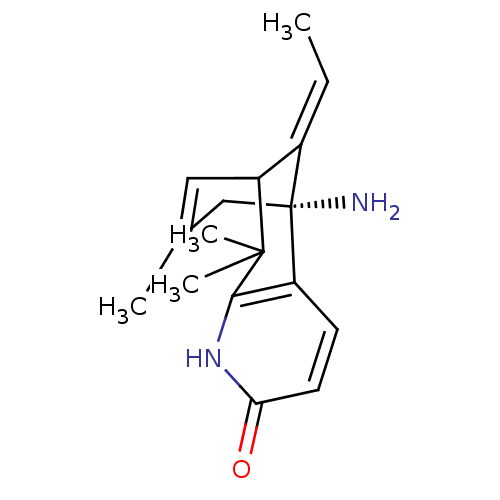 ((R)-1-Amino-13-eth-(E)-ylidene-8,8,11-trimethyl-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity towards Acetylcholinesterase from fetal bovine serum | Bioorg Med Chem Lett 10: 2467-9 (2001) BindingDB Entry DOI: 10.7270/Q2R211X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50093799 ((R)-1-Amino-13-eth-(E)-ylidene-8,8,11-trimethyl-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant for the inhibition of fetal bovine serum acetylcholinesterase was determined.. | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibitory constant against fetal bovine serum (FBS) acetylcholinesterase | Bioorg Med Chem Lett 12: 1521-3 (2002) BindingDB Entry DOI: 10.7270/Q2FX79ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018230 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50093799 ((R)-1-Amino-13-eth-(E)-ylidene-8,8,11-trimethyl-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity towards Acetylcholinesterase from fetal bovine serum | Bioorg Med Chem Lett 10: 2467-9 (2001) BindingDB Entry DOI: 10.7270/Q2R211X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50093799 ((R)-1-Amino-13-eth-(E)-ylidene-8,8,11-trimethyl-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity towards Acetylcholinesterase from fetal bovine serum | Bioorg Med Chem Lett 10: 2467-9 (2001) BindingDB Entry DOI: 10.7270/Q2R211X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018225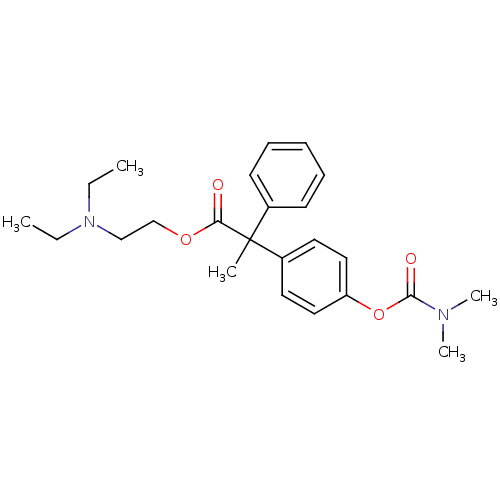 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Binding affinity was determined from the inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 subtype. | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50005685 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the muscarinic acetylcholine r... | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018225 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the muscarinic acetylcholine receptor M1 | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50113645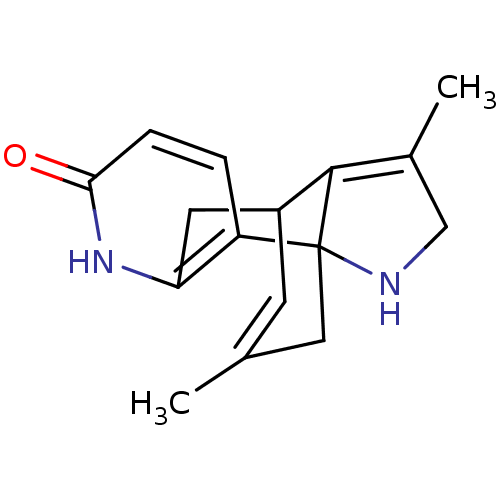 (CHEMBL39918 | Huperzine Analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibitory constant against fetal bovine serum (FBS) acetylcholinesterase | Bioorg Med Chem Lett 12: 1521-3 (2002) BindingDB Entry DOI: 10.7270/Q2FX79ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50113646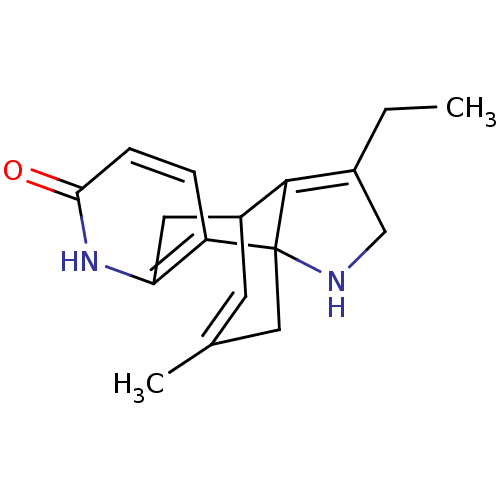 (CHEMBL37105 | Huperzine Analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibitory constant against fetal bovine serum (FBS) acetylcholinesterase | Bioorg Med Chem Lett 12: 1521-3 (2002) BindingDB Entry DOI: 10.7270/Q2FX79ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50199518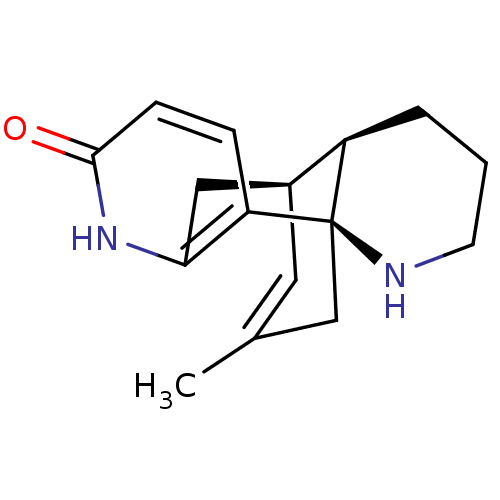 ((-)-huperzine B | CHEMBL245079 | huperzine B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibitory constant against fetal bovine serum (FBS) acetylcholinesterase | Bioorg Med Chem Lett 12: 1521-3 (2002) BindingDB Entry DOI: 10.7270/Q2FX79ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018226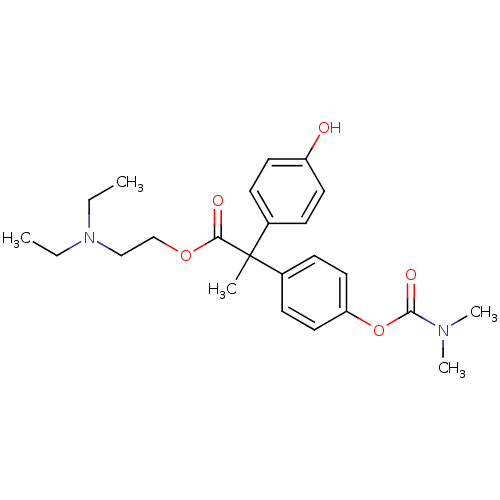 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018225 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018230 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50113648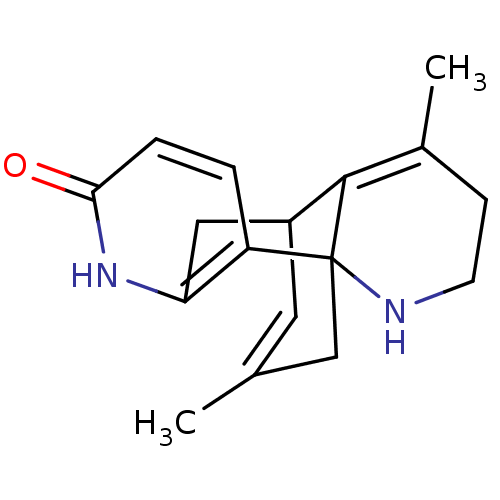 (CHEMBL290113 | Huperzine Analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibitory constant against fetal bovine serum (FBS) acetylcholinesterase | Bioorg Med Chem Lett 12: 1521-3 (2002) BindingDB Entry DOI: 10.7270/Q2FX79ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018227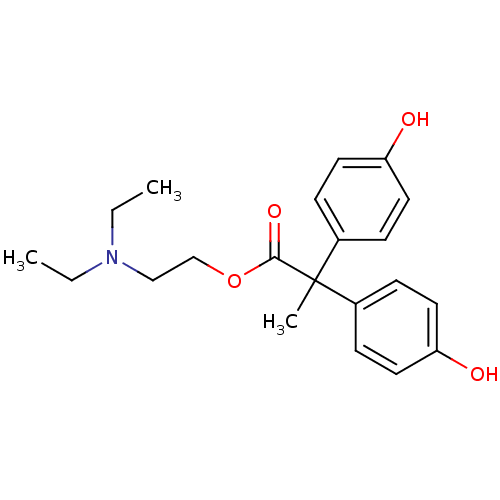 (2,2-Bis-(4-hydroxy-phenyl)-propionic acid 2-diethy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018226 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018226 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50313079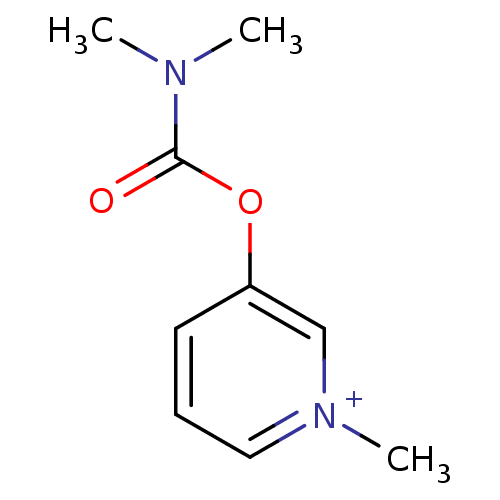 (3-Dimethylcarbamoyloxy-1-methyl-pyridinium; bromid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018228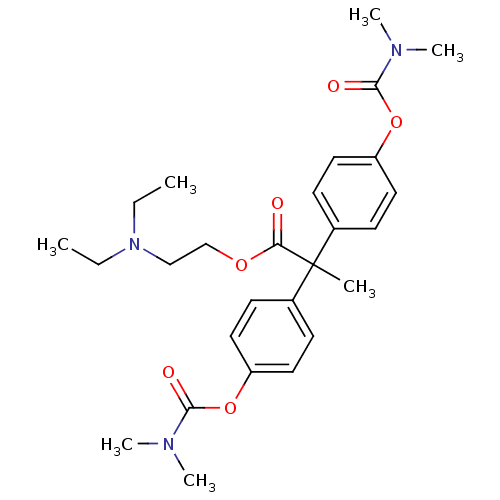 (2,2-Bis-(4-dimethylcarbamoyloxy-phenyl)-propionic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018227 (2,2-Bis-(4-hydroxy-phenyl)-propionic acid 2-diethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50199522 ((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Bioorg Med Chem Lett 23: 1544-7 (2013) Article DOI: 10.1016/j.bmcl.2012.11.083 BindingDB Entry DOI: 10.7270/Q2FB549P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 0.0567 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Kinetic constant for inhibition of fetal bovine serum acetylcholinesterase | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.000367 | 0.0155 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant for the inhibition of fetal bovine serum acetylcholinesterase was determined.. | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 0.0570 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Kinetic constant for inhibition of fetal bovine serum acetylcholinesterase | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50093799 ((R)-1-Amino-13-eth-(E)-ylidene-8,8,11-trimethyl-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant for the inhibition of fetal bovine serum acetylcholinesterase was determined.. | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.000333 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant for the inhibition of fetal bovine serum acetylcholinesterase was determined.. | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50093799 ((R)-1-Amino-13-eth-(E)-ylidene-8,8,11-trimethyl-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.000217 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Kinetic constant for inhibition of fetal bovine serum acetylcholinesterase | Bioorg Med Chem Lett 6: 259-262 (1996) Article DOI: 10.1016/0960-894X(96)00012-1 BindingDB Entry DOI: 10.7270/Q2R49R8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||